Abstract
Objective: To address gaps in the literature on healthcare resource utilization (HRU) and costs among patients with schizophrenia and prior hospitalization who transition from oral risperidone or paliperidone (oral ris/pali) to once-monthly paliperidone palmitate (PP1M) in a real-world setting by comparing treatment patterns, HRU, and costs 12-months pre- and post-transition to PP1M among Veterans Health Administration (VHA) patients affected by schizophrenia who have had ≥1 hospitalization.
Methods: VHA patients with schizophrenia (aged ≥18 years) who initiated oral ris/pali, had ≥1 all-cause inpatient stay, and transitioned to PP1M from January 2015–March 2017 were included from the VHA database. The first transition date to PP1M was identified as the index date. Patients were required to have continuous health plan eligibility for 12 months pre- and post-PP1M. Outcomes were compared using the Wilcoxon signed-rank and McNemar’s test, as appropriate.
Results: The study included 319 patients (mean [SD] age = 51.6 [4.2] years) during 12 months of baseline and follow-up. During pre-PP1M transition, 7.2% of the patients were adherent (proportion of days covered [PDC] ≥ 80%) to oral ris/pali. Post-PP1M transition, 27.6% of the patients were adherent to PP1M. Comparison of HRU outcomes from the pre- to post-PP1M transition revealed significantly lower all-cause inpatient stays (3.5 vs 1.4, p < .0001) and shorter inpatient length of stay (43.4 vs 18.3 days, p < .0001). Similar trends were seen for mental health and schizophrenia-related HRU. Cost outcome comparison indicated significantly lower all-cause inpatient costs ($64,702 vs $24,147, p < .0001), total medical costs ($87,917 vs $56,947, p < .0001), and total costs ($91,181 vs $69,106, p < .0001). A similar trend was observed for mental health and schizophrenia-related costs.
Conclusions: Transitioning from oral ris/pali to PP1M may significantly improve HRU and provide potential cost savings in VHA patients with schizophrenia and ≥1 prior hospitalization.
Introduction
Schizophrenia is one of the top 20 leading causes of disability worldwide and is characterized by a diminished capacity for learning, working, self-care, and interpersonal relationshipsCitation1–5. The pervasive, chronic mental disorder affects less than 1% of adults in the US, and the estimated prevalence of schizophrenia and related psychotic disorders in the US is between 0.25–0.64%Citation6,Citation7. Schizophrenia is not only considered a difficult-to-treat, complex illness, but is also considered one of the costliest mental illnessesCitation8–10. The estimated overall economic burden of schizophrenia in the US for 2013 was $155.7 billion, with most costs stemming from indirect costs ($117.3 billion) driven by caregivingCitation11.
A key treatment strategy for patients with schizophrenia is to prevent relapses. Relapses can delay disease progress and increase rates of hospitalizations, in addition to driving higher healthcare costsCitation9,Citation12,Citation13. Patients with schizophrenia who relapse can incur healthcare costs up to 2–5-times higher compared to patients who do not relapseCitation12. These higher costs are mainly driven by a higher number of hospitalizations and longer hospital staysCitation9,Citation14. Previous studies support the notion that patients with schizophrenia who relapse or are admitted to the hospital tend to have more serious symptoms and thus incur higher healthcare costs and utilization. Furthermore, patients with schizophrenia and previous hospital admission for relapse are at risk for future relapses and subsequent hospitalizations (or readmissions) due to severe symptoms or poor disease management (i.e. non-adherence)Citation9,Citation12.
Before the advent of long-acting injectable (LAI) therapies, antipsychotic (AP) therapies such as oral atypical antipsychotics (OAAs) were most commonly recommended. Adherence for patients with schizophrenia is critical, as non-adherence is often associated with adverse clinical and economic outcomes. Further, with oral medications such as OAAs, non-adherence is a common problem and may lead to relapses and hospitalization (or rehospitalization)Citation15. In 2009, the US Food and Drug Administration approved once-monthly paliperidone palmitate (PP1M), an LAI therapy, to treat patients with schizophrenia. Compared to OAAs, PP1M can lead to adherence improvement, alleviate clinical burdens, reduce hospitalizations, and lower medical costsCitation1,Citation9,Citation15. Lafeuille et al.Citation9 reported that, compared to patients prescribed PP1M, OAA users incurred higher medical costs and healthcare resource utilization (HRU); OAA use was also linked to more frequent, unplanned inpatient visits.
Prior studies have demonstrated the clinical and economic advantage of LAIs, such as PP1M, over OAA use in patients with schizophrenia, specifically through the reduction of experienced relapses, hospitalizations, and significantly lower HRU and healthcare costsCitation16,Citation17. However, there is a lack of evidence specifically discussing patients with schizophrenia and at least one prior hospitalization who have transitioned from oral risperidone or paliperidone (oral ris/pali) to PP1M in a real-world setting. Oral ris/pali’s receptor sub-types are alike in their binding affinities in addition to paliperidone being an active metabolite of risperidoneCitation18,Citation19. Furthermore, PP1M is the LAI form of oral paliperidone. Upon comparison, PP1M has exhibited greater effectiveness in the maintenance of schizophrenia symptoms due to adherence advantagesCitation20,Citation21.
This study aims to assess treatment patterns, HRU, and costs among patients with schizophrenia who have had at least one hospitalization and who transitioned directly from oral ris/pali to PP1M in a real-world setting using the most recent Veterans Health Administration (VHA) data.
Methods
Objective
The main objective was to compare treatment patterns, HRU, and costs related to the 12 months pre- and post-transition from oral ris/pali to PP1M among VHA patients diagnosed with schizophrenia who had ≥1 all-cause inpatient stay.
The study also compared the main objective outcomes using 6-months pre- and post-transition from oral ris/pali to PP1M among VHA patients with schizophrenia who had ≥1 all-cause inpatient stay.
Data source
Data from the VHA database from January 1, 2014 to March 31, 2018 (the study period) was utilized to perform this retrospective cohort study.
The VHA is the largest integrated healthcare system in the US. In 2014, the US Department of Veterans Affairs (VA) provided medical services to nearly 6 million veterans and to over 700,000 non-veterans (e.g. active duty military and reserve, spousal collateral, consultations and instruction, CHAMPVA workload, reimbursable workload with affiliates, humanitarian care, and employees receiving occupational immunizations such as Hepatitis A and B and flu vaccinations)Citation22. The VHA Medical Statistical Analysis System (SAS) datasets are national administrative data for VHA-provided healthcare utilized primarily by veterans, but also by some non-veterans (e.g. employees, research participants). The stability of VHA data sources allows for superior evaluation of the continuity of care of patients over multiple years.
Since the core study did not involve the collection, use, or transmittal of individual identifiable data, institutional review board approval to conduct this study was not required. No identifiable patient information or medical records were disclosed for the purposes of this study except in compliance with applicable law.
Study population
VHA patients included in the study were adults aged 18 years and older. Those selected had ≥1 healthcare encounter that included a schizophrenia diagnosis (International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9/10-CM] code: 295.XX [excluding 295.7 schizoaffective disorder], ICD-10-CM: F20.XX, F21) during the study period (January 1, 2014–March 31, 2018); had initiated treatment with oral ris/pali during the identification period (January 1, 2015–March 31, 2017); had continuous health plan enrollment for 12 months pre- (baseline) and post-index (follow-up) date; and had ≥1 all-cause inpatient stay during the baseline period. Patients must have transitioned directly from oral ris/pali to PP1M during the identification period. The first dispensing of PP1M was defined as the index date. Patients were excluded from the study if they had evidence of PP1M during the 12-month baseline period.
Furthermore, the analysis was repeated using the same inclusion and exclusion criteria for the 6-month group (with 6 months pre- and post-index period) and an identification period ranging between July 1, 2014–September 30, 2017.
Demographic and baseline clinical characteristics
Patient demographics including age, sex, and race were assessed. Clinical characteristics including the Quan-Charlson comorbidity index (Q-CCI) score, mental health-related comorbidities (including post-traumatic stress disorder, personality disorder, anxiety, suicide attempt and intentional injuries, tobacco use, bipolar disorder, any depressive disorder, and substance abuse), and non-mental health (MH) related comorbidities (including obesity, diabetes mellitus, cardiovascular disease [CVD]-hyperlipidemia, CVD-hypertension, CVD-coronary artery disease, hepatitis C, and chronic obstructive pulmonary disease) were assessed using ICD-9/10-CM codes. The ICD-9-CM codes are listed in Supplementary Appendix 1, and all ICD-9 diagnostic and procedure codes were mapped to their corresponding ICD-10 codes based on the General Equivalence Mappings (GEMs) published by the Centers for Medicare and Medicaid Services (CMS)Citation23.
Outcome measures
Outcome measures included treatment patterns, HRU, and costs during the 12-month and 6-month pre- and post-PP1M initiation periods. Treatment patterns among VHA patients who transitioned directly from oral ris/pali to PP1M were measured as the proportion of patients prescribed APs (including any oral, LAI, and short-acting injectable [SAI]; Supplementary Appendices 2 and 3), and other MH-related medications (antidepressants, anxiolytics, and mood stabilizers; Supplementary Appendix 4). The proportion of days covered (PDC), defined as the number of days in the follow-up period covered by medication divided by follow-up time (i.e. 12 months and 6 months), was used to calculate medication adherence. PDC was reported with ≥80% as adherent and <80% as non-adherentCitation24. The medication possession ratio (MPR), defined as the number of days of supply (i.e. number of days a prescription is supposed to last) within the entire exposure to therapy divided by the exposure to therapy, was also used to assess adherence. The exposure was defined as the number of days between the date of the first drug fill and the last drug refill plus the number of days of supply of the last refill. The MPR was then computed as the sum of the days of supply divided by the exposure to therapyCitation25. The difference between PDC and MPR is that PDC represents a more accurate representation of adherence due to the elimination of the possibility of ostensible adherence being elevated if patients obtain medications earlier than expected. In addition, PDC can be used to calculate the number of days a patient is covered by multiple therapiesCitation26.
During the 12-month and 6-month pre- and post-PP1M transition, HRU (e.g. inpatient length of stay, inpatient stays, outpatient visits, outpatient pharmacy visits) and costs (e.g. inpatient, outpatient, pharmacy, total medical, and total costs) were assessed and compared for all-cause, MH-related, and schizophrenia-related outcomes. Medical claims were considered MH-related if there was a mental health disorder (Supplementary Appendix 5) and/or schizophrenia diagnosis (as defined previously) in any position on the claim. MH-related pharmacy costs included costs for any AP (Supplementary Appendices 2 and 3) and/or other MH-related (Supplementary Appendix 4) medications. If there was a schizophrenia diagnosis in any position on the claim, costs were considered schizophrenia-related. Schizophrenia-related pharmacy costs included costs for any AP (Supplementary Appendices 2 and 3). All costs were adjusted to 2017 US dollars using the medical care component of the Consumer Price Index (CPI).
Statistical analysis
Descriptive analyses were performed for demographics and baseline clinical characteristics among oral ris/pali users who transitioned to PP1M. To compare the 12-month (and 6-month) pre- and post-PP1M transition outcomes (e.g. treatment patterns, HRU, and healthcare costs), the Wilcoxon signed-rank test for continuous variables and McNemar’s test for categorical variables were utilized. The level of significance was set at α = 0.05. All the analyses were conducted using SAS statistical software (Version 9.3, SAS Institute, Cary, NC, 2012).
Results
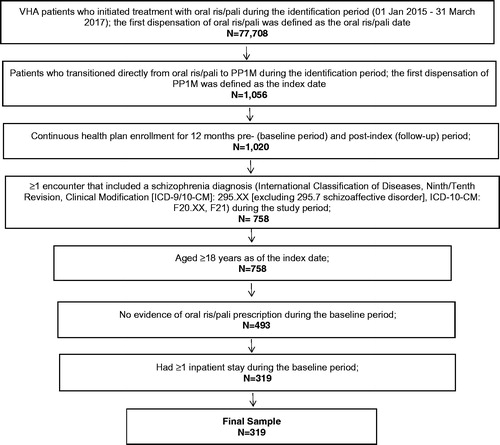
Based on the inclusion and exclusion criteria, there were 77,708 VHA patients who initiated treatment with oral ris/pali during the identification period. Among the 77,708 patients identified, using the 12-month pre- and post-transition period, there was a total of 319 patients who met the remaining inclusion and exclusion criteria, as shown in . Furthermore, there was a total of 401 VHA patients who met the same inclusion and exclusion criteria when implementing the 6-month pre- and post-transition time period.
Figure 1. Patient selection criteria for 12-months pre- and post-PP1M pransition among VHA patients with schizophrenia and ≥1 prior hospitalization. Abbreviations. ICD-9/10-CM, International Classification of Diseases—Ninth/Tenth Revision—Clinical Modification; oral ris/pali, oral risperidone or paliperidone; PP1M, once-monthly paliperidone palmitate; VHA, Veterans Health Administration.
Demographic and baseline characteristics
Findings using the 12-month pre–post transition period revealed that the average age of oral ris/pali users who transitioned to PP1M was 51.6 years (standard deviation [SD] = 14.2 years). Most VHA patients belonged to the age group between 55–64 years (36.4%) followed by the 65 years and older (18.5%) group. Patients were mostly male (90.9%) and most patients were white (48.6%) followed by black (38.9%). The mean Q-CCI score of patients was 1.1. The most common MH-related comorbidities were substance abuse (61.1%) followed by any depressive disorder (58.9%), tobacco use (55.8%), and anxiety (31.3%). Meanwhile, the most common non-MH-related comorbidities included CVD-hypertension (49.8%), CVD-hyperlipidemia (44.5%), obesity (24.5%), and diabetes mellitus (24.5%) ().
Table 1. Demographic and baseline clinical characteristics of PP1M VHA patients with schizophrenia and prior hospitalization.
Similar demographics and baseline clinical characteristics were observed using the 6-month pre- and post-transition criteria, as shown in .
Treatment patterns during pre- and post-transition to PP1M
6 months pre- and post-PP1M
During the 6-month pre-PP1M transition, findings on PDC and MPR revealed that 12.5% and 20.7% of VHA patients with schizophrenia were adherent (≥80%) to oral ris/pali, respectively. The mean PDC and MPR for oral ris/pali during the pre-PP1M transition was 0.4 (standard deviation [SD] = 0.3; median = 0.3) and 0.4 (SD = 0.3; median = 0.3), respectively. Furthermore, PDC and MPR results also exhibited that 40.6% and 50.4% of VHA patients with schizophrenia were adherent (≥80%) to PP1M post-transition. The mean PDC and MPR for PP1M during the post-PP1M transition was 0.6 (SD = 0.3; median = 0.7) and 0.7 (SD = 0.4; median = 0.8), respectively ().
Table 2. Comparison of treatment patterns 12-months and 6-months pre- and post-PP1M transition among VHA patients with schizophrenia treated with oral Ris/pali.
12 months pre- and post-PP1M
The use of mood stabilizers declined significantly from the 12 months pre- to post-PP1M transition period (58.9% vs 53.0%, p = .0104). No significant difference was observed in the use of any APs, antidepressants, or anxiolytics upon comparison of the 12 months pre- and post-PP1M initiation.
During the 12-month pre-PP1M transition, patient adherence to ris/pali (≥80%) was 7.2% (PDC) and 19.4% (MPR). The mean PDC and MPR for oral ris/pali during the pre-PP1M transition was 0.3 (SD = 0.3; median = 0.2) and 0.4 (SD = 0.3; median = 0.2), respectively (). Post-PP1M transition, PDC, and MPR results exhibited that 27.6% and 37.0% of VHA patients with schizophrenia were adherent (≥80%) to PP1M, respectively. Mean PDC and MPR for PP1M during the post-PP1M transition was 0.5 [SD = 0.3; median = 0.5] and 0.6 [SD = 0.4; median = 0.5], respectively.
HRU during pre- and post-transition to PP1M
6 months pre- and post-PP1M
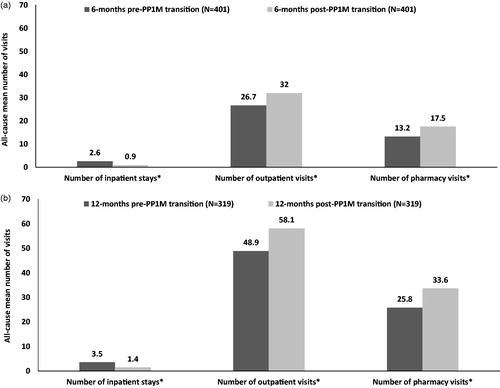
All-cause number of inpatient stays (2.6 vs 0.9, p < .0001) and inpatient LOS (30.7 vs 11.0 days, p < .0001) significantly decreased from pre- to post-PP1M transition; while the number of outpatient visits (26.8 vs 32.0, p < .0001) and pharmacy visits (13.2 vs 17.5, p < .0001) significantly increased post-PP1M transition (. MH- and schizophrenia-related HRU followed similar trends (results not shown).
Figure 2. Comparison of all-cause HRU (a) 6-months, and (b) 12-months pre- and post-PP1M transition among VHA patients with schizophrenia treated with oral ris/pali. *Significant at p < .05. Abbreviations. HRU, healthcare resource utilization; oral ris/pali, oral risperidone or paliperidone; PP1M, once-monthly paliperidone palmitate; VHA, Veterans Health Administration.
12 months pre- and post-PP1M
VHA patients with schizophrenia and ≥1 all-cause inpatient stay had a significantly longer inpatient length of stay (LOS) during the 12-month pre- compared to the post-PP1M period (43.4 vs 18.3 days, p < .0001; results not shown). Furthermore, VHA patients with schizophrenia and ≥1 all-cause inpatient stay also had a significantly lower number of all-cause inpatient stays (3.5 vs 1.4, p < .0001), higher number of outpatient visits (48.9 vs 58.1, p < .0001), and a higher number of pharmacy visits (25.8 vs 33.6, p < .0001) upon comparison of the 12-month pre- and post-PP1M transition (. Similar findings were observed for MH- and schizophrenia-related HRU during the 12-month pre–post period among VHA patients with schizophrenia and ≥1 all-cause inpatient stay such as a significantly lower number of MH- and schizophrenia-related inpatient stays and number of inpatient stays (results not shown).
Costs during pre- and post-transition to PP1M
6 months pre- and post-PP1M
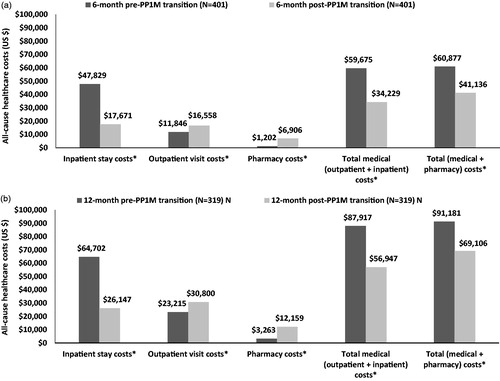
All-cause costs for inpatient stays ($47,829 vs $17,671, p < .0001), total medical ($59,675 vs $34,229, p < .0001), and total costs ($60,877 vs $41,136, p < .0001) significantly declined from the pre- to post-PP1M transition period. However, there was a significant increase in outpatient visit costs ($11,846 vs $16,558, p < .0001) and pharmacy costs ($1202 vs $6906, p < .0001) (. MH- and schizophrenia-related costs also followed mostly similar trends (results not shown).
Figure 3. Comparison of all-cause healthcare costs (a) 6-month, and (b) 12-month pre- and post-PP1M transition among VHA patients with schizophrenia treated with oral ris/pali. *Significant at p < .05. Abbreviations. ris/pali, risperidone or paliperidone; PP1M, once-monthly paliperidone palmitate; VHA, Veterans Health Administration.
12 months pre- and post-PP1M
Among VHA patients with schizophrenia and ≥1 all-cause inpatient stay, a significant increase was observed for all-cause outpatient visits ($23,215 vs $30,800, p < .0001) and pharmacy costs ($3263 vs $12,159, p < .0001) from pre- to post-PP1M transition. However, there was a significant decline in the all-cause total medical costs ($87,917 vs $56,947, p < .0001) from pre- to post-PP1M transition. The difference in costs was mainly driven by the significant decrease pre- to post-PP1M transition in all-cause inpatient costs ($64,702 vs $24,147, p < .0001). As a result, there was also a significant decline observed in the all-cause total costs ($91,181 vs $69,106, p < .0001) during the post-PP1M transition (. Similar findings were also observed for MH- and schizophrenia-related costs from pre- to post-PP1M transition for VHA patients with schizophrenia and ≥1 all-cause inpatient stay, regarding inpatient, outpatient, and pharmacy costs (results not shown).
Discussion
The current retrospective claims-based study comprehensively assessed the periods of pre- and post-transition to PP1M directly from oral ris/pali among VHA patients with schizophrenia and at least one prior hospitalization. The study revealed findings such as a reduction in inpatient stays along with shorter inpatient length of stay, as well as reduction in inpatient costs, total medical costs, and total costs after the transition to PP1M. The findings of this current study are consistent with numerous previous studies that have compared the use of PP1M and OAAs in general by comparing treatment cohorts and have reported similar outcomes to the current study, such as reductions in inpatient stay, improved adherence, increased pharmacy cost due to PP1M, and lower medical costsCitation9,Citation17,Citation32,Citation33. Moreover, a recent study using a decision-tree model to grasp patient pathways related to costs reported that patients with schizophrenia on OAA with a recent relapse who switched to PP1M resulted in reduced relapse rates and net healthcare cost savings, which offset the pharmacy cost associated with PP1MCitation27.
The pre–post design of the current study helps reduce bias since the design has allowed us to examine the exposure-outcome relations and has improved internal validity. Through specific observation of how outcomes for the same patients are affected by the transition to PP1M directly from oral ris/pali. As previously noted, oral ris/pali was specifically chosen to compare because the two are alike in their binding affinities. Furthermore, because paliperidone is an oral form of the LAI PP1M and they are similar in their components, we were able to observe how the outcomes of interest varied with a difference in the route of administration (i.e. oral vs injection administration) and frequency of dosage.
Adherence plays a critical role in schizophrenia treatment since non-adherence to medication can lead to increased hospitalization with longer inpatient stays and subsequent increases in healthcare system costsCitation9,Citation13–15,Citation28. Prior studies have shown that, upon comparison of patients treated with either PP1M or OAAs, patients treated with PP1M have been found to have a longer duration of continuous treatment along with a higher proportion of patients being adherentCitation16,Citation33. The findings from the current study also confirmed higher adherence to any antipsychotic among VHA patients with schizophrenia and at least one prior hospitalization after the transition to PP1M.
However, associations between adherence and costs should be interpreted with caution because prior studies have reported adherence is associated with higher pharmacy costs but not overall cost savingsCitation29. Although findings in the literature are mixed, the current study has found evidence of decreased total medical and total costs in spite of an increase in pharmacy costs. LAIs, such as PP1M, give patients and providers a better way to track medication administration since it must be administered by a medical provider. Patients will have to make an outpatient visit for administration, but may still have improved adherence (due to a sense of responsibility not to miss outpatient appointments for PP1M and the lack of the burden of remembering daily oral medications), which leads to better management of schizophreniaCitation30,Citation31. However, adherence is a multifaceted aspect of schizophrenia and there could be other factors that influence adherence (e.g. environmental factors, patient education, or other interventions in addition to medication)Citation13.
Improvements in all-cause HRU were observed with significantly shorter inpatient LOS and a smaller number of inpatient stays; however, higher outpatient and pharmacy visits were also revealed in the results. These findings were also reflected in similar trends for MH- and schizophrenia-related HRU (results not shown). Shah et al.Citation17 compared recently-diagnosed schizophrenia patients prescribed LAIs or OAAs and observed that patients prescribed LAIs (such as PP1M) had fewer inpatient admissions, ER visits, and days in the hospital compared to patients prescribed OAAs. In alignment with our results, Shah et al.Citation17 also noted that patients prescribed LAIs had higher schizophrenia-related outpatient visits and more prescription fills, which can be explained by the frequent provider visits for LAI administration. Similar findings were discovered in other studies such as reduced risk of hospitalization and fewer inpatient days when comparing patients on PP1M vs OAAsCitation32–34. The reduction in inpatient stays and LOS for PP1M patients is further supported by findings from a study by Lafeuille et al.Citation9, which found that, upon transitioning to PP1M from OAA, patients benefitted from a 36% relative risk reduction of hospitalization along with subsequent lower inpatient costs. The reduced hospitalizations and shorter length of stay may be attributed to improved adherence triggered by the mode of medication administration (i.e. an injectable as opposed to oral medication) and the longer duration of an LAI compared to OAAsCitation9. If inpatient stays can be reduced upon transition to PP1M, patients with schizophrenia and at least one prior hospitalization can avoid future costly hospitalizations and benefit from a greater quality-of-life clinically, economically, and personally.
Significantly lower all-cause inpatient, total medical, and total costs were also observed post-PP1M initiation; however, higher costs were observed for pharmacy and outpatient visits. These findings are further supported by previous studies and can be attributed to the higher cost of PP1M compared to OAAs in addition to more patients having a higher level of adherence and making more outpatient visits to receive the PP1M administrationCitation8,Citation17,Citation31,Citation32. Additionally, MH- and schizophrenia-related costs exhibited similar trends. Xiao et al.Citation32 reported that, upon comparison of PP1M and OAA users, PP1M use was linked to significantly lower medical costs, which offset the higher pharmacy cost of PP1M. Similar findings were also revealed by Pesa et al.Citation34 and Pilon et al.Citation33, who investigated patients recently diagnosed with schizophrenia. The lower medical costs for the PP1M cohorts in previous studies appeared to be driven by the significantly lower inpatient costsCitation32–34. These findings are in accordance with the current study wherein a majority of the total medical costs were driven by the inpatient costs, which were significantly higher during the pre- compared to the post-PP1M transition. Despite the lower medical costs in the previous studies mentioned, there was no significant difference in total healthcare costs for the previous studiesCitation9,Citation17,Citation32,Citation33. The current study, however, observed a significantly lower total cost post-PP1M transition. The difference in findings may be attributed to the magnitude of significant inpatient cost-savings for VHA patients with schizophrenia and at least one prior hospitalization that transitioned to PP1M from oral ris/pali.
Edwards et al.Citation35 investigated the threshold at which PP1M is more cost efficient than OAAs, and reported that PP1M patients saved $1486 in healthcare costs compared to patients with schizophrenia treated with OAAs. Furthermore, Pennington and McCroneCitation36 reported that patients with schizophrenia can experience excess relapse costs of $6033–$32,753, attributable to hospitalization. Overall, based on the current and previous studies, PP1M has been shown to provide a clinical benefit to patients while generating cost offsets.
Claims data can provide extremely valuable real-world information to assess healthcare outcomes, treatment patterns, HRU, and costs; but they are not without limitations. Coding errors are possible, and diagnoses could be entered for administrative processing as opposed to being linked to clinical outcomes. Specific information, such as clinical parameters, are not readily available in claims data and could have the potential to influence outcomes. Furthermore, the evaluation of adherence was based on the presence of a claim for a filled prescription; however, this does not indicate if the medication was consumed or taken (e.g. oral medications) as prescribed.
Limitations of the retrospective cohort study design include that patient data was not assessed outside of the defined study period along with less study control over acquired data as opposed to a prospective study designCitation37. Furthermore, with retrospective administrative claims data, unmeasured confounders may cause the study results to be subject to residual confoundingCitation9,Citation32. Because of the retrospective nature of the study, it will be important to compare these findings as newer data becomes available.
The baseline period of 12 months (and 6 months) may not capture the first oral ris/pali use for the sub-set of VHA patients who may have been on the medication for a longer period. The pre–post study also did not account for possible changes in patient characteristics before and after PP1M initiation when assessing the change in the outcomes. The pre–post design came with limitations such as the lack of comparison between oral ris/pali patients who did and did not switch to PP1M. Pre–post cost analyses may also be subject to bias due to costs that are incurred after a medication switch due to the failure of initial treatmentCitation38. Further, the differences in the outcome of interest may not be fully credited to the specific intervention when using the pre–post design. However, a strength of the pre–post design was the ability to use patients as their own controls. Furthermore, the study analyses did not utilize a t-test and chose to use a Wilcoxon signed-rank, as with similar previous studies, due to the smaller sample sizeCitation39.
Another limitation is that the current study only focused on the transition from oral risperidone or paliperidone to PP1M as opposed to considering additional oral medications. In addition, the study focused on patients who have had at least one prior hospitalization as opposed to VHA patients with schizophrenia as a whole. Moreover, the findings may not be generalizable to the overall US population, since the study utilized data from patients obtaining healthcare through the VHA system. Additionally, the current study sample consisted of a higher proportion of males with schizophrenia who had at least one prior hospitalization, were aged 55 years and older, and may have had different comorbidities compared to the general population.
Conclusion
Transitioning from oral ris/pali to PP1M was associated with fewer all-cause inpatient stays along with shorter inpatient length of stay among VHA patients with schizophrenia and at least one prior hospitalization. Additionally, all-cause inpatient, total medical, and total costs significantly declined after the transition to PP1M. Overall, the study findings indicate that the transition from oral ris/pali to PP1M among VHA patients with schizophrenia and prior hospitalization may lead to improved clinical outcomes and potential cost savings.
Transparency
Declaration of funding
This study was funded by Janssen Scientific Affairs, LLC., but this business entity did not have direct involvement in the conception, design, or conduct of the study, nor the decision to publish the same.
Declaration of financial relationships
Antoine El and Khoury Charmi Patel are employees of Janssen Scientific Affairs, LLC., Titusville, NJ. At the time of the drafting of this manuscript, Ahong Huang, Li Wang, and Richa Bashyal were all employees of STATinMed Research, Inc., a paid consultant to Janssen Scientific Affairs, LLC. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (45.5 KB)Acknowledgements
The authors would like to acknowledge, with their permission, Catherine Callan and Jieni Li from STATinMED Research for writing and programming services, which were funded by Janssen Scientific Affairs, LLC.
References
- Lafeuille MH, Grittner AM, Fortier J, et al. Comparison of rehospitalization rates and associated costs among patients with schizophrenia receiving paliperidone palmitate or oral antipsychotics. Am J Health Syst Pharm. 2015;72:378–389.
- Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586.
- DerSarkissian M, Lefebvre P, Joshi K, et al. Health care resource utilization and costs associated with transitioning to 3-month paliperidone palmitate among US veterans. Clin Ther. 2018;40:1496–1508.
- Emond B, Joshi K, Khoury Ac E, et al. Adherence, healthcare resource utilization, and costs in medicaid beneficiaries with schizophrenia transitioning from once-monthly to once-every-3-months paliperidone palmitate. Pharmacoecon Open. 2019;3:177–188.
- Lafeuille MH, Gravel J, Lefebvre P, et al. Patterns of relapse and associated cost burden in schizophrenia patients receiving atypical antipsychotics. J Med Econ. 2013;16:1290–1299.
- Parekh R. What is Schizophrenia? American Psychiatric Association. [cited 2019 Jan 18]. Available from: https://www.psychiatry.org/patients-families/schizophrenia/what-is-schizophrenia.
- Schizophrenia. National Institude of Mental Health. [cited 2019 Jan 18]. Available from: https://www.nimh.nih.gov/health/statistics/schizophrenia.shtml.
- Baser O, Xie L, Pesa J, et al. Healthcare utilization and costs Of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J Med Econ. 2015;18:357–365.
- Lafeuille MH, Tandon N, Tiggelaar S, et al. Economic impact in Medicaid beneficiaries with schizophrenia and cardiometabolic comorbidities treated with once-monthly paliperidone palmitate vs oral atypical antipsychotics. Drugs Real World Outcomes. 2018;5:81–90.
- Olivares JM, Sermon J, Hemels M, et al. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry. 2013;12:32.
- Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77:764–771.
- Masand PS, Roca M, Turner MS, et al. Partial adherence to antipsychotic medication impacts the course of illness in patients with schizophrenia: A review. Prim Care Companion J Clin Psychiatry. 2009;11:147–154.
- Velligan DI, Weiden PJ, Sajatovic M, et al. Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: Adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70:1–46.
- Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2.
- Zhang W, Amos TB, Gutkin SW, et al. A systematic literature review of the clinical and health economic burden of schizophrenia in privately insured patients in the United States. CEOR. 2018;10:309–320.
- Manjelievskaia J, Amos TB, El Khoury AC, et al. A comparison of treatment patterns, healthcare resource utilization, and costs among young adult Medicaid beneficiaries with schizophrenia treated with paliperidone palmitate or oral atypical antipsychotics in the US. J Med Econ. 2018;21:1221–1229.
- Shah A, Xie L, Kariburyo F, et al. Treatment patterns, healthcare resource utilization and costs among schizophrenia patients treated with long-acting injectable versus oral antipsychotics. Adv Ther. 2018;35:1994–2014.
- Corena-McLeod M. Comparative pharmacology of risperidone and paliperidone. Drugs R D. 2015;15:163–174.
- Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962.
- Markowitz M, Fu DJ, Levitan B, et al. Long-acting injectable paliperidone palmitate versus oral paliperidone extended release: a comparative analysis from two placebo-controlled relapse prevention studies. Ann Gen Psychiatry. 2013;12:22.
- Hsia SL, Leckband SG, Rao S, et al. Dosing strategies for switching from oral risperidone to paliperidone palmitate: Effects on clinical outcomes. Ment Health Clin. 2017;7:95–100.
- Bagalman E. The number of veterans that use VA health care services: a fact sheet. [cited 2019 March 19]. Available from: https://fas.org/sgp/crs/misc/R43579.pdf.
- Centers for Medicare & Medicaid Services (CMS). General Equivalence Mappings: ICD-9-CM to and from ICD-10-CM and ICD-10-PCS. [cited 2018 Dec 20]. Available from: https://www.cms.gov/Medicare/Coding/ICD10/downloads/ICD-10_GEM_fact_sheet.pdf.
- Campagna EJ, Muser E, Parks J, et al. Methodological considerations in estimating adherence and persistence for a long-acting injectable medication. JMCP. 2014;20:756–766.
- Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidem Dr S. 2006;15:565–574.
- Patel N. The difference between primary measures of medication adherence: PDC and MPR. [cited 2019 June 26]. Available from: https://www.usciences.edu/blog/noteworthy/posts/the-difference-between-primary-measures-of-medication-adherence-pdc-and-mpr.html.
- El Khoury AC, Pilon D, Morrison L, et al. The prospective economic impact of once-monthly paliperidone palmitate versus oral atypical antipsychotics in Medicaid patients with schizophrenia. Curr Med Res Opin. 2019;35:395–405.
- Higashi K, Medic G, Littlewood KJ, et al. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3:200–218.
- Pennington M, McCrone P. Does Non-Adherence Increase Treatment Costs in Schizophrenia? Pharmacoeconomics. 2018;36:941–955.
- Brasso C, Bellino S, Bozzatello P, et al. Role of 3-monthly long-acting injectable paliperidone in the maintenance of schizophrenia. NDT. 2017;13:2767–2779.
- Joshi K, Lafeuille MH, Kamstra R, et al. Real-world adherence and economic outcomes associated with paliperidone palmitate versus oral atypical antipsychotics in schizophrenia patients with substance-related disorders using Medicaid benefits. J Comp Eff Res. 2018;7:121–133.
- Xiao Y, Muser E, Fu DJ, et al. Comparison of Medicaid spending in schizoaffective patients treated with once monthly paliperidone palmitate or oral atypical antipsychotics. Curr Med Res Opin. 2016;32:759–769.
- Pilon D, Muser E, Lefebvre P, et al. Adherence, healthcare resource utilization and Medicaid spending associated with once-monthly paliperidone palmitate versus oral atypical antipsychotic treatment among adults recently diagnosed with schizophrenia. BMC Psychiatry. 2017;17:207.
- Pesa JA, Doshi D, Wang L, et al. Health care resource utilization and costs of California Medicaid patients with schizophrenia treated with paliperidone palmitate once monthly or atypical oral antipsychotic treatment. Curr Med Res Opin. 2017;33:723–731.
- Edwards NC, Muser E, Doshi D, et al. The threshold rate of oral atypical anti-psychotic adherence at which paliperidone palmitate is cost saving. J Med Econ. 2012;15:623–634.
- Pennington M, McCrone P. The cost of relapse in schizophrenia. Pharmacoeconomics. 2017;35:921–936.
- Song JW, Chung KC. Observational studies: Cohort and case-control studies. Plast Recon Surg. 2010;126:2234–2242.
- Faries DE, Nyhuis AW, Ascher-Svanum H. Methodological issues in assessing changes in costs pre-and post-medication switch: A schizophrenia study example. Cost Eff Resour Alloc. 2009;7:11.
- Berger A, Edelsberg J, Treglia M, et al. Change in healthcare utilization and costs following initiation of benzodiazepine therapy for long-term treatment of generalized anxiety disorder: A retrospective cohort study. BMC Psychiatry. 2012;12:177.
