Abstract
Objective: Asthma is a common heterogeneous disease characterized by airway inflammation and bronchoconstriction. Current treatment guidelines provide recommendations for categorizing disease severity, asthma control and management. This paper reviews asthma assessment in primary care and describes the pathophysiology, clinical characteristics and new targeted treatments available for patients with severe eosinophilic asthma.
Methods: A non-systematic PubMed literature search was conducted and articles, primarily from the last 5 years, were selected based on relevance to primary care practice, asthma pathophysiology and biologic therapies.
Results: Despite optimal therapy including high-dose inhaled corticosteroids (ICS), long-acting β2-agonists and tiotropium, ∼4–10% of all patients with severe asthma continue to have poor asthma control. These patients have impaired quality of life, frequent exacerbations and are exposed to the side effects of repeated courses of oral steroids. Approximately 50% of patients with severe uncontrolled asthma have eosinophilic asthma, with increased airway expression of type 2 cytokines IL-4, IL-5 and IL-13. Eosinophilic asthma is identified in primary care by having eosinophils ≥150–300 cells/μL on a complete blood count with differential.
Conclusions: A new class of agents is available for patients with moderate to severe eosinophilic asthma. Four biologic therapies – mepolizumab, reslizumab, benralizumab and dupilumab – that interfere with the regulation and activity of eosinophils have been approved by the FDA for patients with moderate to severe asthma with an eosinophilic phenotype. Primary care physicians should be familiar with these medications to explain part of the rationale for referral to specialist care and manage patient expectations for treatment.
Introduction
According to 2015 data, approximately 8% of the total US population has asthmaCitation1. In 2014, asthma was among the 20 most common diagnoses in primary care office visitsCitation2, with an expected increase worldwideCitation3. Most patients with asthma in the US are managed by primary care physiciansCitation4,Citation5, highlighting the need for primary care physicians to be familiar with identification, classification and therapeutic management.
Severity and control are crucial, but separate, aspects of asthma management. Asthma control is the extent to which a patient’s asthma symptoms can be observed or have been reduced by therapy, while asthma severity refers to the level of treatment required to control symptoms and prevent exacerbationsCitation6. Frequent exacerbations are also a sign of uncontrolled asthma; patients with frequent exacerbations despite maximal maintenance therapy should be reassessed for control to determine why it was not achieved or was lostCitation6. A patient with mild asthma may still have uncontrolled disease, and a patient with severe asthma may have good control of their symptoms. Asthma is a dynamic disease and, as such, the severity of a patient’s asthma may change over timeCitation6. It is therefore important to regularly assess whether current therapy is appropriate or whether modifications should be made to increase or decrease the level of intervention using structured reviews to ensure treatment optimization. Furthermore, making the correct initial diagnosis is critical: two different studies have estimated that 12% to 33% of patients diagnosed with uncontrolled asthma suffered from non-asthmatic conditionsCitation7,Citation8.
In 2013, the total cost of asthma in the US was estimated to be $82 billionCitation9. Four to ten percent of patients with asthma are classified as severe, and they account for a disproportionate amount of health care utilization and costsCitation10–13. When severe asthma is uncontrolled, patients are at higher risk for serious exacerbations with subsequent decreased lung function, as well as emergency department visits and extended hospitalizationsCitation14–16. Patients with severe uncontrolled asthma are also more likely to miss work or school because of their disease than those with less severe disease, leading to lost productivity and reduced quality of lifeCitation17,Citation18. The hospitalization rate for patients with controlled asthma in the US is not different from those without asthma, but patients with uncontrolled asthma were hospitalized 4.5-fold more frequently and visited the emergency department 1.8-fold more often than patients without asthmaCitation19. Socioeconomic factors further complicate asthma control, as lower-income populations often have less access to regular care for chronic diseasesCitation20,Citation21. In addition, the burden of asthma varies country to country; for example, a structured approach to care significantly reduced the morbidity and costs associated with asthma in FinlandCitation22.
Patients with asthma have airway inflammation, bronchoconstriction, and symptoms of wheezing and shortness of breath, though the underlying pathophysiology of asthma may vary. A large number of patients with severe asthma that remains uncontrolled despite appropriate therapy with inhaled corticosteroids (ICS) have been found to have high eosinophil counts in their sputum, blood and/or bronchial biopsiesCitation23,Citation24. This eosinophilic phenotype of asthma accounts for approximately 5% of all asthma cases, but occurs in 30% to 50% of all patients with severe asthmaCitation23,Citation25. Eosinophils are a sub-type of white blood cells that migrate to defend against infections, mediate allergic response and contribute to tissue inflammationCitation25–27. Eosinophils contain cytoplasmic granules which, when activated, lead to the production of proinflammatory cytokinesCitation28. Eosinophilic asthma is associated with a higher risk of exacerbation and worse lung function than non-eosinophilic asthmaCitation29.
The goal of this paper is to review the pathophysiology, clinical characteristics and new targeted treatments available for patients with severe, uncontrolled eosinophilic asthma. All patients with severe asthma that remains uncontrolled despite appropriate first-line treatment should be referred to a specialist for further evaluation and treatment. Some patients with severe uncontrolled asthma have an eosinophilic phenotype and may be candidates for therapy with one of the new classes of biologic agents.
Methods
We conducted a non-systematic PubMed literature search for articles, primarily published in the last 5 years, relevant to primary care practice, asthma pathophysiology and biologic therapies. Articles focusing on the mechanism of eosinophilic asthma were of particular interest. In cases where more recent data were not available, we extended this search to include studies published within the last 20 years.
Results
Guideline-based identification and treatment of severe asthma
The 2018 Global Initiative for Asthma (GINA) reportCitation6, consistent with but updated from the 2007 National Asthma Education and Prevention Program (NAEPP) treatment guidelinesCitation30, outlines a stepwise process for the treatment of asthma with increasing degrees of intervention for increasing severity of illness ()Citation6. The 2018 GINA report makes an important distinction between severity and control. Asthma may be considered uncontrolled either because of persistence of symptoms despite appropriate treatment, or due to poor adherence to therapy or use of inhaler devices. Regardless of treatment step, a patient who has experienced three or four of the following characteristics over the past month has asthma that is “uncontrolled” according to GINA: daytime symptoms more than twice per week; reliever medication (e.g. short-acting β2-agonists) needed more than twice per week; any nighttime awakening due to asthma; any activity limitation due to asthmaCitation6. The occurrence of multiple exacerbations is an indicator of severe uncontrolled asthma; having ≥2 exacerbations in the previous year has been part of the inclusion criteria for clinical trials, and may be useful in clinical practice as wellCitation31,Citation32. In a patient with a confirmed diagnosis of asthma, asthma severity is based on the level of treatment required to maintain control of asthma symptoms and exacerbations. Patients who require Step 4 or 5 therapy (medium- or high-dose ICS/long-acting β2-agonist [LABA]), whether or not their asthma symptoms are controlled, are considered to have “severe asthma”. The 2018 GINA report suggests that the level of treatment may need to be adjusted periodically, especially if a patient is not responding to their current treatmentCitation6. Similar evaluations will be crucial as expensive biologic therapies become more widely used. Furthermore, if a patient appears to have severe asthma on optimal non-biologic therapy and the disease is still uncontrolled, the initial diagnosis of asthma may require re-evaluationCitation33. It is possible that some patients may have other diseases that are not asthma but present with persistent asthma-like symptoms such as vocal cord dysfunction. Patients should therefore be carefully evaluated for other underlying conditionsCitation34. Thus, it is essential to review and confirm an asthma diagnosis in patients with persistently uncontrolled asthma.
Figure 1. GINA stepwise asthma treatmentCitation6.
Asthma management requires assessment of current symptoms, adjustment of treatment accordingly and review of the response; assessment should be repeated periodically to ensure optimal treatment. GINA asthma treatment is separated into five steps, with increasing step number corresponding to increasing level of intervention.
Abbreviations. FEV1, Forced expiratory volume in 1 s; GINA, Global Initiative for Asthma; HDM, House dust mite; ICS, Inhaled corticosteroid; IgE, Immunoglobulin-E; IL, Interleukin; LABA, Long-acting β2-agonist; LTRA, Leukotriene receptor antagonist; OCS, Oral corticosteroid; SABA, Short-acting β2-agonist; SLIT, Sublingual immunotherapy. *Not for children <12 years. **For children 6 to 11 years, the preferred Step 3 treatment is medium-dose ICS. †Tiotropium by mist inhaler is an add-on treatment for patients with a history of exacerbations; it is not indicated in children <12 years of age.
From Global Initiative for Asthma (GINA), ‘Global Strategy for Asthma Management and Prevention,’ (2017). Reprinted with permission from GINA, http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/
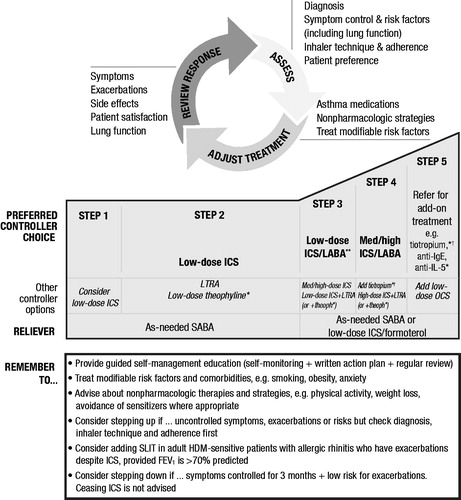
Most patients can achieve good symptom control with standard treatments including low-dose ICS alone, or combination therapy with low-dose ICS and LABA (ICS/LABA) bronchodilators (GINA Step 2–3; )Citation6. However, some patients have more severe underlying disease, and their symptoms may remain uncontrolled despite adherence to high-dose ICS and multiple classes of controller medicationsCitation11. Antimuscarinic agents such as tiotropium are now FDA approved and recommended by GINA for treatment of asthma as add-on therapy for patients aged ≥6 years at GINA Step 4Citation35–37.
In order to identify a patient with uncontrolled asthma, a number of objective measures of asthma symptoms and control have been developed to quantify a patient’s responsiveness to prescribed therapy. Lung function can be measured by spirometry, giving a forced expiratory volume in 1 s (FEV1), and this can be done pre-bronchodilation and post-bronchodilation. FEV1 is compared with predicted values based on the patient’s age, sex, height and ethnicityCitation38. FEV1 alone does not define asthma and can be normal even when a patient with severe asthma is being assessed, but the 2007 NAEPP guidelines state that, in patients aged ≥12 years, reduced FEV1 can indicate moderate (60% < FEV1 < 80% predicted) or severe (FEV1 < 60% predicted) asthmaCitation39. A patient’s symptom control can be assessed by questionnaires such as the Asthma Control Questionnaire (ACQ)Citation40 or the Asthma Control Test (ACT)Citation41, which quantify factors such as frequency of symptoms, frequency of β2-agonist use and activity limitationsCitation42.
Challenges in treating patients with severe asthma in primary care
There are numerous challenges in caring for patients with asthma. Patients may be unwilling or unable to make lifestyle changes, such as avoiding triggers or stopping smoking. There may be problems with medication adherence, which contribute to poor control of asthmaCitation43. An estimated 50% of patients (both adults and children) have poor adherence to taking their medicationsCitation6. In addition, incorrect inhaler technique can result in the patient receiving an insufficient dose of medicationCitation44. Regardless of treatment step, prior to stepping up therapy for a patient with uncontrolled asthma, it is important to determine whether lack of control is due to poor adherence, incorrect inhaler technique or easily addressable environmental exposures, and take appropriate steps to address these issuesCitation45. Providing patients with information about asthma, a personalized asthma action plan and reinforcing proper inhaler technique over the course of multiple visits have been shown to improve control and reduce the risk of exacerbationsCitation46.
Although GINA Step 4 therapy is effective for a large number of patients, some patients with severe disease have asthma that remains uncontrolled despite appropriate therapyCitation6. Chronic or frequent intermittent oral corticosteroid (OCS) use is associated with increased risk of adverse effects, including osteoporosis and diabetes mellitusCitation47–49. A recent analysis of real-world data illustrates that risk is elevated even at relatively low cumulative exposure to OCSCitation50. Once appropriate adherence and technique have been confirmed, it is then helpful to classify asthma by phenotype in order to determine whether referral to an allergist or pulmonologist for consideration of biologics may be usefulCitation6,Citation51.
Immunology of asthma: the role of eosinophils
An understanding of the basic immunology and molecular mechanism contributing to the pathogenesis of asthma is important for appropriate utilization of new biologic therapies approved for the treatment of severe asthma. Type 1 and type 2 immune responses describe different pathways of the immune system that are regulated by CD4 T cellsCitation52. Type 1 immune responses are directed against intracellular bacteria, protozoa and viruses, and are the primary defense against infection. Type 2 immune responses are typically allergic mediated and are associated with atopic diseases such as allergic rhinitis and asthma. In Type 2 immune responses, Type 2 T helper cells secrete cytokines IL-4, IL-5 and IL-13, and stimulate type 2 immunity. Type 2 immunity is characterized by high antibody titers and eosinophilia. Recently, cells associated with the innate immune system, innate lymphoid cells, have been identified and found to occur in three lineages producing similar cytokines to T helper cellsCitation53. The group 2 innate lymphoid cells (ILC2) produce the type 2 cytokines, including IL-4, IL-5 and IL-13Citation54.
In many patients with asthma, the molecular response of airway epithelial cells to a stimulant or trigger is through type 2 inflammation. This type of asthma has been called “type 2 high”. These patients have increased airway expression of type 2 cytokines IL-4, IL-5 and IL-13 compared with the levels typically found in a healthy control populationCitation52,Citation55. These cytokines have effects on many different cell types, but two pathways have emerged as particularly relevant to severe asthma. First, IL-4 and IL-13 switch on B cells to produce immunoglobulin E (IgE), which leads to degranulation of mast cells mediating allergic responsesCitation56. Second, IL-5 promotes eosinophil maturation, differentiation and migration from the bone marrow into the lungs through binding to IL-5 receptors on the eosinophil surfaceCitation28,Citation57,Citation58. Thus, eosinophilic asthma is a result of type 2 inflammationCitation25–27. In the lungs, eosinophils contribute to ongoing inflammation and airway hyperresponsiveness, and may lead to tissue damage and airway remodeling, in patients with asthmaCitation59. Eosinophils can also be recruited into the lungs by IL-5 and other factors associated with the allergic response to an antigenCitation60.
Clinical characteristics associated with eosinophilic asthma
Eosinophilic asthma is associated with increased eosinophil counts in a patient’s sputum, but sputum testing is not widely availableCitation6,Citation23,Citation61. In patients with asthma, there is a correlation between sputum eosinophil counts and the amount of circulating eosinophils in the bloodCitation62,Citation63. Elevated eosinophil count on a peripheral blood smear may serve as a surrogate marker for sputum eosinophilia. In addition, peripheral blood eosinophil counts have been correlated with the severity of asthma exacerbationsCitation64. Although a standard definition has not emerged, peripheral blood eosinophil counts of ≥150 cells/μLCitation65–67, ≥300 cells/μLCitation31,Citation32,Citation68 or ≥400 cells/μLCitation69,Citation70 have been used in registration trials of biologic agents to describe eosinophilic asthma and can readily be identified in a primary care settingCitation69–73. Fractional exhaled nitric oxide (FeNO) is another biomarker that can indicate eosinophilic airway inflammation; FeNO >50 ppb is considered elevated in adults, whereas in children the cutoff is >35 ppbCitation74.
The clinical characteristics of eosinophilic asthma are shown in . After verifying adherence to GINA Step 4 or 5 therapy in patients with uncontrolled asthma, evaluating eosinophil count as a biomarker for underlying type 2 inflammation is a straightforward way to identify eosinophilic asthma, in order to determine whether the patient may potentially benefit from new biologic therapies.
Table 1. Clinical profile of late-onset eosinophilic asthmaCitation23.
New biologic therapies for severe asthma
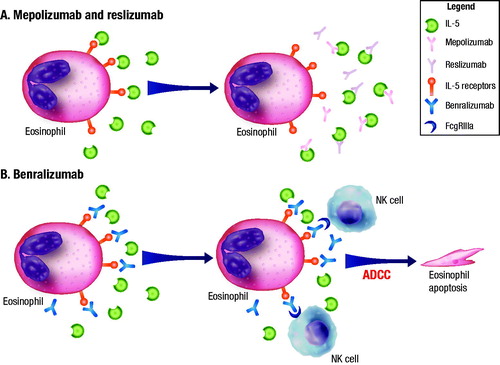
Four biologic therapies that interfere with the regulation and activity of eosinophils are currently FDA approved for severe asthma. Three of these therapies rely on the role of IL-5 in eosinophil maturation, migration, proliferation, differentiation and activationCitation75. Mepolizumab and reslizumab are humanized monoclonal antibodies against IL-5 that inhibit the binding of IL-5 to the IL-5 receptor that is expressed on the surface of both eosinophils and basophils, thereby disrupting the pathway regulating eosinophil maturation and activation, resulting in a decrease in eosinophil counts. Benralizumab is a humanized, afucosylated, monoclonal antibody against the α subunit of the IL-5 receptor. Benralizumab is unique in that the afucosylation (removal of the fucose [sugar] residue) of the antibody enhances the binding of natural killer cells to that part of the antibody, which leads to the rapid and direct depletion of both eosinophils and basophils via antibody-dependent cell-mediated cytotoxicity (ADCC)Citation28,Citation75,Citation76. illustrates the mechanistic difference between the anti-IL-5 agents mepolizumab/reslizumab and the anti-eosinophil agent benralizumabCitation76. Sputum and blood eosinophil counts are reduced following treatment with benralizumabCitation77, mepolizumabCitation78 and reslizumabCitation79. In a phase 1 study of benralizumab, treatment of patients with mild asthma resulted in near complete reductions in peripheral blood eosinophil counts within 24 hCitation80. Furthermore, airway biopsies have shown a decrease in eosinophil counts of 96% with benralizumabCitation77; in a separate study, eosinophil counts measured by airway biopsy were reduced by 55% with mepolizumabCitation78.
Figure 2. Mechanistic differences between mepolizumab/reslizumab and benralizumabCitation76. (A) Mepolizumab and reslizumab are monoclonal antibodies against IL-5 that prevent the binding of IL-5 to the receptor, resulting in eosinophil depletion. (B) Benralizumab is a humanized, afucosylated monoclonal antibody that binds to the α subunit of the IL-5 receptor. The absence of fucose on the Fc domain of benralizumab facilitates binding to FcgRIIIa receptors on NKs, leading to apoptosis of eosinophils (and basophils) through ADCC. The apoptosis of eosinophils does not result in the release of granule contentCitation95. Abbreviations. ADCC, Antibody-dependent cell-mediated cytotoxicity; FcgRIIIa, Low affinity immunoglobulin-γ Fc region receptor III-α; IL, Interleukin; NK, Natural killer cell.
Republished with permission from Dove Medical Press Limited, from ‘Benralizumab: a unique IL-5 inhibitor for severe asthma,’ by Tan, Bratt, Godor, et al; Journal of Asthma and Allergy, 2016:9 71-81.
Another biologic therapy recently approved for asthma is dupilumab, a fully human monoclonal antibody against the α subunit of the IL-4 receptor that simultaneously inhibits IL-4 and IL-13 signalingCitation81. IL-4 and IL-13 both promote IgE synthesis and recruitment of eosinophils proximally in the inflammatory cascade, leading to airway inflammationCitation82. The receptor for both IL-4 and IL-13 contains an α subunit of the IL-4 receptor; dupilumab thus inhibits the binding of these cytokines to their receptors and the subsequent signaling cascadeCitation82. Multiple phase 3 trials have been carried out for each of the biologics, the details of which are presented in .
Figure 3. Findings from key anti-IL-5 and anti-eosinophil studies. Phase 3 trial top-line results for anti-IL-5 and anti-eosinophil agents are summarized.
Abbreviations. ACQ-5, Asthma Control Questionnaire-5; AHR, Airway hyperresponsiveness; DB, Double-blind; EOS, Eosinophil count; FEV1, Forced expiratory volume in 1 s; ICS, Inhaled corticosteroid; IL, Interleukin; IV, Intravenous; LABA, Long-acting β2-agonist; OCS, Oral corticosteroid; PC, Placebo-controlled; PG, Parallel-group; Q2W, Administered every 2 weeks; Q4W, Administered every 4 weeks; Q8W, Administered every 8 weeks; SC, Subcutaneous.

MepolizumabCitation83 is indicated for add-on maintenance treatment of patients with severe asthma (≥12 years of age) and with an eosinophilic phenotype. The safety and efficacy of mepolizumab was studied in two phase 3 randomized, double-blind, placebo-controlled trials (DREAM, MENSA) that enrolled patients with evidence of eosinophilic inflammation and a history of recurrent severe asthma exacerbations while taking high-dose ICS and another controller medicationCitation66,Citation68. A third phase 3 trial (SIRIUS) evaluated the ability of mepolizumab to reduce maintenance OCS dose while maintaining asthma control in patients with eosinophilic inflammation on high-dose ICS plus additional controller medications and taking maintenance OCS for ≥6 monthsCitation65.
Mepolizumab demonstrated significant reductions in annualized exacerbation rate in patients with severe eosinophilic asthma, and reduction in OCS dose in patients with severe asthma requiring maintenance OCS treatment. For the DREAM and MENSA trials, the percentage reduction in annualized exacerbation rate versus placebo ranged between 32% and 53%Citation66,Citation68. In the SIRIUS trial that enrolled patients who required maintenance OCS therapy for at least 6 months prior to the study, the median percentage reduction from baseline in maintenance OCS dose was 50% for mepolizumab, compared with 0% for placebo ()Citation65. Overall, the frequency of adverse events was similar between mepolizumab and placebo in these studies, and the long-term safety profile was similar to that of the initial studies throughout the additional year of mepolizumab treatment with no reports of mepolizumab-related anaphylaxis during the clinical studiesCitation84.
ReslizumabCitation85 is indicated for add-on maintenance treatment of patients with severe asthma ≥18 years of age and with an eosinophilic phenotype. The safety and efficacy of reslizumab was investigated in two identical phase 3 randomized, double-blind, placebo-controlled trials that enrolled patients with evidence of eosinophilic inflammation and a history of exacerbations requiring treatment with an OCS burst while taking medium-dose ICS with or without an additional controllerCitation69. Another phase 3 trial enrolled patients with eosinophilic asthma taking medium-dose ICS with or without an additional controllerCitation70, and a different phase 3 trial enrolled patients taking medium-dose ICS with or without an additional controller regardless of eosinophil countCitation86.
Reslizumab demonstrated reductions in exacerbation frequency as well as improvements in FEV1. Among patients with high blood eosinophil counts (≥400 cells/μL), reslizumab treatment resulted in a 54% reduction in exacerbation frequency compared with placeboCitation69, and resulted in improvements in FEV1 ranging from 115 mL to 242 mL compared with placeboCitation69,Citation70. The effects of reslizumab on the overall population (unselected for eosinophil count) were more modestCitation86. Reslizumab was generally well tolerated; rates of common adverse events with reslizumab were similar to or lower than placeboCitation69,Citation70,Citation86, although two cases of anaphylaxis were reportedCitation69. Reslizumab was well tolerated in a 2-year extension study, with no new safety signals associated with the prolonged exposureCitation87.
BenralizumabCitation88 is indicated for the add-on maintenance treatment of patients with severe asthma ≥12 years of age, and with an eosinophilic phenotype. The safety and efficacy of benralizumab have been investigated in two phase 3 randomized, double-blind, placebo-controlled trials (SIROCCO, CALIMA) that enrolled patients with asthma with a history of exacerbations requiring an OCS burst while taking medium- or high-dose ICS and an additional controller for ≥1 year, and FEV1 <80% predicted (<90% for patients aged 12–17 years)Citation31,Citation32. A third phase 3 trial (ZONDA) enrolled patients with evidence of eosinophilic inflammation, a history of maintenance OCS use and exacerbations while taking medium- or high-dose ICS with an additional controller for ≥1 year (medium) or ≥6 months (high)Citation67.
Benralizumab demonstrated significant reductions in annual exacerbation rates as well as reductions in maintenance OCS dosage while maintaining asthma control. In patients on high-dosage ICS/LABA with blood eosinophil counts ≥300 cells/μL (SIROCCO and CALIMA), the percentage reduction in exacerbation rate relative to placebo ranged from 28% to 51% and lung function significantly increased from baseline by 106–159 mL (pre-bronchodilator FEV1), compared with placeboCitation31,Citation32. In patients with blood eosinophil counts ≥150 cells/μL using daily OCS for management of severe asthma (ZONDA), benralizumab resulted in a median reduction from baseline in OCS dose of 75% compared with 25% for placeboCitation67. Benralizumab was also associated with reductions in exacerbation rate of 55% to 70% compared with placebo in this same populationCitation67. Overall, benralizumab was well tolerated, with similar or fewer adverse events than placeboCitation31,Citation32,Citation67. An analysis of data from the first year of the BORA long-term safety and efficacy extension study (N = 1576; treatment period, 56 weeks) found that the safety and tolerability profile of benralizumab was consistent with that observed in SIROCCO and CALIMA, and the improvements in efficacy outcomes observed with benralizumab in the placebo-controlled studies were maintainedCitation89.
Dupilumab is indicated for add-on maintenance treatment of patients with moderate to severe asthma aged ≥12 years with an eosinophilic phenotype or OCS-dependent asthmaCitation90. The safety and efficacy of dupilumab have been investigated in two phase 3 randomized, double-blind, placebo-controlled trials (QUEST, VENTURE). QUEST enrolled patients aged ≥12 years with asthma currently treated with medium- to high-dosage ICS plus up to two additional controllers, pre-bronchodilator FEV1 <80% predicted (<90% for patients aged 12–17 years), ACQ-5 score ≥1.5, FEV1 reversibility of ≥12% and 200 mL, and ≥1 exacerbation in the past year resulting in hospitalization, emergency medical care or OCS treatment for ≥3 daysCitation91. VENTURE enrolled patients aged ≥12 years with asthma currently treated with maintenance OCS for 6 months, high-dosage ICS plus a second controller for ≥3 months, pre-bronchodilator FEV1 <80% predicted (<90% for patients aged 12–17 years), and either documented FEV1 reversibility of ≥12% and 200 mL or airway hyperresponsiveness in the prior 12 monthsCitation92.
In QUEST, dupilumab 200 mg reduced severe exacerbations by 47.7% compared with placebo, and by 65.8% for patients with baseline eosinophil counts ≥300 cells/μLCitation91. The increase in FEV1 at Week 12 was 140 mL greater than with placebo (p < .001). Patients with eosinophil counts ≥300 cells/μL had improvements in FEV1 of 210 mL more than placebo with dupilumab 200 mg (p < .001)Citation91. In VENTURE, 80% of patients treated with dupilumab reduced their OCS dosage by at least 50%, and 48% of patients in the dupilumab group completely discontinued OCS therapy compared with 50% and 25% in the placebo group, respectivelyCitation92. The rate of severe exacerbations was 59% lower in the dupilumab group compared with placebo and FEV1 increased by 220 mL. Across the two studies, dupilumab treatment resulted in transient hypereosinophilia for slightly more patients (4–14%) than placebo (0.6–1%)Citation91,Citation92.
Considerations for appropriate patient selection for biologic therapy
Biologic therapies are costly, so it is important to ensure they are only prescribed for the patients who will experience the most benefit. Prior to specialist referral for consideration of biologic therapy (GINA Step 5), the diagnosis of severe uncontrolled asthma should first be confirmed (e.g. pre- and post-bronchodilator spirometry)Citation6. Patients with confirmed asthma who have frequent exacerbations or persistent symptoms despite compliance with medium- to high-dosage combination therapy, with or without additional therapies of a leukotriene modifier and/or a long-acting muscarinic antagonist, or who require OCS for control of their symptoms may be candidates for biologic therapyCitation93. Patients with severe asthma who have evidence of type 2 inflammation (e.g. elevated blood or sputum eosinophil counts, elevated FeNO and elevated IgE) may be candidates for the new FDA-approved class of biologic anti-interleukin (IL) medicationsCitation23,Citation94.
Conclusion
Patients with severe asthma that is uncontrolled on GINA Step 4 treatments (medium- to high-dose combination ICS/LABA therapy, with or without additional therapies of a leukotriene modifier and/or a long-acting muscarinic antagonist) face a higher risk of exacerbations and lower quality of life compared with patients with well controlled asthma. A new class of biologic agents, which includes mepolizumab, reslizumab, benralizumab and dupilumab, has emerged as a helpful advance in therapy for these patients. Proper identification of eosinophilic asthma defines a population of patients with severe asthma who may benefit from referral for consideration of these new biologic therapies.
Transparency
Declaration of funding
Medical writing support was provided by Katie Gersh PhD of MedErgy (Yardley, PA, USA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE, USA).
Declaration of financial/other relationships
N.S.S. has disclosed that he has participated on advisory boards for AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi and Janssen Pharmaceuticals; has served a speaker for AstraZeneca and Boehringer Ingelheim; and has received research support from Sanofi, AstraZeneca and Boehringer Ingelheim. No potential conflict of interest was reported by S.P.C. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Both authors were involved in the analysis and interpretation of the literature search results. Both authors participated in the development and critical review of the manuscript, provided final approval to submit for publication and agree to be accountable for all aspects of the work.
Acknowledgements
Medical writing support was provided by Katie Gersh PhD of MedErgy (Yardley, PA, USA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE, USA).
References
- Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) Data [Internet] [cited 2018 Mar 6]. Available from: https://www.cdc.gov/asthma/nhis/2015/table4-1.htm
- Rui P, Hing E, Okeyode T. National ambulatory medical care survey: 2014 state and national summary tables [Internet]. 2014 [cited 2018 Mar 7]. Available from: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
- Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS data brief, no 94. Hyattsville, MD: National Center for Health Statistics. 2012.
- Murphy KR, Meltzer EO, Blaiss MS, et al. Asthma management and control in the United States: results of the 2009 Asthma Insight and Management survey. Allergy Asthma Proc. 2012;33:54–64.
- Price D, Bjermer L, Bergin DA, et al. Asthma referrals: a key component of asthma management that needs to be addressed. J Asthma Allergy. 2017;10:209–223.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2018 update) [Internet]. 2018 [cited 2018 Mar 16]. Available from: http://www.ginasthma.org
- Colodenco D, Palomares O, Celis C, et al. Moving toward consensus on diagnosis and management of severe asthma in adults. Curr Med Res Opin. 2018;34:387–399.
- Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269–279.
- Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15:348–356.
- Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373.
- Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm. 2016;22:848–861.
- Suruki RY, Daugherty JB, Boudiaf N, et al. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17:74.
- Luskin AT, Chipps BE, Rasouliyan L, et al. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2:544–552, e541–542.
- Lee LK, Obi E, Paknis B, et al. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55:208–219.
- Chipps BE, Zeiger RS, Dorenbaum A, et al. Assessment of asthma control and asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) observational cohort. Curr Respir Care Rep. 2012;1:259–269.
- Godard P, Chanez P, Siraudin L, et al. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19:61–67.
- Peters SP, Ferguson G, Deniz Y, et al. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100:1139–1151.
- Sullivan PW, Slejko JF, Ghushchyan VH, et al. The relationship between asthma, asthma control and economic outcomes in the United States. J Asthma. 2014;51:769–778.
- Malhotra K, Baltrus P, Zhang S, et al. Geographic and racial variation in asthma prevalence and emergency department use among Medicaid-enrolled children in 14 southern states. J Asthma. 2014;51:913–921.
- Lozano P, Grothaus LC, Finkelstein JA, et al. Variability in asthma care and services for low-income populations among practice sites in managed Medicaid systems. Health Serv Res. 2003;38:1563–1578.
- Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663–670.
- de Groot JC, Ten Brinke A, Bel EH. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1:00024–2015.
- Zhang JY, Wenzel SE. Tissue and BAL based biomarkers in asthma. Immunol Allergy Clin North Am. 2007;27:623vi–626vi.
- Carr TF, Berdnikovs S, Simon HU, et al. Eosinophilic bioactivities in severe asthma. World Allergy Organ J. 2016;9:21.
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2016;50:214–227.
- Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750.
- Bagnasco D, Ferrando M, Varricchi G, et al. Anti-interleukin 5 (IL-5) and IL-5Ra biological drugs: efficacy, safety, and future perspectives in severe eosinophilic asthma. Front Med. 2017;4:135.
- Miranda C, Busacker A, Balzar S, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108.
- National Heart Lung and Blood Institute. National asthma education and prevention program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Full report 2007. Bethesda (MD): NHLBI Health Information Center; 2007.
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127.
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141.
- Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. 2014;111:847–855.
- Hetherington KJ, Heaney LG. Drug therapies in severe asthma – the era of stratified medicine. Clin Med. 2015;15:452–456.
- Spiriva Respimat (tiotropium bromide) inhalation spray, for oral inhalation [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2017.
- Casale TB, Bateman ED, Vandewalker M, et al. Tiotropium respimat add-on is efficacious in symptomatic asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. 2017;6:923–935, e9.
- Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343.
- National Asthma Education Prevention Panel. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Summary Report 2007 [Internet]. 2007 [cited 2017 Jan 4]. Available from: http://www.nhlbi.nih.gov/files/docs/guidelines/asthsumm.pdf
- Qoltech. Measurement of health-related quality of life & asthma control [Internet] [cited 2017 Nov 19]. Available from: http://www.qoltech.co.uk/index.htm
- Optum. Asthma control test [Internet]. 2017 [cited 2017 Nov 27]. Available from: https://campaign.optum.com/optum-outcomes/what-we-do/disease-specific-health-surveys/asthma-control-test-act.html
- Juniper EF, Bousquet J, Abetz L, et al. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621.
- Makela MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938.
- Boulet LP, Vervloet D, Magar Y, et al. Adherence: the goal to control asthma. Clin Chest Med. 2012;33:405–417.
- Plaza V, Peiro M, Torrejon M, et al. A repeated short educational intervention improves asthma control and quality of life. Eur Respir J. 2015;46:1298–1307.
- Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103:975–994.
- Wang JJ, Rochtchina E, Tan AG, et al. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116:652–657.
- Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–346.
- Price D, Trudo F, Ling Zhi Jie J, et al. Oral corticosteroids increase risks of onset of diabetes mellitus and osteoporosis in a UK patient population. Chest. 2017;152:A14.
- Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–360.
- Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
- Eberl G, Colonna M, Di Santo JP, et al. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566
- Karta MR, Broide DH, Doherty TA. Insights into group 2 innate lymphoid cells in human airway disease. Curr Allergy Asthma Rep. 2016;16:8
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395.
- Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570.
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22.
- Hashimoto S, Bel EH. Targeting IL-5 in severe asthma: a DREAM come true? Lancet. 2012;380:626–627.
- Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367:551–569.
- Possa SS, Leick EA, Prado CM, et al. Eosinophilic inflammation in allergic asthma. Front Pharmacol. 2013;4:46.
- Louis R, Lau LC, Bron AO, et al. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16.
- de Groot JC, Storm H, Amelink M, et al. Clinical profile of patients with adult-onset eosinophilic asthma. ERJ Open Res. 2016;2:00100-2015.
- Aleman F, Lim HF, Nair P. Eosinophilic endotype of asthma. Immunol Allergy Clin North Am. 2016;36:559–568.
- Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3:849–858.
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207.
- Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366.
- Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789–798.
- Busse W, Spector S, Rosen K, et al. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132:485–486, e411.
- Ferguson GT, FitzGerald JM, Bleecker ER, et al. Benralizumab for patients with mild to moderate, persistent asthma (BISE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2017;5:568–576.
- Goldman M, Hirsch I, Zangrilli JG, et al. The association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: subanalyses of the phase III SIROCCO and CALIMA studies. Curr Med Res Opin. 2017;33:1605–1613.
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615.
- Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–1353, e1342.
- Tan LD, Bratt JM, Godor D, et al. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71–81.
- Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086–1096, e1085.
- Flood-Page PT, Menzies-Gow AN, Kay AB, et al. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204.
- Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132.
- Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–1244, e1232.
- Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466.
- Vatrella A, Fabozzi I, Calabrese C, et al. Dupilumab: a novel treatment for asthma. J Asthma Allergy. 2014;7:123–130.
- Nucala (mepolizumab injection), solution for subcutaneous use [package insert]. Philadelphia, PA: GlaxoSmithKline LLC; 2017.
- Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058–2070, e2051.
- Cinqair (reslizumab injection), solution for subcutaneous use [package insert]. Frazer (PA): Teva Respiratory LLC; 2016.
- Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799–810.
- Murphy K, Jacobs J, Bjermer L, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol Pract. 2017;5:1572–1581, e1573.
- Fasenra (benralizumab) [package insert]. Södertälje, Sweden: AstraZeneca AB; 2017.
- Busse WW, Bleecker ER, FitzGerald JM, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2018;7:46–59.
- Dupixent (dupilumab) injection, for subcutaneous use [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc.; 2018.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–2496.
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485.
- Manka LA, Wechsler ME. Selecting the right biologic for your patients with severe asthma. Ann Allergy Asthma Immunol. 2018;121:406–413.
- Robinson D, Humbert M, Buhl R, et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47:161–175.
- Pham TH, Damera G, Newbold P, et al. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. 2016;111:21–29.
