Abstract
Objective: STELLA-LONG TERM is an ongoing post-marketing surveillance study examining the safety and effectiveness of ipragliflozin in real-world clinical practice in Japan. This interim report of STELLA-LONG TERM examined the safety and effectiveness of ipragliflozin in non-elderly and elderly Japanese patients with type 2 diabetes mellitus (T2DM) using data up to 12 months.
Methods: Data from T2DM patients who were first prescribed ipragliflozin between July 2014 and October 2015 and whose 12 month data were locked by January 2018 were analyzed and compared between non-elderly (<65 years) and elderly patients (≥65 years).
Results: The safety and efficacy analysis sets included 11,051 and 8788 patients, respectively. Elderly patients accounted for 28.6% (n = 3157) of the study population. The mean body mass index was 29.9 kg/m2 and 26.8 kg/m2, the percentage of patients with glycated hemoglobin (HbA1c) <8.0% was 50.1% and 59.5%, and the percentage of patients with complications was 83.2% and 87.3% in the non-elderly and elderly groups, respectively. Mean HbA1c and body weight decreased significantly from baseline to 12 months in both age groups, regardless of baseline HbA1c and body weight (all p < .05). The incidence of adverse drug reactions (ADRs) was 14.8% and 14.2% and that of serious ADRs was 0.8% and 1.4% in non-elderly and elderly patients, respectively (p = .002 for serious ADRs).
Conclusion: The incidence of serious ADRs was higher in elderly patients than non-elderly patients. Ipragliflozin was effective in both non-elderly and elderly patients with T2DM in the real-world clinical setting.
Introduction
Since the approval of the sodium–glucose cotransporter 2 (SGLT2) inhibitor ipragliflozin for the treatment of type 2 diabetes mellitus (T2DM) in Japan in 2014Citation1, two post-marketing surveillance studies of ipragliflozin have been conducted (the STELLA-ELDER and STELLA-LONG TERM studies) in JapanCitation2–4. Although the safety and effectiveness of ipragliflozin were evaluated in six pre-approval trialsCitation5–10, a pooled analysis of these trials showed that only 32.6% (325/996) of the studied population was ≥65 years of ageCitation11. This raised concerns regarding the lack of long-term postmarketing data on the safety and effectiveness of ipragliflozin in the elderly population.
The STELLA-ELDER study was a 1 year post-marketing surveillance study that assessed the safety and effectiveness of ipragliflozin among elderly Japanese patients aged ≥65 years who were first prescribed ipragliflozin within 3 months after its launch in April 2014Citation2,Citation3. Improvements in glycated hemoglobin (HbA1c) and body weight were shown, and no new safety concerns were raised.
The STELLA-LONG TERM study is an ongoing post-marketing surveillance study examining the safety and effectiveness of ipragliflozin in real-world clinical practice in Japan. Several analyses based on interim cutoff data and various subgroup analyses have been conducted so farCitation12–16. One of the published subgroup analyses evaluated the safety and effectiveness of ipragliflozin in non-elderly vs elderly patients (<65 vs ≥65 years) using data from 3, 12 and 24 months. Although greater improvements in some measures of safety and effectiveness were observed in non-elderly compared with elderly patients, it is not clear whether these should be attributed to the less favorable baseline characteristics that were typically observed in the non-elderly patients. In other words, higher HbA1c and body mass index (BMI) present in the non-elderly group may have facilitated a greater improvement with treatment, as these patients presumably would have had greater potential for improving these parameters from the beginning of the study. Therefore, the safety and effectiveness of ipragliflozin with respect to patient age and baseline characteristics remain to be fully clarifiedCitation16.
Because the data of many patients followed up for 24 months or more were unlocked in the previous report, the present interim report of STELLA-LONG TERM, therefore, focuses on the safety and effectiveness of ipragliflozin in elderly versus non-elderly Japanese T2DM patients using data up to 12 months. Our findings will be compared with those of the STELLA-ELDER study.
Methods
Study design and patients
STELLA-LONG TERM is an ongoing 3 year post-marketing surveillance study in Japanese T2DM patients. The study design, patient selection criteria and methods have been described previouslyCitation4. Ipragliflozin was administered at a dose of 50 mg, once daily before or after breakfast, in accordance with the package insert. In this subgroup analysis, the cutoff date for the receipt of survey forms was 16 January 2018. Only patients whose 12 month data were locked at this time were included in this subgroup analysis.
Data from T2DM patients who were first prescribed ipragliflozin between 17 July 2014 and 16 October 2015 were analyzed and compared between non-elderly and elderly patients (<65 vs ≥65 years). Baseline characteristics and safety data were also compared among three age groups (<65, 65 to <75, and ≥75 years). The survey items included demographic characteristics, body weight, BMI, complications, use of concomitant drugs and laboratory data (HbA1c and estimated glomerular filtration rate [eGFR]).
This study was conducted in compliance with Good Post-marketing Study Practice and involved the collection of anonymized data from a clinical setting as required by the regulatory body; therefore, informed consent was waived. All medical institutions that agreed to provide data signed a contract with the study sponsor (Astellas Pharma Inc.). The study was registered at ClinicalTrials.gov (NCT02479399) on 24 June 2015.
Safety
Safety was evaluated based on adverse drug reactions (ADRs) that occurred during treatment with ipragliflozin. All ADRs were categorized according to system organ class (SOC) and preferred term (PT) using MedDRA/J Version 20.1. ADRs of special interest were also evaluated.
Effectiveness
Effectiveness outcome measures were the changes in HbA1c and body weight from baseline up to 12 months. The changes in HbA1c were evaluated in patients with a baseline HbA1c level <8.0% and ≥8.0%. The changes in body weight were evaluated in patients with baseline BMI <25.0 kg/m2 and ≥25.0 kg/m2.
Statistical analysis
Sample size calculations for the primary analysis have been described previouslyCitation4. No sample size calculation was considered regarding the subgroup comparisons of non-elderly and elderly patients. The safety analysis set included all patients from whom survey forms were collected, who received at least one dose of the study drug, without any registration violations and with data from at least one post-baseline visit. The efficacy analysis set included all patients in the safety analysis set, except for those who were noncompliant with the study drug, had unclear efficacy assessment or had missing HbA1c, serum fasting insulin or fasting plasma glucose data at baseline or post-baseline. Categorical variables are shown as n (%) and continuous variables are shown as mean ± standard deviation. The chi-squared test or two-sample t-test (one-way analysis of variance) was used to compare patient demographic and clinical data and treatments used between non-elderly and elderly patients (between three age categories; <65, 65 to <75, and ≥75 years). Changes in HbA1c and body weight from baseline to 1, 3, 6 and 12 months were assessed using one-sample t-tests. Two-sided p values <.05 were considered statistically significant. We did not perform adjustments for type I error based on multiple hypothesis testing. Furthermore, we only tested the overall null hypothesis for comparisons between the three age categories (<65, 65 to <75, and ≥75 years) (i.e. we did not perform any pairwise comparisons). SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis.
Results
Patient disposition
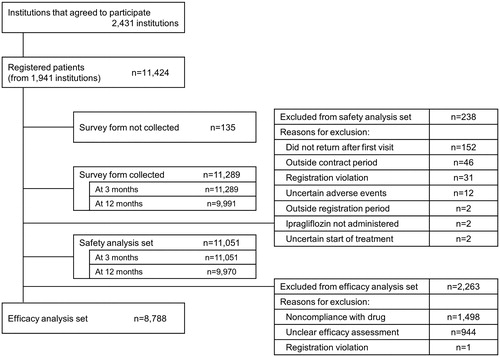
shows the disposition of patients. A total of 11,424 patients were registered from 1941 institutions. Among the participating institutions, 73.6% were clinics and 24.5% were hospitals and universities. Survey forms were collected from 9991 patients for 12 months of follow-up. The safety analysis set included 11,051 patients. Among them, 2263 patients were excluded from the efficacy analysis set mainly because of noncompliance with the study drug (e.g. starting dose other than 50 mg once daily without severe hepatic impairment), unclear efficacy assessment, missing data for HbA1c, serum fasting insulin, or fasting plasma glucose level at baseline or post-baseline. Therefore, the efficacy analysis set included 8788 patients.
Patient characteristics
shows patient characteristics at baseline in the safety analysis set (n = 11,051). Elderly patients (≥65 years) accounted for 28.6% (n = 3157) of the study population. Elderly patients had a significantly lower BMI than non-elderly patients (26.8 ± 4.1 vs 29.9 ± 5.4 kg/m2, respectively; p < .001). The percentage of patients with HbA1c <8.0% and that of patients with complications were significantly higher among elderly vs non-elderly patients (HbA1c <8.0%: 59.5% vs 50.1%, respectively; complications: 87.3% vs 83.2%, respectively; both p < .001). When comparing our findings in elderly patients with those of the STELLA-ELDER study, baseline characteristics such as BMI, eGFR and percentage of patients with HbA1c ≥8.0% were similar between the two groups. The percentage of patients with complications was higher among elderly patients in the present study vs patients in STELLA-ELDER (87.3% vs 81.3%).
Table 1. Baseline characteristics.
shows the treatments used at baseline and/or during the survey period. Although the percentage of patients using concomitant antidiabetic drugs was significantly lower among elderly vs non-elderly patients (80.1% vs 82.0%, respectively; p = .010), the magnitude of this difference was small. The percentage of patients using concomitant diuretics or drugs other than antidiabetics and diuretics was significantly higher among elderly vs non-elderly patients (concomitant diuretics: 11.1% vs 6.2%; other concomitant drugs: 77.7% vs 68.7%; both p < .001). When comparing our findings in elderly patients with those of the STELLA-ELDER study, the percentage of patients using concomitant antidiabetic drugs and diuretics was marginally higher in the STELLA-ELDER study. The percentage of patients using other concomitant drugs was notably higher in the elderly group in the present study vs the STELLA-ELDER study patients (77.7% vs 69.4%, respectively). Supplementary Tables S1 and S2 show the baseline characteristics of patients and treatments used at baseline and/or during the survey period stratified by three age categories (<65, 65 to <75, and ≥75 years). Mean BMI and HbA1c values were significantly higher among patients aged <65 years compared with that in patients aged 65 to <75 years and ≥75 years (both p < .001). The proportion of patients taking concomitant antidiabetic drugs was significantly higher among patients aged <65 years and 65 to <75 years compared with that among patients aged ≥75 years (p < .001). The proportion of patients taking concomitant diuretics and that of patients taking concomitant drugs other than antidiabetic drugs and diuretics was significantly lower among patients aged <65 years compared with that among patients aged 65 to <75 years and ≥75 years (both p < .001).
Table 2. Treatments used at baseline and/or during the survey period.
Safety
ADRs were assessed in the safety analysis set (n = 11,051). The incidence of ADRs was similar between non-elderly and elderly patients (14.8% vs 14.2%, respectively) (). The incidence of serious ADRs was significantly higher in elderly vs non-elderly patients (1.4% vs 0.8%, respectively; p = .002). The incidence of ADRs in elderly patients in this survey was lower than that reported in the STELLA-ELDER study (14.2% vs 16.9%).
Table 3. ADRs, serious ADRs and ADRs of special interest.
ADRs and serious ADRs stratified by three age categories (<65, 65 to <75, and ≥75 years) are shown in Supplementary Table S3. The incidence of ADRs was similar across the three age categories. However, the incidence of serious ADRs was significantly higher (p = .008) in patients aged 65 to <75 years (1.4%) and ≥75 years (1.5%) compared with that in patients aged <65 years (0.8%).
Supplementary Tables S4 and S5 show ADRs and serious ADRs by system organ class and preferred term, as well as the incidences reported in the pre-approval clinical trials and in the STELLA-ELDER study. The incidence of ADRs was 32.9% in pre-approval clinical trials on ipragliflozin, and the most frequently reported ADRs were those related to renal and urinary disorders (10.6%), investigations (8.0%), general disorders and administration site conditions (6.1%), gastrointestinal disorders (6.1%), infections and infestations (3.8%), skin and subcutaneous tissue disorders (2.9%), and nervous system disorders (2.5%) (Supplementary Table S4). In the present survey, no unreported safety concerns were observed. The most common serious ADR in elderly patients was cerebral infarction (0.3%) (Supplementary Table S5).
Regarding ADRs of special interest, the incidences of hypoglycemia, renal disorder and skin complications were significantly higher among elderly vs non-elderly patients (p = .037, .021 and .012, respectively), while those of genital infection, polyuria/pollakiuria, and hepatic disorder were significantly higher among non-elderly vs elderly patients (p = .043, p < .001 and p = .002, respectively) (). Elderly patients in the current STELLA-LONG TERM interim report had a lower incidence of volume depletion and skin complications than patients in STELLA-ELDER, while the incidence of other ADRs was almost comparable. In both age groups (elderly vs non-elderly), hematocrit increased significantly from baseline at each time point evaluated (Supplementary Figure S1); however, there were no significant differences between these increases in elderly vs non-elderly patients, except at 1 month of ipragliflozin treatment.
Supplementary Table S6 shows ADRs of special interest stratified by three age categories (<65, 65 to <75, and ≥75 years). Volume depletion was most frequently observed in patients ≥75 years compared with those <65 years and 65 to <75 years, although the difference was not statistically significant. The incidence of polyuria/pollakiuria progressively and significantly increased in the lower age groups (<65 years, 5.7%; 65 to <75 years, 4.3%; and ≥75 years, 2.1%; p < .001).
Effectiveness
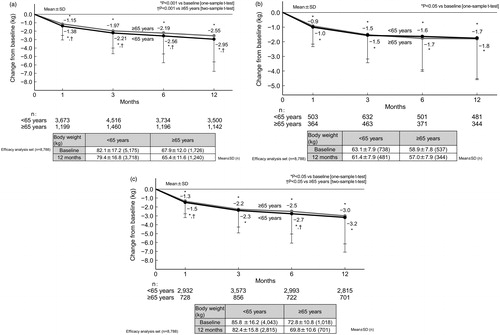
The effectiveness of ipragliflozin was assessed up to 12 months (n = 8788 [n = 6453 and n = 2335 in the non-elderly and elderly subgroups, respectively]). Overall, the HbA1c level was 8.1 ± 2.8% (n = 8754) at baseline and 7.3 ± 1.1% (n = 6436) at 12 months. Glycemic control improved significantly (p < .001 vs baseline) at 12 months, with sustained mean reductions from baseline in HbA1c (−0.8 ± 1.2%). shows the time course changes in HbA1c among patients by age group (regardless of baseline HbA1c), and then in groups further stratified by baseline HbA1c (<8.0% and ≥8.0%). The mean HbA1c decreased significantly from baseline to 12 months in both age groups, regardless of their baseline HbA1c (p < .05 vs baseline). At 12 months, no statistically significant difference was found in HbA1c between non-elderly and elderly patients among those with baseline HbA1c <8.0% and ≥8.0%. Overall, the body weight was 78.5 ± 17.2 kg (n = 6901) at baseline and 75.9 ± 16.8 kg (n = 4958) at 12 months. Significant sustained reductions (p < .001 vs baseline) in body weight (−2.9 ± 3.7 kg) were also observed. shows the time course changes in body weight in patients by age group (regardless of baseline BMI), and then in patients further stratified by baseline BMI (<25.0 and ≥25.0 kg/m2). The mean body weight decreased significantly from baseline to 12 months in both age groups, regardless of their baseline BMI (p < .05 vs baseline). At 12 months, no statistically significant difference was found in body weight between non-elderly and elderly patients among those with baseline BMI <25.0 kg/m2 and ≥25.0 kg/m2.
Figure 2. Changes in HbA1c (NGSP) from baseline to 12 months in patients by age (elderly vs non-elderly) (a), and further stratified by baseline HbA1c <8.0% (b) and HbA1c ≥8.0% (c). Abbreviations. HbA1c, Glycated hemoglobin; NGSP, National Glycohemoglobin Standardization Program; SD, Standard deviation.
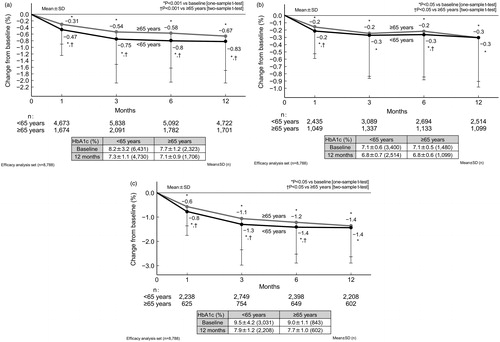
Figure 3. Changes in body weight from baseline to 12 months in patients by age (elderly vs non-elderly) (a), and further stratified by baseline BMI <25.0 kg/m2 (b) and BMI ≥25.0 kg/m2 (c). Patients with missing height data (i.e. for whom BMI could not be calculated) were excluded from panels (b) and (c). Abbreviations. BMI, Body mass index; SD, Standard deviation.
Discussion
The STELLA-LONG TERM study is an ongoing 3 year post-marketing surveillance study. In this subgroup analysis, we presented the safety and effectiveness results up to 12 months, stratified by age groups (<65 vs ≥65 years). We also compared our findings with those of the previous subgroup analysis in which data were pooled from 3, 12 and 24 monthsCitation16, and the STELLA-ELDER studyCitation3.
Patient demographic characteristics were consistent with those reported in the previous subgroup analysisCitation16. The elderly group in the present study had some favorable background characteristics, including a significantly lower BMI and a higher percentage of patients with HbA1c <8.0% compared with the non-elderly group. As expected, the percentage of patients with complications was significantly higher in the elderly group vs the non-elderly group.
The number of concomitant antidiabetic drugs used by patients in the STELLA-ELDER study was higher compared with the elderly patients in the present subgroup analysis (2.1 vs 1.5). Ipragliflozin was the second most commonly used antidiabetic drug in the STELLA-LONG TERM study and the third most commonly used antidiabetic drug in the STELLA-ELDER study. This could be a reflection of the recommendation issued in 2015, after the introduction of SGLT2 inhibitors, that these agents should be used with caution and preferably in patients with more favorable background characteristicsCitation17.
The STELLA-ELDER study was conducted as an all-patient registration survey, while the STELLA-LONG TERM study involved physicians who had an interest in the survey. Because of the differences in the study settings, patients included in the latter study may have been treated more cautiously and monitored more closely than in the former study.
The incidence of ADRs was 16.9% in STELLA-ELDER, which was slightly higher than that in the elderly group of the present study (14.2%). The incidence of serious ADRs was significantly higher in elderly patients vs non-elderly patients in the present study (1.4% vs 0.8%; p = .002). A similar result was obtained in the STELLA-ELDER study. The incidence of serious ADRs among the elderly group in the present study was consistent with that reported in the STELLA-ELDER study (1.5%).
Regarding ADRs of special interest, hypoglycemia, renal disorder and skin complications were more common among the elderly group while genital infection, polyuria/pollakiuria and hepatic disorder were more common among the non-elderly group. Multivariable regression analysis in the STELLA-ELDER study showed that patients with low BMI tend to be affected by hypoglycemiaCitation3. A cross-sectional survey conducted in China reported an association between increasing BMI and lower risk of hypoglycemiaCitation18. Also, hypoglycemia incidence reportedly increases with lower BMI following canagliflozin treatmentCitation19. In this interim report, elderly patients tended to have a lower BMI, which could explain the higher incidence of hypoglycemia in this group. Kidney function tends to deteriorate with age, which may explain the high incidence of renal disorders among elderly patientsCitation20. Skin complications in general, and dry skin in particular, are common problems among the elderlyCitation21. These may be related to lower water intake and more frequent use of diuretics among older vs younger subjects.
Volume-depletion-related events, including dehydration, were more common in patients in the STELLA-ELDER study than the elderly group of the present study (3.1% vs 1.8%, respectively). As the changes in hematocrit from baseline to 12 months between elderly and non-elderly patients were generally similar, with only a single statistical difference, it is unlikely that the increase observed is involved in the development of volume-depletion-related events. Elevated hematocrit levels generally indicate an increase in hemoconcentration owing to the diuretic action of SGLT2 inhibitors but, as reported previouslyCitation22–29, this effect is not considered sufficient to increase the risk of stroke. However, as the data reported here are derived from an interim analysis of this study, we will continue to monitor this point. Polyuria/pollakiuria was less common in patients in the STELLA-ELDER study vs the elderly group of the present study (2.0% vs 3.8%, respectively). The reason for these differences may be that physicians provided patients with instructions on adequate fluid intake as a result of the recommendations issued after the completion of the STELLA-ELDER studyCitation17. Because the changes in hematocrit from baseline up to 12 months were generally similar among elderly and non-elderly patients in the present study, it is unlikely that increased hematocrit is of any additional clinical concern in elderly patients.
Regarding serious ADRs of special interest, the incidence of cerebrovascular disease in elderly patients was significantly higher than that in non-elderly patients and was consistent with that reported in the STELLA-ELDER study (0.4%). Some previously reported risks of treatment with other SGLT2 inhibitors include amputation, fracture and serious infection of the genital areaCitation30. In a recent retrospective, single-center, case–control study of T2DM patients, the incidence of lower limb amputations were compared between patients with active diabetic foot wounds who were receiving an SGLT2 inhibitor and those who were notCitation31. However, no statistically significant increase in the risk of lower limb amputation was shown with SGLT2 inhibitor use and the total number of lower limb amputations did not significantly differ between the two groups. Similarly, a recent meta-analysis reported that increased risk of fracture was not observed among T2DM patients treated with SGLT2 inhibitors vs placeboCitation32. Although serious infection of the genital area is rare, the Food and Drug Administration has issued a warning on the occurrence of this ADR with the use of SGLT2 inhibitorsCitation33. Further study is warranted to assess the occurrence of such ADRs of special interest beyond 1 year of treatment with ipragliflozin. Regarding the general outcomes of ADRs in the present study, there were no clear differences between the two age groups. However, the incidence of death was higher in elderly patients aged ≥75 years vs those aged <65 years and 65 to <75 years. Overall, no clinically relevant differences in the effectiveness (change in HbA1c and body weight) and safety (incidence of ADRs) of ipragliflozin were shown between non-elderly and elderly patients in the present study.
Regarding effectiveness, as the comparison by age groups in the present study did not account for differences in baseline characteristics, the change in HbA1c over time was examined in patients according to baseline HbA1c levels (<8.0% and ≥8.0%). In both HbA1c categories, no significant difference was observed in the change in HbA1c between the age groups at 12 months. Similarly, the change in body weight over time was examined in patients according to baseline BMI (<25.0 and ≥25.0 kg/m2). There was no significant difference in the change in body weight between the age groups at 12 months once stratified by baseline BMI (<25.0 and ≥25.0 kg/m2). The mean change in body weight from baseline to 12 months in the elderly group in the present study with baseline BMI ≥25.0 kg/m2 (−3.0 kg) was consistent with those included in the STELLA-ELDER study (−2.9 kg, baseline mean BMI 27.0 kg/m2).
The present study has some limitations, including those inherent to survey reports and the lack of an active comparator group. Furthermore, the participating centers were chosen based on the interest of the physicians from these centers, and this could have contributed to a potential bias toward favorable results. Additionally, the potential for bias due to confounding by unadjusted/unmeasured factors owing to the non-randomized, observational study design should be noted.
In conclusion, the incidence of serious ADRs was higher in elderly patients than non-elderly patients. Hypoglycemia, renal disorder and skin complications were more common in elderly patients, while genital infection, polyuria/pollakiuria and hepatic disorder were more common in non-elderly patients. Ipragliflozin was effective in both non-elderly and elderly patients with T2DM in the real-world clinical setting.
Transparency
Declaration of funding
This study was sponsored by Astellas Pharma Inc., Japan.
Author contributions
H.M., K.T. and S.U. contributed to the study design, data analysis and writing of the manuscript. I.N. contributed to the study design, study conduct, data analysis and writing of the manuscript. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Declaration of financial/other relationships
H.M. has disclosed that he has received lecture fees from Astellas Pharma Inc., MSD KK, Daiichi Sankyo Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Kissei Pharmaceutical Co., Mitsubishi Tanabe Pharma Corporation, Sanofi KK, Kowa Pharmaceutical Co. Ltd. and Takeda Pharmaceutical Co. Ltd.; research support from Astellas Pharma Inc., AstraZeneca KK and Boehringer Ingelheim Co. Ltd.; and grants from Takeda Pharmaceutical Co. Ltd., Astellas Pharma Inc., MSD KK, Teijin Limited, Nippon Boehringer Ingelheim Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., Kowa Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Sanofi KK, Mitsubishi Tanabe Pharma Corporation, Shionogi & Co. Ltd., Otsuka Pharmaceutical Co. Ltd. and Sanwa Kagaku Kenkyusho Co. Ltd.
K.T. has disclosed that he has received lecture fees from Astellas Pharma Inc., AstraZeneca KK, Kowa Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd. and Novo Nordisk Pharma Ltd.; and grants from Kowa Pharmaceutical Co. Ltd., Astellas Pharma Inc., Ono Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Takeda Pharmaceutical Co. Ltd., Sanofi KK and Fuji Chemical Industries Co. Ltd.
I.N. and S.U. have disclosed that they are employees of Astellas Pharma Inc.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
We would like to thank the study investigators and participating patients. Medical writing and editorial support was funded by Astellas and provided by Dr. Michelle Belanger (Edanz Medical Writing) and EMC KK. This manuscript has not been published elsewhere and is not under consideration in whole or in part by another journal, except as a poster presented at the 61st Annual Meeting of the Japan Diabetes Society (2018).
Data availability statement
Data sharing is not applicable to this article as informed consent was waived and data sharing with external researchers is restricted per our standard operating procedures.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/03007995.2021.1936911)
References
- Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014;74:611–617.
- Terauchi Y, Yokote K, Nakamura I, et al. Safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): interim results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17:463–471.
- Yokote K, Terauchi Y, Nakamura I, et al. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17:1995–2003.
- Maegawa H, Tobe K, Tabuchi H, et al. Baseline characteristics and interim (3-month) efficacy and safety data from STELLA-LONG TERM, a long-term post-marketing surveillance study of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice. Expert Opin Pharmacother. 2016;17:1985–1994.
- Kadokura T, Akiyama N, Kashiwagi A, et al. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2014;106:50–56.
- Kashiwagi A, Akiyama N, Shiga T, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2015;6:125–138.
- Kashiwagi A, Kazuta K, Goto K, et al. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17:304–308.
- Kashiwagi A, Kazuta K, Takinami Y, et al. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015;6:8–18.
- Kashiwagi A, Shiga T, Akiyama N, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study). Diabetol Int. 2015;6:104–116.
- Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–160.
- Kashiwagi A, Yoshida S, Kawamuki K, et al. Effects of ipragliflozin, a selective sodium–glucose co-transporter 2 inhibitor, on blood pressure in Japanese patients with type 2 diabetes mellitus: a pooled analysis of six randomized, placebo-controlled clinical trials. Diabetol Int. 2017;8:76–86.
- Nakamura I, Maegawa H, Tobe K, et al. Safety and efficacy of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice: interim results of the STELLA-LONG TERM post-marketing surveillance study. Expert Opin Pharmacother. 2018;19:189–201.
- Nakamura I, Maegawa H, Tobe K, et al. Safety and efficacy of ipragliflozin for type 2 diabetes in Japan: 12-month interim results of the STELLA-LONG TERM post-marketing surveillance study. Adv Ther. 2019;36:923–949.
- Tobe K, Maegawa H, Tabuchi H, et al. Impact of body mass index on the efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a subgroup analysis of 3-month interim results from the Specified Drug Use Results Survey of Ipragliflozin Treatment in Type 2 Diabetic Patients: Long-term Use study. J Diabetes Investig. [cited 2019 Aug 20]. DOI:10.1111/jdi.13021.
- Tabuchi H, Maegawa H, Tobe K, et al. Effect of ipragliflozin on liver function in Japanese type 2 diabetes mellitus patients: a subgroup analysis of the STELLA-LONG TERM study (3-month interim results). Endocr J. 2019;66:31–41.
- Maegawa H, Tobe K, Tabuchi H, et al. Safety and efficacy of ipragliflozin in elderly versus non-elderly Japanese patients with type 2 diabetes mellitus: a subgroup analysis of the STELLA-LONG TERM study. Expert Opin Pharmacother. 2018;19:327–336.
- Yabe D, Nishikino R, Kaneko M, et al. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf. 2015;14:795–800.
- Gu W, Ren Y, Ji L, et al. Non-linear associations of risk factors with mild hypoglycemia among Chinese patients with type 2 diabetes. J Diabetes Complications. 2016;30:462–468.
- Inagaki N, Goda M, Yokota S, et al. Safety and efficacy of canagliflozin in Japanese patients with type 2 diabetes mellitus: post hoc subgroup analyses according to body mass index in a 52-week open-label study. Expert Opin Pharmacother. 2015;16:1577–1591.
- Yoon HE, Choi BS. The renin–angiotensin system and aging in the kidney. Korean J Intern Med. 2014;29:291–295.
- White-Chu EF, Reddy M. Dry skin in the elderly: complexities of a common problem. Clin Dermatol. 2011;29:37–42.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128.
- US Food and Drug Administration Center for Drug Evaluation and Research. Invokana (canaglifozin) NDA 204042 summary review [Internet]. 2013 Mar 26 [cited 2019 July 17]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000SumR.pdf
- US Food and Drug Administration Center for Drug Evaluation and Research. Farxiga (dapaglifozin) NDA 202293 summary review [Internet]. 2014 Jan 11 [cited 2019 July 17]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/202293Orig1s000SumR.pdf
- Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385:2107–2117.
- Wu JH, Foote C, Blomster J, et al. Effects of sodium–glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4:411–419.
- Guo M, Ding J, Li J, et al. SGLT2 inhibitors and risk of stroke in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1977–1982.
- Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract. 2019;150:8–16.
- Kaku K, Lee J, Mattheus M, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease – results from EMPA-REG OUTCOME. Circ J. 2017;81:227–234.
- Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13:84–91.
- Sung J, Padmanabhan S, Gurung S, et al. SGLT2 inhibitors and amputation risk: real-world data from a diabetes foot wound clinic. J Clin Transl Endocrinol. 2018;13:46–47.
- Ruanpeng D, Ungprasert P, Sangtian J, et al. Sodium–glucose cotransporter 2 (SGLT2) inhibitors and fracture risk in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Metab Res Rev. 2017;33:e2903.
- US Food and Drug Administration. SGLT2 (sodium–glucose cotransporter-2) inhibitors for diabetes: drug safety communication – regarding rare occurrences of a serious infection of the genital area [Internet] [cited 2018 Oct 22]. Available from: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm618908.htm