Abstract
Objective: Type 2 diabetes (T2D) is associated with insulin resistance and deteriorated glycemic control that can be restored with insulin injections. Choice of insulin pen injector may affect complexity, adherence, efficacy of treatment and health-related quality of life. We describe detailed patient-reported outcomes (PROs) on treatment impact and preference comparing insulin degludec (degludec) using FlexTouch1 versus insulin glargine U100 (glargine U100) with SoloStar2 pen injector.
Methods: In this randomized, multicenter (USA), open-label, crossover, treat-to-target study (NCT01570751), patients with T2D using high-dose insulin (≥81 U/day from vials) were randomized (n = 145) 1:1 to 16 weeks of degludec U200 (3 mL FlexTouch) followed by 16 weeks of glargine U100 (3 mL SoloStar) or vice versa. PRO questionnaires assessed treatment impact and patient preference of pen injectors.
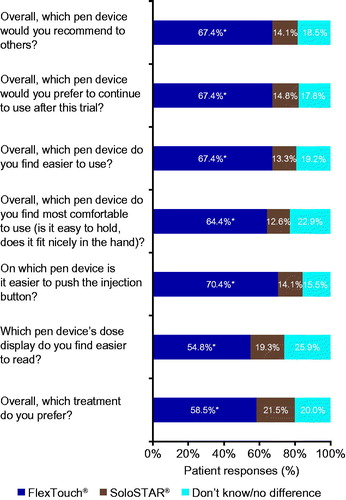
Results: Significantly more patients (p < .01) considered FlexTouch “extremely easy” for learning (62.5 vs. 43.0%), maintaining (63.2 vs. 42.2%) and adjusting the dose (63.2 vs. 44.4%), and significantly more were “very” or “extremely confident” in using the device (60.3 vs. 36.3%) and in its accuracy (50.7 vs. 30.4%) versus SoloStar. Significantly more were “not at all bothered” by device discomfort (74.3 vs. 54.1%), whereas device size (83.8 vs. 80.0%) or public use (69.9 vs. 60.7%) were numerically in favor of FlexTouch. Significantly more patients preferred degludec treatment with FlexTouch (59 vs. 22%), preferred to continue (67 vs. 15%) and recommend (67 vs. 14%) use of FlexTouch compared with SoloStar with glargine U100.
Conclusions: In this randomized, crossover trial, lower treatment impact and higher patient preference were reported for FlexTouch versus SoloStar pen injectors.
Introduction
Type 2 diabetes (T2D) is a progressive disease characterized by insulin resistance and beta-cell dysfunction, resulting in hyperglycemiaCitation1 and the risk of an assortment of complicationsCitation2. While initial lifestyle modifications and oral antidiabetic drugs (OADs) can help control blood glucose, the progressive nature of T2D typically results in inadequate glycemic control requiring intensification of treatment from monotherapy to dual and combination therapies with OADs, glucagon-like peptide-1 agonists and insulinCitation1,Citation3, the latter often using a long-acting basal insulin product delivered by pen injector.
Insulin pen injectors offer the potential to address well recognized barriers to adherence such as perceived burden, injection pain, the embarrassment of injections and other practical difficulties with injectionsCitation4,Citation5. Consequently, insulin pen injectors are preferred by patients when compared with vial and syringeCitation6, offering demonstrable and immediate benefits as shown by the improved persistence and adherence to basal insulin therapy in insulin-naïve patients with T2DCitation7,Citation8. The relative features of different pen injectors, impacting their ease of use and acceptability, as well as improved accuracy of dosingCitation9,Citation10, should therefore be a consideration when choosing insulin therapy, as these may influence patient preference and adherence to treatmentCitation9,Citation11–14.
Two devices available for the administration of two commonly used basal insulins, insulin degludec (degludec) and insulin glargine 100 units/mL (glargine U100), are FlexTouch1 (capable of delivering 160 U of degludec U200 in a single injection) and SoloStar2 (capable of delivering 80 U of glargine U100 in a single injection)Citation13. These pen injectors have been compared in laboratory and usability studies which have suggested advantages for FlexTouch, e.g. greater accuracy delivering the lowest doseCitation15, lower injection forceCitation16, and greater patient confidence and preference versus SoloStar, particularly at higher dosesCitation13. Greater patient confidence and preference may be partly explained by the benefit of the U200 formulation of degludec permitting higher doses to be given without the need for additional injections, but may also be attributed to the differences in the mechanism of the injection process for the two devices: the manual push-button extension of SoloStar versus the spring-loaded mechanism of FlexTouch, the benefits of which have been described previouslyCitation13,Citation14,Citation16,Citation17.
There is a paucity of evidence, however, concerning patient attitudes towards use of these pen injectors in the clinical setting, which would allow patients to become more familiar with their device and adapt to any of its advantages or disadvantages. One study that did collect data pertaining to patient attitudes towards these insulin pens was BEGIN: HIGH DOSE (ClinicalTrials.gov NCT01570751). This study compared the U100 formulation of glargine with the U200 formulation of degludec, which is bioequivalent and clinically equivalent to degludec U100. The aim of the present analysis was therefore to report, in detail, the patient-reported outcomes (PROs), treatment preference and effect on PROs, when switching from glargine U100 with SoloStar to degludec with FlexTouch, or vice versa.
Methods
Study population and setting
The present study reports detailed PRO results from a randomized, multicenter (28 sites in the USA), open-label, crossover, treat-to-target study of patients with T2D requiring high-dose insulin (≥81 U/day from vials) after a 16 week run-in period (BEGIN: HIGH DOSE [NCT01570751]). These previously unpublished PROs relate to the individual items from the Treatment-Related Impact Measure – Diabetes Device (TRIM-DD), which may clarify the drivers of the overall and previously reported PRO results for FlexTouch versus SoloStar. The primary objective of the parent study, which was metCitation18, was to confirm the efficacy of degludec U200 (3 mL FlexTouch) in controlling glycemia in high-dose users (≥81 U/day) of insulin. This was done by establishing the non-inferiority of degludec to glargine U100 (Lantus3 100 units/mL, 3 mL SoloStar) with regard to change from baseline in HbA1c at the end of each 16 week treatment period.
Eligible patients were aged ≥18 years with T2D for ≥6 months, using glargine 65–100 U/day once daily from a vial and syringe in combination with metformin ± one other OAD for at least 3 months, and with HbA1c levels ≥7.5%.
Prior to randomization, treatment with glargine U100 using vial and syringe was optimized during a 16 week run-in period, to ensure stable and improved HbA1c. The use of syringe and vial during the run-in also ensured all patients were naïve to both pen injectors tested during the study, thereby reducing the risk of pre-trial pen injector bias. Patients discontinued use of OADs, with the exception of metformin. Those requiring ≥81 U/day of glargine U100 (N = 145) were randomized 1:1 to 16 weeks of degludec U200 (3 mL FlexTouch) followed by 16 weeks of glargine U100 (3 mL SoloStar) or vice versa. Both degludec U200 and glargine U100 were administered once daily with the possibility of taking the first dose at any time of the day as preferred by the patient, but with this dosing time subsequently maintained throughout the remainder of the study. If a patient’s insulin dose was too large for a single injection, the dose was split across two or more injections delivered at the same time.
Informed written consent was obtained from all participants before any study-related activities and was conducted in compliance with local requirements, International Conference on HarmonizationCitation19 and the Declaration of HelsinkiCitation20. All necessary institutional review board approval was obtained for the research.
Study design, procedures and materials
In this randomized, open-label, crossover trial, two questionnaires were used to specifically focus and capture only patients’ perceptions of the pen injectors, thus no questions were asked that concerned treatment satisfaction with clinical outcomes. Therefore we shall describe the two populations by the devices used (FlexTouch and SoloStar) and not the treatment (degludec and glargine U100). The crossover design was beneficial to the study as all patients used both devices and this consequently increased the power of the analysis. A TRIM-DD questionnaire was utilized to assess pen injector function and bother and was completed at the end of each 16week treatment period. This questionnaire has been previously validated for use in clinical trialsCitation21 and linguistic validation was provided by researchers in the Mapi Institute. To assess patient preference, a 7 item questionnaire developed internally by Novo Nordisk was also provided to the subjects at the end of the trial/following completion of both 16 week treatment periods (Supplementary Material). Linguistic validation of the patient preference questionnaire was provided by Oxford Outcomes.
Statistical analysis
Treatment-Related Impact Measure – Diabetes Device questionnaire
Responses to individual Treatment-Related Impact Measure – Diabetes Device (TRIM-DD) questions were completed on a raw 5 point scale (“not at all”, “a little”, “somewhat”, “very”, “extremely”), reflecting patient experience regarding the ease of device use, bother incurred during device use and confidence in using the devices. Data are reported descriptively and the numbers of subjects giving the most positive response (extremely easy – device use and confidence; not at all – device bother) were compared using a chi-square test. TRIM-DD scores after 16 weeks of treatment were calculated by first transforming raw 5 point scores to a 0–100 scale. Higher scores indicated higher insulin device satisfaction. The estimated treatment difference (ETD) in the total and domain score (device bother and function) was analyzed using a linear mixed model with treatment and period as fixed effects and subject as random effect. These data have been reported previouslyCitation18.
Patient preference questionnaire
Patient preference was reported descriptively based on the presence or absence of preference (“don’t know” or “no difference”). The proportions of responses reporting a preference for either FlexTouch or SoloStar were compared using a z-statistic test, as proportions within one group are analyzed.
Results
Participant characteristics
Baseline characteristics have been described previouslyCitation18 and demonstrate an equal distribution of characteristics between the patients randomized (n = 145) to each treatment sequence ().
Table 1. Patient baseline characteristics.
Treatment-Related Impact Measure – Diabetes Device questionnaire
As previously reported, FlexTouch had a significantly higher TRIM-DD score for device function (ETD: 8.40 [95% confidence interval (CI): 5.15; 11.65], p < .05) and bother (6.01 [95% CI: 2.23; 9.78], p < .05) compared with SoloStar, and this resulted in a higher total TRIM-DD score (ETD: 7.50 [95% confidence interval (CI) 4.79; 10.21]; p < .05 (). The previously published results within the TRIM-DD domains of pen-injector function and device bother are supplemented here () with the eight individual TRIM-DD items, consisting of five questions assessing function and three questions assessing botherCitation21,Citation22. In general, patients rated both pen injectors highly, but significantly more patients gave responses in the most positive indicators for questions related to device function (extremely easy and extremely confident, for all questions) for FlexTouch ().
Figure 1. Assessment of pen-injector device function (a,b) and bother (c) from TRIM-DD. Patient percentages and p values correspond to comparisons between the two pen injectors for the most positive indicators for device function and bother comparison (“extremely easy”, “not at all bothered” and “extremely confident”). The two-sided p value is based on chi-square test for comparing number of patients responding to the responses. Abbreviation, TRIM-DD, Treatment-Related Impact Measure – Diabetes Device.
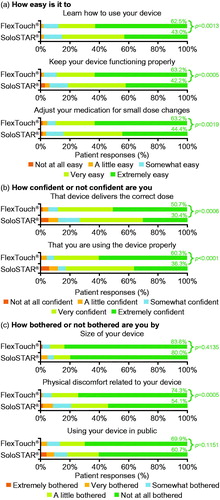
Table 2. Overall TRIM-DD data – change from baseline statistical analysis.
Regarding device bother (), similarly high proportions between the device treatment groups were “not at all bothered” by either the size of the device or using the device in public; however, significantly more patients using FlexTouch were “not at all bothered” by the physical discomfort related to the device, compared with SoloStar.
The novel results from the 7 item patient preference questionnaire () found that significantly more patients (p < .0001) indicated a preference for FlexTouch versus SoloStar. Among the questions, the greatest preference for FlexTouch concerned the ease of pushing the injection button, with 70.4% preferring FlexTouch compared with only 14.1% SoloStar. A similarly large divide was also noted for ease of use with 67.4 vs. 13.3% preferring FlexTouch versus SoloStar. Lastly, significantly more patients preferred treatment with degludec using FlexTouch and also preferred to continue to use this pen injector and would recommend to others when compared with SoloStar.
Discussion
It is generally accepted that insulin pen injectors are easier to read and operate, more discreet and convenient, and less painfulCitation23 than traditional vial and syringe insulin delivery, hence are the preferredCitation23,Citation24 and most popular method of insulin delivery globallyCitation25. The results from this randomized controlled crossover trial also highlight the generally highly positive results for both FlexTouch and SoloStar pen injectors regarding the potential benefit of treatment adherence and management of blood glucoseCitation26,Citation27.
When comparing the two pen injectors, we found that patients with T2D considered FlexTouch easier to learn, adjust and maintain, in addition to providing them with greater confidence during use and less bother due to device discomfort when compared with SoloStar. Furthermore, in assessing patient preference, we found patients preferred FlexTouch, with more patients finding the device more comfortable, and easier to read and inject the dose, and this was reflected by a preference to use, continue to use and recommend the device compared with SoloStar.
While the present study is the first to report detailed PROs for the two pen injectors in a randomized, crossover clinical trial, it cannot be excluded that there might have been confounding due to differences in the insulin formulations. This may be mitigated to some extent by the focus of the questionnaires; indeed, the TRIM-DD was specifically designed and validated to investigate PROs for “diabetes devices”, and in the patient preference questionnaire only one of the questions asked was not specifically aimed at the device features and preference (“overall which treatment do you prefer”). While the open-label design comparing the basal insulin analogs in their respective and unique pen injectors may have resulted in some treatment bias, device-specific bias was mitigated through use of vial and syringe prior to randomization.
The main potential confounding factor is the fact that the data are derived from a study that compared the more concentrated degludec U200 formulation with glargine U100 in a population requiring high insulin doses that could often not be delivered in a single injection regardless of insulin or formulation strength. Consequently, FlexTouch was associated with fewer injections compared with glargine U100 SoloStar. This was evident in the proportion of patients requiring multiple daily injections (50.4% with FlexTouch; 94.1% with SoloStar) and when comparing the average number of injections at 16 weeks (1.62 with degludec U200 FlexTouch versus 2.40 with glargine U100 SoloStar)Citation18. Therefore a comparison between degludec U100 with FlexTouch and glargine U100 with SoloStar may have negated this potential confounder due to the identical formulation strengths and maximum deliverable doses (80 units)Citation28,Citation29.
As well as affecting the number of injections, the higher formulation strength of degludec would also have permitted lower injection volumes at the same unit dose and this may also have biased the results in favor of FlexTouch (e.g. for injection discomfort and preference). A sub-analysis of patients using the same number of injections on both treatments, while possible, would be unable to confirm this assumption of bias. This is partly a result of the limited number of patients with the same number of injections with both pen injectors, but also a result of the crossover design of the study which would mean that patients with the same number of injections with both devices were more likely to have started with SoloStar and crossed over to FlexTouch – given that the basal insulin dose increased over the course of the study. As a result, this sub-analysis would likely introduce a selection bias towards the first or the last pen injector used.
Another potential confounding factor is that the BEGIN: HIGH DOSE study showed that treatment with degludec U200 (using FlexTouch) resulted in equivalent glycemic control but a reduced rate of confirmed hypoglycemia when compared with glargine U100 (with SoloStar)Citation18. This treatment difference regarding hypoglycemia may also have contributed in part to the patients’ preference for degludec U200 with FlexTouch, despite most questions being specifically about aspects of the device. Notwithstanding these potential confounding factors, the generally higher ratings for the FlexTouch device in this clinical study are consistent with results from a previous study conducted in a laboratory settingCitation13.
A final limitation of this study is that it has not measured patient adherence, preventing any conclusion regarding pen injectors that are preferred by patients resulting in improved adherence. However, BEGIN: HIGH DOSE demonstrated non-inferior glycemic control between degludec and glargine U100, suggesting a similar adherence to treatment with the two basal insulinsCitation18. We can still speculate that despite a lack of studies investigating patient preference and adherence in pen injectors, patients prefer devices which are easier to useCitation9,Citation13,Citation30–32, and previous studies have demonstrated that ease of use corresponds to improved adherenceCitation12,Citation13,Citation30,Citation33. Therefore, it may be inferred that patient preference may be a marker for adherence. An example of this would be comparisons of pens versus vials and syringes, in which pens are preferred by patients and have improved adherenceCitation9,Citation32–34.
A strength of the present study is that it was conducted as a randomized, crossover clinical trial and as such the trial design has contributed to a more robust comparison with reduced between-subject variability. This was also aided by the large number of subjects included in the trial (n = 145) and by the high completion rate (93.1%) of the BEGIN: HIGH DOSE studyCitation18.
A potential driver of the patient preferences expressed in this study may be the difference in the mechanism of the injection process for the two devices: the manual push-button extension of SoloStar versus the spring-loaded mechanism of FlexTouchCitation14. This difference has been discussed previously in a clinical study comparing PROs for the FlexTouch and FlexPen4 pen injectorsCitation31. In that comparison, 40% of patients reported that it was “sometimes hard to push” or that it was “difficult to push the injection plunger” with the FlexPen, illustrating that push-button extension in pen injectors can cause difficultiesCitation31. Thus, an advantage of FlexTouch for patients requiring high doses of insulin is that it is the only prefilled pen with no push-button extension at any dose, hence patients requiring higher doses do not face a more difficult injection due to the larger injection volumeCitation14. With SoloStar, however, a larger injection volume will extend the push-button more, increase difficulty of use at the correct angle, and require more force to deliver the dose, potentially leading to injection instability and bruisingCitation14. The increased difficulty of administering higher doses using SoloStarCitation13,Citation35,Citation36 could be a driver of the relatively increased discomfort and lower patient preference in the present study. This would explain the greater proportion reporting FlexTouch as more comfortable, easier to use and easier to push the injection button compared with SoloStar. These benefits may have influenced other results such as those in the TRIM-DD regarding physical discomfort and confidence that the device delivers the correct dose.
In summary, our results suggest that patient preferences in favor of FlexTouch reported in laboratory studies are preserved in a clinical setting when patients have the ability to use both devices and adapt to them during daily therapeutic use. Further studies are indicated, however, to unambiguously isolate patient preferences concerning their pen injectors from the insulin formulations therein, since these may have impacted treatment preferences if fewer injections were required.
Conclusions
These data are the first from a randomized controlled crossover trial comparing detailed PROs for the pen injectors associated with degludec and glargine U100. FlexTouch rated higher in the TRIM-DD, indicating a greater ease of use and lower burden of use compared with SoloStar, findings that were mirrored in the patient preference questionnaire where the FlexTouch was the preferred device. These results suggest that the FlexTouch pen injector may alleviate the burden of frequent injections and potentially lead to improved treatment adherence and glycemic control. Future studies are warranted to determine whether this preference is mirrored when potential confounding factors are removed, such as differences in the dose concentrations of the insulin formulations administered.
Transparency
Declaration of funding
This study was funded by Novo Nordisk A/S.
Author contributions
J.H.-B., L.B.C., M.L.W. and T.S. were involved in the conception and design; M.B. and T.S. were involved in the analysis and interpretation of the data; J.H.-B., L.B.C., M.B., M.L.W. and T.S. were involved in the drafting of the paper or revising it critically for intellectual content and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Declaration of financial/other relationships
M.L.W. has disclosed that he has participated in advisory boards for Novo Nordisk and Eli Lilly, and in speakers’ bureau for Novo Nordisk, Janssen, Eli Lilly, AstraZeneca, Sanofi, Vivus, Merck Sharp & Dohme and Boehringer Ingelheim; he has also received research support from Novo Nordisk, Eli Lilly, Janssen, NPS Pharmaceuticals, Merck Sharp & Dohme, Forest Laboratories, Pfizer, Mylan, Sanofi, Takeda, VPI and Boehringer Ingelheim. M.B. has disclosed that she is a paid consultant to Novo Nordisk A/S. J.H.-B. has disclosed that he is an employee at Novo Nordisk A/S. T.S. has disclosed that he is an employee and stakeholder at Novo Nordisk A/S. L.B.C. has disclosed that he has participated in speakers’ bureau for Novo Nordisk. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material - Patient Questionnaire
Download PDF (21.4 KB)Acknowledgements
The authors thank Charlotte Thim Hansen (Novo Nordisk A/S), Deniz Tutkunkardas (Novo Nordisk A/S) and Lars Lynne Hansen (Novo Nordisk A/S) for assistance with article preparation. The authors acknowledge medical writing and submission support provided by Dr Sam Mason and Beverly La Ferla of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc. This support was funded by Novo Nordisk A/S. This was previously presented at: 77th Scientific Sessions of American Diabetes Association (ADA); 2017 Jun 9–13; San Diego, CA, USA, as an abstract and poster.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Notes
Notes
1 FlexTouch is a registered trade name of Novo Nordisk, Bagsvaerd, Denmark
2 SoloStar is a registered trade name of Sanofi US LLC, St Louis, MO, USA
3 Lantus is a registered trade name of Sanofi US LLC, St Louis, MO, USA
4 FlexPen is a registered trade name of Novo Nordisk, Bagsvaerd, Denmark
References
- Sorli C. New developments in insulin therapy for type 2 diabetes. Am J Med. 2014;127:S39–S48.
- Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16.
- Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, et al. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164:542–552.
- Peyrot M, Rubin RR, Kruger DF, et al. Correlates of insulin injection omission. Diabetes Care. 2010;33:240–245.
- Peyrot M, Barnett AH, Meneghini LF, et al. Factors associated with injection omission/non-adherence in the global attitudes of patients and physicians in insulin therapy study. Diabetes Obes Metab. 2012;14:1081–1087.
- Korytkowski M, Bell D, Jacobsen C, et al. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–2848.
- Buysman E, Conner C, Aagren M, et al. Adherence and persistence to a regimen of basal insulin in a pre-filled pen compared to vial/syringe in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin. 2011;27:1709–1717.
- Slabaugh SL, Bouchard JR, Li Y, et al. Characteristics relating to adherence and persistence to basal insulin regimens among elderly insulin-naive patients with type 2 diabetes: pre-filled pens versus vials/syringes. Adv Ther. 2015;32:1206–1221.
- Pfützner A, Bailey T, Campos C, et al. Accuracy and preference assessment of prefilled insulin pen versus vial and syringe with diabetes patients, caregivers, and healthcare professionals. Curr Med Res Opin. 2013;29:475–481.
- Lteif AN, Schwenk WF. Accuracy of pen injectors versus insulin syringes in children with type 1 diabetes. Diabetes Care. 1999;22:137–140.
- Chandran A, Bonafede MK, Nigam S, et al. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8:148–158.
- Pearson TL. Practical aspects of insulin pen devices. J Diabetes Sci Technol. 2010;4:522–531.
- Pfützner A, Forst T, Niemeyer M, et al. Assessment for ease of use and preference of a new prefilled insulin pen (FlexTouch Degludec U100/U200) versus the SoloStar insulin pen by patients with diabetes and healthcare professionals. Expert Opin Drug Deliv. 2014;11:1381–1389.
- Bailey T, Campos C. FlexTouch for the delivery of insulin: technical attributes and perception among patients and healthcare professionals. Expert Rev Med Devices. 2012;9:209–217.
- Bohnet J, Schmitz M, Kamlot S, et al. Dosing accuracy and insulin flow rate characteristics of a new disposable insulin pen, FlexTouch, compared with SoloStar. J Diabetes Sci Technol. 2013;7:1021–1026.
- Götzche D, Rasmussen BO, Pedersen MT, et al. Injection force and dose accuracy of FlexTouch for the delivery of a new basal insulin. Expert Opin Drug Deliv. 2013;10:1613–1619.
- Nadeau DA, Campos C, Niemeyer M, et al. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28:3–13.
- Warren ML, Chaykin LB, Jabbour S, et al. Insulin degludec 200 units/ml is associated with lower injection frequency and improved patient-reported outcomes compared with insulin glargine 100 units/ml in patients with type 2 diabetes requiring high-dose insulin. Clin Diabetes. 2017;35:90–95.
- International Conference on Harmonisation. ICH Harmonised Tripartate Guideline. Good clinical practice [Internet]. 1996 May 1 [2018 Jun 18]. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
- World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. Last amended by the 59th WMA Assembly Seoul, October 2008 [Internet]. 2008 [2018 Jun 18]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Brod M, Christensen T, Hammer M, et al. Examining the ability to detect change using the TRIM–Diabetes and TRIM–Diabetes Device measures. Qual Life Res. 2011;20:1513–1518.
- Brod M, Hammer M, Christensen T, et al. Understanding and assessing the impact of treatment in diabetes: the treatment-related impact measures for diabetes and devices (TRIM–Diabetes and TRIM–Diabetes Device). Health Qual Life Outcomes. 2009;7:83.
- Anderson BJ, Redondo MJ. What can we learn from patient-reported outcomes of insulin pen devices? J Diabetes Sci Technol. 2011;5:1563–1571.
- Ahmann A, Szeinbach SL, Gill J, et al. Comparing patient preferences and healthcare provider recommendations with the pen versus vial-and-syringe insulin delivery in patients with type 2 diabetes. Diabetes Technol Ther. 2014;16:76–83.
- Perfetti R. Reusable and disposable insulin pens for the treatment of diabetes: understanding the global differences in user preference and an evaluation of inpatient insulin pen use. Diabetes Technol Ther. 2010;12:S79–S85.
- Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: a review of the evidence. Diabetes Technol Ther. 2010;12:S101–S108.
- Seggelke SA, Hawkins RM, Gibbs J, et al. Effect of glargine insulin delivery method (pen device versus vial/syringe) on glycemic control and patient preferences in patients with type 1 and type 2 diabetes. Endocr Pract. 2014;20:536–539.
- Novo Nordisk A/S. Tresiba 100 units/mL, 200 units/mL Pre filled (FlexTouch), 100 units/mL Cartridge (Penfill) Summary of Product Characteristics [Internet]. 2017 [2018 Jun 18]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002498/WC500138940.pdf
- Sanofi-Aventis Deuchland GmBH. Lantus Summary of Product Characteristics [Internet]. 2017 [2018 Jun 18]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000284/WC500036082.pdf
- Asakura T, Jensen KH. Comparison of intuitiveness, ease of use, and preference in two insulin pens. J Diabetes Sci Technol. 2009;3:312–319.
- Garg S, Bailey T, DeLuzio T, et al. Preference for a new prefilled insulin pen compared with the original pen. Curr Med Res Opin. 2011;27:2323–2333.
- Lajara R, Guerrero G, Thurman J. Healthcare professional and patient perceptions of a new prefilled insulin pen versus vial and syringe. Expert Opin Drug Deliv. 2012;9:1181–1196.
- Lee WC, Balu S, Cobden D, et al. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28:1712–1725.
- Asakura T, Seino H, Nakano R, et al. A comparison of the handling and accuracy of syringe and vial versus prefilled insulin pen (FlexPen). Diabetes Technol Ther. 2009;11:657–661.
- Bailey T, Thurman J, Niemeyer M, et al. Usability and preference evaluation of a prefilled insulin pen with a novel injection mechanism by people with diabetes and healthcare professionals. Curr Med Res Opin. 2011;27:2043–2052.
- Oyer D, Narendran P, Qvist M, et al. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv. 2011;8:1259–1269.