Abstract
Objective: Despite guidelines that identify potential patients eligible for preventive migraine medications, their underutilization leaves patients at risk of acute medication overuse, disease progression, and higher healthcare resource utilization and disability. This exploratory, retrospective, observational study aimed to identify which factors predict preventive migraine medication initiation. Demographics and initiation of acute medication use were hypothesized to be predictive of initiation of preventive migraine medication.
Methods: The Truven Health Analytics MarketScan1 U.S. Commercial and Medicare Supplemental claims database (2011-2013) was used to identify adults newly diagnosed with migraine. Patients were divided into 2 subgroups: initiated a preventive migraine medication (antidepressants, anti-epileptics, beta-blockers, or neurotoxins) within 1 year of migraine diagnosis and did not initiate a preventive migraine medication. Logistic regression models were constructed to identify factors associated with preventive migraine medication initiation.
Results: Study population included 147,923 patients: 43,660 preventive migraine medication initiators and 104,263 non-preventive migraine medication patients. Best-fit model for predicting preventive migraine medication initiation included: female gender (odds ratio = 1.181 [95% CI = 1.144,1.218]; measured at date of first migraine diagnosis); headache diagnosis prior to migraine diagnosis (odds ratio = 1.538 [95% CI = 1.498,1.579]; measured 1-year before first migraine diagnosis); and sleep disorder (odds ratio = 1.206 [95% CI = 1.161,1.252]), headache/migraine-specific Emergency Department (ED) visit (odds ratio = 1.224 [95% CI = 1.168,1.283]), neurologist visit (odds ratio = 1.502 [95% CI = 1.459,1.547]), and acute medication refills with <90-day gap (odds ratio = 1.509 [95% CI = 1.470,1.549]) each measured at 1-year before first preventive migraine medication.
Conclusions: In addition to consistent acute medication refills, specific comorbidity diagnoses, headache/migraine-specific ED utilization, and neurologist care are predictive of preventive migraine medication initiation in the 1-year post-incident migraine diagnosis.
Introduction
Migraine is one of the most common neurological diseases, affecting nearly 12% of the U.S. populationCitation1–3. It is associated with effects on day-to-day functioning that can be severely debilitatingCitation1,Citation4,Citation5. Recent studies have shown that burden of disease is increased, including an increase in the number of comorbidities, among patients with migraine who failed acute treatmentCitation6; burden also increased with the addition of each preventive migraine medications (PMMs)Citation6,Citation7.
Recommendations for preventive treatments for migraine based on the U.S., Canadian, and European evidence-based guidelines have been published and include both recommendations for PMMs and behavioral interventionsCitation8–11. The following pharmacologic agents have been recommended by the American Academy of Neurology/American Headache Society as PMMs based on evidence, and expert consensus: Established efficacy (Level A) for metoprolol, timolol, propranolol, divalproex sodium, sodium valproate, topiramate, frovatriptan; probably effective (Level B) for nadolol, amitriptyline, venlafaxine, atenolol, naratriptan, zolmitriptan; possibly effective (Level C) for candesartan, lisinopril, clonidine, guanfacine, carbamazepine, nebivolol, pindolol; and inadequate or conflicting data to support or refute use for gabapentin, fluoxetine, fluvoxamine, protriptyline, acenocoumarol, warfarin, picotamide, bisoprolol, nicardipine, nifedipine, nimodipine, verapamil, acetazolamide, cyclandelateCitation11. OnabotulinumtoxinA is approved by the U.S. Food and Drug Administration for the prevention of chronic migraine. As per Canadian Headache Society guidelines, 11 drugs received a “strong” recommendation for use (topiramate, propranolol, nadolol, metoprolol, amitriptyline, gabapentin, candesartan, Petasites [butterbur], riboflavin, coenzyme Q10, and magnesium citrate)Citation10.
Treatment recommendations for migraine clearly document when a preventive treatment should be initiated, as well as specific recommended medications that should be prescribedCitation8–12. However, original findings from the American Migraine Prevalence and Prevention study estimated that only 13% of all patients with migraine received PMM, and an estimated 39% were appropriate candidates for PMMCitation3. More recent data suggest that an estimated 25% of patients with episodic migraine and only 41% with chronic migraine are current users of a preventive treatment in the U.S.Citation13. Patients who are eligible for PMM but not using any often manage their disease solely with acute medications, including over-the-counter medications, opioids, and/or barbituratesCitation14–17, which places them at significant risk or may be a marker for disease progressionCitation18,Citation19. This pattern of undertreatment often is associated with higher migraine-related disability (e.g. increased number of days of missed work or school per month)Citation4,Citation20, reduced patient functioning (i.e. restriction or prevention of activities across multiple areas of life)Citation4,Citation21,Citation22, and higher healthcare resource utilization (HCRU)Citation23,Citation24. Research has indicated that the use of PMM decreases the patient burden and reduces HCRUCitation25, suggesting that PMM should be initiated when clinically warrantedCitation26.
The overall goal of this study was to examine available information in the U.S. claims database to identify and execute predictive modeling for patients who initiated preventive migraine medications. Although data on headache frequency and severity were not available in this database, many other patient-specific variables are likely predictive. Specifically, this exploratory study aimed to identify which patients in the medical claims database were most likely eligible for prevention, by determining variables that are predictive of PMM initiation within 12 months of migraine diagnosis. Of specific interest was the association of PMM initiation with acute migraine medication (i.e. opioids, triptans, barbiturates, isometheptenes, and ergots) use patterns. Other variables of interest included patient demographics, type of health insurance plan, selected comorbidities and symptoms (i.e. obesity, sleep disorders, cardiovascular disease, allodynia, diabetes mellitus), and HCRU. Specifically, this research addressed the following objectives: (1) determine if acute medication refills is a predictor of PMM initiation and (2) identify other predictors that are associated with PMM initiation. This is important to address given that administrative claims datasets have limited clinical variables, and identifying a predictive model with the available data could enable changes in health insurance policies to facilitate earlier interventions for patients with a greater risk. Early use of an efficacious and tolerable PMM has the potential to reduce the overuse of acute medications, decrease the risk of disease progression, prevent unnecessary HCRU, and improve patient outcomesCitation13,Citation25.
Methods
Study design and data sources
This retrospective observational cohort study extracted and analyzed claims data from the Truven Health Analytics MarketScan1 U.S. Commercial and Medicare Supplemental claims database claims database from 01 January 2010 to 31 December 2014. This was an exploratory study with pre-specified objectives and analysis plan. The Truven patient population consists of more than 240 million commercially insured patients, covered dependents, and retirees with employer-sponsored supplemental Medicare coverage. These data contain de-identified administrative claims capturing patient-level data on age, gender, geographic region, and HCRU (e.g. emergency department [ED] visits, inpatient/outpatient visits, prescriptions), expenditures, and enrollment across inpatient, outpatient, prescription drug, and carve-out services. The Truven Health Analytics MarketScan research databases link paid claims and encounter data, capturing when services occurred, and diagnosis codes via the International Classification of Diseases, Tenth Revision (ICD-9); Healthcare Common Procedure Coding System; and Current Procedural Terminology codes. Institutional review board approval and patient consent to release information were not required due to the de-identified nature of this existing data source, and methods to protect both patients and healthcare sites.
Patient selection
Patients of at least 18 years of age with at least 1 migraine diagnosis during an index period from 01 January 2011 to 31 December 2013 were identified for cohort inclusion (index date = first migraine diagnosis during this period) (Supplementary Figure). In addition, patients where this index migraine diagnosis was on an outpatient claim were required to have an additional migraine diagnosis in the 3-year time period after the index event. In the case that the index migraine diagnosis was on an inpatient claim, no additional migraine diagnosis was required. For the purpose of this study, patients with a migraine diagnosis or claims for any PMM (established, probably, or possibly effective) per the U.S. treatment guidelinesCitation9,Citation11 in the 1-year pre-index were excluded. This exclusion limited the cohort to newly diagnosed (incident) patients with migraine (i.e. patients who received their diagnosis from a healthcare professional). The patients were permitted to have a (non-migraine) headache diagnosis during the 1-year pre-index period.
This study included 2 index events: date of first migraine diagnosis described above (hereafter referred to as migraine diagnosis date) and date of first PMM (hereafter referred to as PMM initiation date). The PMM initiation date for patients not initiating a PMM (hereafter referred to as non-PMM) was a random date post-migraine diagnosis date that met the distribution of the PMM initiation date for the PMM initiators for every 30-day interval. The inclusion criteria required 12 months of pre- and post-migraine diagnosis date continuous medical and prescription enrollment.
All patients receiving Medicaid assistance were excluded due to the heterogeneity of the patient population and a notably different health services and reimbursement structure compared with commercial providers. Patients with claims for human immunodeficiency virus infection or cancer from 1-year pre-migraine diagnosis date to 1-year post-migraine diagnosis date were not included in this study. Patients with an ICD-9 code for a non-migraine disease treated by that PMM class (i.e. received an anti-epileptic/antihypertensive/beta-blocker/antidepressant drug during the 1-year post-migraine diagnosis AND received an epilepsy/hypertension/congestive heart failure/depression diagnosis in the 1 year before receiving the specific drug class) were also excluded. In addition, patients with a claim for any PMM without proven efficacy (Level C) per the treatment guidelines during the 1-year post-migraine diagnosis date period were excluded in an effort to increase the validity of medication use specific to treating migraineCitation9,Citation11. Finally, patients prescribed naratriptan and zolmitriptan (drugs only recommended for short-term use associated with menstrual headache) were also excluded.
This migraine cohort was then subgrouped into (1) patients receiving an established or probably effective (Level A or B) PMM in the 1-year post-migraine diagnosis date period and (2) non-PMM initiators in the 1-year post-migraine diagnosis date periodCitation9,Citation11. These 2 subgroups were mutually exclusive.
Descriptive analyses
A series of descriptive analyses were performed for each subgroup (PMM and non-PMM), including gender, disease-relevant comorbidity frequencies and symptoms (i.e. obesity [ICD-9: 278.0, 278.00, 278.01, 278.02, 278.03], sleep disorders [ICD-9: 780.5x, 307.4x, 327.xx,], cardiovascular disease [see supplement], allodynia [ICD-9: 782.0], diabetes mellitus [(ICD-9: 250.xx)]), age, insurance type, geographic region, and receipt of a headache diagnosis (ICD-9: 784.0, 339.xx, 307.81) in the 1-year pre-migraine diagnosis date period (ICD-9: 346.xx). The number of headache diagnosis codes (defined as ICD-9: 784.0, 339.xx, 307.81 in this study) per patient in the 1-year pre-migraine diagnosis date period and measures of HCRU (all-cause and migraine-related) were also analyzed. The results of these descriptive analyses were reviewed for clinically meaningful differences in order to identify variables for inclusion in the predictive model. Cohort attrition was based on patient selection criteria. Average and median time to PMM initiation was also calculated. Analyses were performed using SAS, Version 9.2 (SAS Inc, Cary, NC, USA).
Predictive modeling
Logistic regression models were constructed comparing patients that initiated a PMM vs non-PMM initiators (within 1 year of first migraine diagnosis during 2011–2013) as the outcome variable; all variables were measured during the 1-year pre-PMM initiation date period, except where noted. Modified purposeful selection techniquesCitation27 were used to build the final multivariate logistic regression models, which used stepwise methods to evaluate predictors for inclusion. Both clinical and theoretical judgment based on the existing literature were applied to select the predictors in the initial model, which at the migraine diagnosis date included gender and at the PMM initiation date included age, geographic region, and plan type (Commercial vs Medicare). Predictors during the 1-year pre-migraine diagnosis date period included headache diagnosis; and predictors during the 1-year pre-PMM initiation date period included number of outpatient visits, ED visits, headache/migraine-specific ED visits (defined as an ED visit with a migraine or headache diagnosis code [346.xx, 307.81, 784.0, 339.1, 339.3] AND 1 of the recommended treatments OR a triptan or opioid)Citation11, neurologist visits, obesity, sleep disorders, cardiovascular disease, allodynia, diabetes mellitus, and consistent acute medication refills (defined as no gap in administrative claims for prescription refills that are received by the patient for any acute medication >90 days in the year pre-index). The acute medication classes included opioids, triptans, barbiturates, isometheptene, and ergots. Nonsteroidal anti-inflammatory drugs were not included due to their common use in both non-migraine pain and migraine disorders, and the inability to capture over-the-counter use.
Results
Patient disposition, demographics, and headache/migraine-specific diagnosis, and comorbidities
From the database, 1,186,112 patients were identified to have at least 2 outpatient (or 1 inpatient) migraine diagnoses claims (ICD-9: 346.xx) from 1 January 2011 to 31 December 2013; reasons for patient exclusion are summarized in . The final sample who met all study inclusion and exclusion criteria consisted of 147,923 patients, including 43,660 patients who initiated a PMM and 104,263 non-PMM patients ().
Table 1. Demographic and medical characteristics at index date and selected pre-index comorbidities.
The migraine cohort (both the subgroups combined) was 82.1% female with a mean age of 40.5 years, and the largest proportion of patients (36.9%) resided in the southern region of the U.S. based on the regional distribution in the Truven database (). Most of the study population (97.0%) had commercial insurance. Overall, 23.7% of the study population had a headache diagnosis (ICD-9 784.0) during the 1-year pre-migraine diagnosis date period (excluding index day), with a notable difference in the subgroup who initiated a PMM (31.6%) vs those who did not initiate a PMM (20.3%). The mean number of non-migraine headache diagnoses during the 1-year pre-migraine diagnosis date period was 0.56 for the overall study population, with a trend towards a higher average in the PMM vs non-PMM subgroups (0.76 vs 0.47, respectively). Among key comorbidities of interest for the overall study population, the most common included sleep disorders (10.2%), cardiovascular disease (8.3%), allodynia (6.8%), obesity (6.5%), and diabetes mellitus (4.5%) during the 1-year pre-PMM initiation date period (including the index day).
All-cause and migraine-specific HCRU characteristics
Most patients (>98%), regardless of subgroup, had at least 1 all-cause outpatient visit during the 1-year pre-PMM initiation date period; the mean number of total outpatient visits per patient trended higher for the PMM subgroup (18.39) vs the non-PMM subgroup (16.34) (). For migraine-specific HCRU, 63% had at least 1 outpatient visit in the 1-year pre-PMM initiation date period; however, the mean number of total outpatient visits per patient was similar for the PMM and non-PMM subgroups (1.17 and 1.12, respectively).
Table 2. Healthcare resource utilization characteristics during the 1-year pre-PMM initiation date period.
For all-cause HCRU, at least 1 ED/inpatient visit was reported for 20.2%/8.8% of the PMM subgroup vs 17.8%/7.1% of the non-PMM subgroup, respectively; the percentage of patients with ED or inpatient admissions visits trended higher for the PMM subgroup vs the non-PMM subgroup. The mean number of total ED and inpatient visits per patient during the 1-year pre-PMM initiation date period was also notably different for the PMM vs non-PMM subgroups (0.75 and 0.13 vs 0.57 and 0.10, respectively). For migraine-specific HCRU, the percentages of all patients with at least 1 ED or inpatient visit was relatively low (≤8.8%) and the mean number of total ED and inpatient visits was similar between the PMM and non-PMM subgroups.
Patients who initiated a PMM were more likely to visit a neurologist or a pain management/specialist in the 1-year pre-PMM initiation date period (25% and 1.9%, respectively) vs those who did not initiate a PMM (16.1% and 1.3%, respectively). The mean number of neurologist and pain management/specialist visits per patient trended higher for the PMM (0.54 and 0.08, respectively) vs non-PMM subgroup (0.33 and 0.05, respectively).
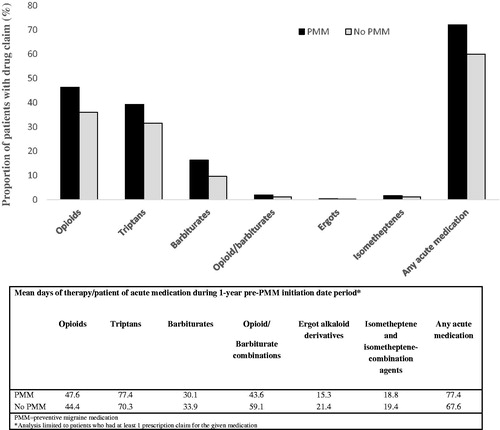
Acute medication refill patterns
During the 1-year pre-PMM initiation date period, 72.1% of PMM initiators and 60.0% of non-PMM initiators refilled a prescription for at least 1 of 5 acute medication classes (). The most commonly filled prescription claims for acute medications for the PMM vs non-PMM groups, respectively, were opioids (46.4% and 36.0%) followed by triptans (39.3% and 31.5%) and barbiturates (16.3% and 9.6%) (, ). Patients initiating a PMM appeared to have increased refills of the most acute medication classes assessed, especially opioids. The average refill of an acute medication (any class) for the PMM subgroup was 77.4 days (SD 105.7) during the 1-year pre-PMM initiation date period vs 67.6 days (SD 99.5) for the non-PMM subgroup. Patients who initiated a PMM also had an observed difference in the proportion of consistent acute medication refills during the 1-year pre-PMM initiation date period (15.3%) vs those who did not initiate a PMM (11.1%). Among PMM initiators, a nonsignificant trend was observed in consistent acute medication refills (more days of therapy) during the 90 days leading up to PMM initiation.
Table 3. Headache/migraine-specific acute medication refills.
Predictors for PMM
Three different exploratory logistic regression modelsCitation27 were examined for predicting PMM initiation. The predictors were identical across the 3 models with the exception of how consistent acute medication refills were captured. The first 2 models examined the number of acute medication classes in the 1-year pre-PMM initiation date period OR acute medication days of therapy in the 3-month pre-index period (model data not shown). Ultimately, a third option that examined consistent acute medication refills (i.e. <90-day gap 1-year pre-PMM initiation date period) provided a slightly better fit for prediction of PMM initiation ().
Figure 3. Forest plots of odds ratios/confidence intervals for the model (consistent acute medication refills with <90-day gap). All variables measured in the 1-year pre-PMM initiation date except for age, region, and health plan that were measured at PMM initiation date and headache diagnosis that was measured in the 1-year pre-migraine diagnosis date. The following variables were not included in the final model (geographic region, cardiovascular disease, diabetes, outpatient visit, and Emergency Department visit); All remaining variables were significant (p < 0.0001). Abbreviations. CI, confidence interval; CV, cardiovascular; ED, emergency department.
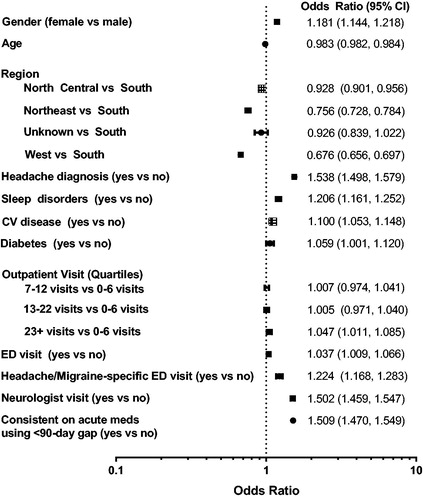
As shown in , the most significant factors predicting PMM initiation (all p < .0001) included female gender (odds ratio [OR] = 1.181 [95% CI = 1.144, 1.218; measured at migraine diagnosis date]), younger age (OR = 0.983 [95% CI = 0.982, 0.984; measured at migraine diagnosis date]); headache diagnosis (OR = 1.538 [95% CI = 1.498, 1.579]; measured 1-year pre-migraine diagnosis date), sleep disorder (OR = 1.206 [95% CI = 1.161, 1.252]), headache/migraine-specific ED visit (OR = 1.224 [95% CI = 1.168, 1.283]), neurologist visit (OR = 1.502 [95% CI = 1.459, 1.547]), and consistent acute medication refills with <90-day gap (OR = 1.509 [95% CI = 1.470,1.549]) each measured at 1-year pre-PMM initiation date unless otherwise noted.
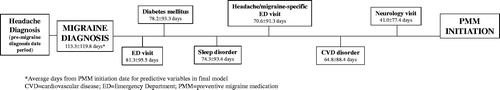
A subsequent analysis explored the timeline from various key events (i.e. average days from PMM initiation date for predictive variable in final model) as the PMM subgroup progressed from diagnosis of migraine to PMM initiation. As shown in , PMM initiators progressed through several key events prior to initiating their first PMM in the following order: ED visit, diabetes mellitus diagnosis, sleep disorder diagnosis, headache/migraine-specific ED visit, cardiovascular disease diagnosis, and neurologist visit. The mean time from migraine diagnosis to PMM initiation was 113.3 ± 119.8 days, and the closest event to PMM initiation was a neurologist outpatient visit at 41.0 ± 77.4 days.
Discussion
This exploratory retrospective observational study recognized several key factors that were associated with preventive migraine medication (PMM) initiation in patients newly diagnosed with migraine by a healthcare professional. This included having a headache diagnosis (other than migraine) in the 1-year prior to the first migraine diagnosis date. This is an important finding as it might point to early misdiagnosis of migraine as headache or that patients had not received a migraine diagnosis code until the date of migraine diagnosis. Therefore, patients with a headache diagnosis before the pre-migraine diagnosis date might not actually have been new patients with migraine at index. This observation has implications for clinical practice (i.e. misdiagnosis)Citation28 and research (i.e. selection of patients to identify incident migraine). While outpatient visits did not predict PMM initiation, a neurologist visit 1-year prior to the index date for start of the first PMM was associated with a greater chance of patients receiving a PMM. Perhaps neurologists have increased knowledge around the need for PMM initiation or a better comfort level at prescribing PMMs. Healthcare professionals would benefit from using existing tools and there may be a need for additional tools to help diagnosis and identify need for PMMsCitation29,Citation30. Alternatively, initiation of a PMM may be due to increased disease severity in patients that seek care from a neurologist. Given that this research study was executed using administrative claims, we were unable to assess severity of disease in terms of headache or migraine day frequency, pain intensity, or treatment responsiveness. Finally, it is noteworthy that a neurologist visit occurred relatively late in the timeline of events between the first diagnosis of migraine and the start of a PMM. Accordingly, involvement of a neurologist at an earlier phase may be beneficialCitation31 and initiate appropriate acute and preventive medications (ones that do not have any relative contraindications to sleep apnea, diabetes mellitus, and cardiovascular disease) as well as empirically supported behavioral treatments and effective lifestyle education. However, due to decreased supply of neurologists, the very small number of headache subspecialists, and the geographic disparities, patients may not be able to see a neurologist or headache expert physician in a timely fashion or access to appropriate care may not be feasibleCitation32,Citation33.
Additional factors found to predict PMM initiation in our study included consistent use of acute medications, especially opioids, during the 1-year prior to initiation of PMM, which could be proxy for migraine severity or lack of migraine control. Use of opioids on at least 10 days per month for more than 3 months is defined as medication overuseCitation34,Citation35 and associated with significant negative outcomesCitation15. Thus, opioids and barbiturates are not recommended for management of migraineCitation8–11, and their use is a red flag suggesting that PMM initiation is warranted. Notably, initiation of a PMM was associated with younger age, it is not clear if this is due to the limitations of the data source or another confounding variable, such as disease severity; natural epidemiology of the disease; association with work, school, and family responsibilities; health insurance status; or other variables. In addition, our model also found that female gender, sleep disorders, and headache-specific ED visits in the 1-year prior to initiation of PMM were highly predictive of PMM initiation. As with other factors previously noted, sleep disorders and presence of a headache-specific ED visit could also signify increased disease severity and, thus, there is more need for a PMM.
Initiation of a PMM may be delayed from the time of first migraine diagnosis due to patient perceptions of adverse events associated with some preventive medicationsCitation36. In this patient-completed survey, two-thirds of respondents delayed or avoided taking a prescription medication for migraine because of concerns about adverse effects, which resulted in a delay in taking medication in more than one-third of patients. Furthermore, <30% of patients with chronic migraine adhere to PMMs at 6 months post-initiationCitation37 and <60% of patients were willing to take PMMs even if they achieve a 50% reduction in frequency of migraine attacksCitation38.
For patients with migraine, one of the most instrumental factors for evaluating the need for clinical interventions is the number of migraine headache days per monthCitation3,Citation39,Citation40. Typical recommendations take into account both attack frequency and related disability for informing the initiation of a PMM treatmentCitation3,Citation41. The number of migraine headache attacks or days per month and migraine-related disability are not captured in administrative claims; however, the identification of other healthcare claims database variables associated with those that lead to initiation of a PMM could serve as a proxy for identifying the subpopulation of patients with migraine that is potentially eligible for this type of care.
Given that headache-specific ED visits were identified as an associated event prior to PMM initiation, there may be an opportunity to intervene early and avoid costs associated with ED useCitation42,Citation43. Escalating acute medication overuse could also be avoided, reducing the risk of disease progression and other poor outcomes associated with this risk factor, including ED useCitation44–46. Migraine care in an ED setting is not ideal; utilization is typically a last resort for patients due to lengthy waits, high noise levels, brightly lighted environments, and non-guideline migraine careCitation14,Citation47. In addition, opioid use is more common in the ED, putting patients at risk of misuse, medication overuse headache and disease progression, and greater healthcare utilization and costsCitation14,Citation15,Citation48–50. Specific comorbidities were also identified in this model; however, the dynamics of the relationship with migraine disease diagnosis and progression to PMM initiation is not clear.
Research to date has indicated that determinants associated with PMM use include migraine symptoms, headache frequency (days per month), headache pain intensity, and headache-related disabilityCitation3,Citation40,Citation51. Similar to our results, initiators of PMM are more likely to have been treated by a neurologistCitation40. HCRU patterns among patients who previously received a preventive initiating a new PMM compared to preventive-naïve patients have been reported using the same commercial claims databaseCitation52. Unlike our research, Bonafede and colleagues evaluated factors post-PMM initiation; however, a noted similarity was that migraine-related ED visits were higher in the pre-index period for PMM-treated patients (14.4%, with 2.0 ± 3.2 visits) than those who were PMM naive (11.7%, with 1.6 ± 2.2 visits).
Limitations
We were limited to the data available from an administrative U.S. healthcare claims database, a system used for medical billing purposes. It was unknown if participants had previous diagnoses, previous PMM use, the patient’s level of headache frequency, and their degree of disability before they were included in that database. However, 2 migraine diagnostic codes (or 1 inpatient) were required to identify the study population, which has been shown to provide higher specificity and sensitivity. Due to underutilization of the ICD-9 code for chronic migraine, this study did not separate episodic and chronic migraineCitation53. Furthermore, this study used a migraine diagnostic code to identify incident patients with migraine; however, they may have received a diagnosis prior to the pre-migraine diagnosis date period. Our findings might not be applicable to patients with long-standing migraine in new need of a PMM. Medication use was based on prescription claims; actual patient consumption and specific indication for use was unknown. We did exclude patients given PMM if they had another disease treated by that medication (e.g. depression and antidepressants), and it was unknown if the subgroup with concurrent illnesses that could benefit from 1 treatment for both disorders would be a predictor of PMM initiation. In addition, the possibility still exists that patients received their preventive medication for a disease other than migraine. Over-the-counter, herbal and neutraceutical, and nonpharmacologic migraine treatments are not captured in this database. Furthermore, our database excluded some patients with comorbidities who may have had migraine (e.g. patients with epilepsy receiving anti-epileptics), thus our findings on prediction of PMM initiation are not generalizable to the whole migraine population. Our study population also may not be representative of patients with migraine because only those with employee-based health insurance or Medicare supplemental insurance were included. Patients on Medicaid were also excluded because they were likely a unique population compared to the commercially insured patients; the results herein cannot be extrapolated to the Medicaid population given their different demographic profiles, disease burden, health-service access, and care practices that would not mimic a commercially insured population. Finally, our findings cannot be extrapolated to patients with migraine who reside outside the U.S. wherein different treatment practices might apply. Characteristics of migraine and PMM use in the general population may be different from the population studied in claims data.
Conclusions
In addition to consistent acute medication refills, specific comorbidity diagnoses, headache/migraine-specific ED utilization, and neurologist care are predictive of PMM initiation in the 1-year post-incident migraine diagnosis among the U.S. patients who participated in this private insurance database. Additional research is needed to expand on the ability to identify patients with migraine who are in need of preventive care within the healthcare system. Receiving PMM at the appropriate time within the course of this neurological disease has the potential to reduce HCRU, prevent disease progression, and improve patient outcomes.
Transparency
Declaration of funding
This work was supported solely by Eli Lilly and Company; the sponsor was responsible for the study design, data collection, data analysis, interpretation of data, and decision to publish the findings.
Declaration of financial/other relationships
DB has received research funding from Amgen, Allergan, and Promeius and honoraria from Allergan, Biohaven, Eli Lilly, and Teva. She also is on the editorial board of Current Pain and Headache Reports. SJ is on the advisory board for Eli Lilly, Pernix, and Avanir and on the speaker’s bureau for Eli Lilly, Depomed, Allergen, Supernus, Avanir, and Teva. JF, SG, SAF, KS, and SKA were employees and minor stockholders of Eli Lilly and Company at the time of the study. A peer reviewer on this manuscript declares multiple previous and current research grants from the UK National Institute for Health Research (NIHR), Arthritis Research UK and grants funded by the Australian NHMRC. They have also received travel expenses for speaking at conferences from the professional organizations hosting the conferences. They are an employee and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. They are a part of an academic partnership with Serco Ltd. They are an editor of the NIHR journal series, and a member of the NIHIR Journal editors Group for which they receive a fee. Finally, they are a co-investigator on a study receiving support in kind from Orthospace Ltd. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Janet H. Ford: Study design, interpretation of the data, wrote the initial draft of the manuscript, and revised the manuscript for content; Krista Schroeder: Contributed to creating and revising the manuscript for content; Dawn C. Buse: Contributed to creating and revising the manuscript for content; Shivang Joshi: Contributed to creating and revising the manuscript for content; Steven Gelwicks: Statistical analyses, interpretation of the data, creating and revising the manuscript for content; Shonda A. Foster: Contributed to creating and revising the manuscript for content; Sheena K. Aurora: Contributed to creating and revising the manuscript for content.
Supplemental Material
Download MS Word (164.2 KB)Acknowledgements
Data analyses were performed by Eli Lilly and Company. Writing support was provided by Teresa Tartaglione, PharmD (Synchrogenix, a Certara Company, Wilmington, Delaware).
Notes
Notes
1 The Truven Health Analytics MarketScan, Ann Arbor, MI, USA.
References
- Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58:496–505.
- Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299.
- Lipton RB, Bigal ME, Diamond M, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349.
- Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31:301–315.
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897.
- Korolainen MA, Kurki S, Lassenius MI, et al. Burden of migraine in Finland: health care resource use, sick-leaves and comorbidities in occupational health care. J Headache Pain. 2019;20:13.
- Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19:115.
- Carville S, Padhi S, Reason T, et al. Guideline Development Group. Diagnosis and management of headaches in young people and adults: summary of NICE guidance. BMJ. 2012;345:e5765.
- Holland S, Silberstein SD, Freitag F, et al. Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the quality standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346–1353.
- Pringsheim T, Davenport W, Mackie G, et al. Canadian Headache Society Prophylactic Guidelines Development Group. Canadian Headache Society guideline for migraine prophylaxis. Can J Neurol Sci. 2012;39:S1–S59.
- Silberstein SD, Holland S, Freitag F, et al. Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–1345. (Erratum in: Neurology. 2013;80:871.)
- García-Azorin D, Santos-Lasaosa S, Gago-Veiga AB, et al. Real world preventative drug management of migraine among Spanish neurologists. J Headache Pain. 2019;20:19.
- Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second International Burden of Migraine Study (IBMS-II). Headache. 2013;53:644–655.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21–34.
- Buse DC, Pearlman SH, Reed ML, et al. Opioid use and dependence among persons with migraine: results of the AMPP study. Headache. 2012;52:18–36.
- Ferrari A, Baraldi C, Sternieri E. Medication overuse and chronic migraine: a critical review according to clinical pharmacology. Expert Opin Drug Metab Toxicol. 2015;11:1127–1144.
- Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53:1300–1311.
- Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821–1828.
- Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16:86–92.
- Torres-Ferrús M, Quintana M, Fernandez-Morales J, et al. When does chronic migraine strike? A clinical comparison of migraine according to the headache days suffered per month. Cephalalgia. 2017;37:104–113.
- Abu Bakar N, Tanprawate S, Lambru G, et al. Quality of life in primary headache disorders: a review. Cephalalgia. 2016;36:67–91.
- Lantéri-Minet M, Duru G, Mudge M, et al. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31:837–850.
- Messali A, Sanderson JC, Blumenfeld AM, et al. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache. 2016;56:306–322.
- Shah AM, Bendtsen L, Zeeberg P, et al. Reduction of medication costs after detoxification for medication-overuse headache. Headache. 2013;53:665–672.
- Wu J, Hughes MD, Hudson MF, et al. Antimigraine medication use and associated health care costs in employed patients. J Headache Pain. 2012;13:121–127.
- Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21:973–989.
- Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Bio Med. 2008;3:17.
- Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS). Headache. 2007;47:213–224.
- Buse DC, Sollars CM, Steiner TJ, et al. Why hurt? A review of clinical instruments for headache management. Curr Pain Headache Rep. 2012;16:237–254.
- Minen MT, Loder E, Tishler L, et al. Migraine diagnosis and treatment: a knowledge and needs assessment among primary care providers. Cephalalgia. 2016;36:358–370.
- Gallagher RM, Alam R, Shah S, et al. Headache in medical education: medical schools, neurology and family practice residencies. Headache. 2005;45:866–873.
- Dall TM, Storm MV, Chakrabarti R, et al. Supply and demand analysis of the current and future US neurology workforce. Neurology. 2013;81:470–478.
- Mauser ED, Rosen NL. So many migraines, so few subspecialists: analysis of the geographic location of United Council for Neurologic Subspecialties (UCNS) certified headache subspecialists compared to United States headache demographics. Headache. 2014;54:1347–1357.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808.
- Thorlund K, Sun-Edelstein C, Druyts E, et al. Risk of medication overuse headache across classes of treatments for acute migraine. J Headache Pain. 2016;17:107.
- Gallagher RM, Kunkel R. Migraine medication attributes important for patient compliance: concerns about side effects may delay treatment. Headache. 2003;43:36–43.
- Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35:478–488.
- Turner DP, Golding AN, Houle TT. Using a graphical risk tool to examine willingness to take migraine prophylactic medications. Pain. 2016;157:2226–2234.
- Ford JH, Jackson J, Milligan G, et al. A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache. 2017;57:1532–1544.
- Shah N, Pike J, Mutebi A, et al. The use of migraine prophylaxis treatments in the United States of America: analysis of data from clinical practice [abstract]. American Headache Society 58th Annual Scientific Meeting, San Diego, CA; 2016 [cited 2017 June 6]. Available from: https://www.mdlinx.com/nursing/conference-abstract.cfm/58197/?nonus=0&searchstring=&coverage_day=&page=1.
- Sarchielli P, Granella F, Prudenzano MP, et al. Italian guidelines for primary headaches: 2012 revised version. J Headache Pain. 2012;13:31–S70.
- Flores NM, Lee LK, Gajria K, et al. Health related quality of life and health care resource use burden in migraine with and without nausea [abstract]. ISPOR 18th Annual European Congress, Milan; November 2015; [cited 2018 Aug 09]. Available from: https://www.valueinhealthjournal.com/article/S1098-3015(15)01581-8/fulltext
- Raval AD, Shah A. National trends in direct health care expenditures among US adults with migraine: 2004 to 2013. J Pain. 2017;18:96–107.
- Friedman BW, Serrano D, Reed M, et al. Use of the emergency department for severe headache. A population-based study. Headache. 2009;49:21–30.
- Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:103–122.
- May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12:455–464.
- Dumas P. What really happens when you go to the ER with migraine [newsletter]; September 28, 2015 [cited 2017 Sep 13]. Available from: https://migraineagain.com/what-really-happens-when-you-go-to-emergency-with-migraine
- Bigal ME, Lipton RB. Overuse of acute migraine medications and migraine chronification. Curr Pain Headache Rep. 2009;13:301–307.
- Cho SJ, Chu MK. Risk factors of chronic daily headache or chronic migraine. Curr Pain Headache Rep. 2015;19:465.
- Jonsson P, Hedenrud T, Linde M. Epidemiology of medication overuse headache in the general Swedish population. Cephalalgia. 2011;31:1015–1022.
- Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47:355–363.
- Bonafede M, Sapra S, Tepper SJ, et al. Healthcare costs and utilization and medication treatment patterns among migraine patients: a retrospective analysis [abstract]. Headache. 2017;57:211. [PS58].
- Kolodner K, Lipton RB, Lafata JE, et al. Pharmacy and medical claims data identified migraine sufferers with high specificity but modest sensitivity. J Clin Epidemiol. 2004;57:962–972.