?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: Neuropathic pain prevalence is estimated between 7% and 10% of the population. International guidelines recommend a variety of drugs at different therapy lines for pain relief. However, side effect profiles, for example, prompted the UK government recently to classify pregabalin and gabapentin as class C drugs. Lidocaine 700 mg medicated plaster (LMP) might be a safer alternative. A systematic review assessed how LMP and pregabalin compared in terms of efficacy and safety. The review focused on pain reduction, quality of life and adverse events in peripheral neuropathic pain (PNP) i.e. post-herpetic neuralgia, diabetic peripheral neuropathy, post-surgical/trauma, or other PNP conditions.
Methods: Electronic databases were searched as well as a number of other sources up to November 2018. Sensitive strategies were used, with no restriction by language or publication status. Two independent reviewers screened records and extracted data with consensus determining final decisions. Risk of bias was assessed using the Cochrane Collaboration 2011 checklist for RCTs. Full network meta-analysis was conducted to compare LMP to pregabalin 300/600 mg in terms of pain reduction, quality of life, as well as serious adverse events and selected adverse events. Trials with enriched enrolment design were excluded.
Results: Searches retrieved 7,104 records. In total 111 references pertaining to 43 RCTs were included for data extraction. Bayesian network meta-analysis of several pain outcomes showed no clear difference in efficacy between treatments However, LMP was clearly advantageous in terms of dizziness and any adverse event vs. pregabalin 600 mg/day and discontinuations vs. pregabalin 300 mg/day or 600 mg/day, as well as being associated with improved quality of life (albeit in this case based on weak evidence).
Conclusions: LMP was found to be similar to pregabalin in reducing pain in all populations but had a better adverse events profile.
Introduction
Neuropathic pain has been estimated to affect between about 7% and 10% of the general populationCitation1. It has been identified as a priority by the International Association for the Study of Pain (IASP) https://www.iasp-pain.org/GlobalYear/NeuropathicPain. Chronic peripheral neuropathic pain (PNP) is chronic pain caused by a lesion or disease of the peripheral somatosensory nervous systemCitation2. This includes, although is not limited to, diabetic peripheral neuropathic (DPN), post-herpetic neuralgia (PHN) and posttraumatic and postsurgical pain.
A number of drugs have been recommended at each line of therapy to treat various types of PNP as part of several major guidelines, including the Canadian Pain Society, the European Federation of Neurological Societies (EFNS), the Neuropathic Pain Special Interest Group and the National Institute for Health and Care ExcellenceCitation3. As a first-line therapy, three drug classes have received strong recommendations in all guidelines: tricyclic antidepressants, particularly amitriptyline; serotonin-norepinephrine reuptake inhibitors (SNRIs), such as duloxetine; and calcium channel alpha-2-delta ligands, such as gabapentin and pregabalin. In support of these recommendations, the most recent Cochrane Review of pregabalin concluded that it was effective in PNP, specifically PHN, DPN and “mixed or unclassified” posttraumatic neuropathic pain. However, it also showed that it was associated with an increased risk of adverse events such as somnolence and dizzinessCitation4. The most recent systematic review similarly highlighted the risk of adverse events associated with pregabalinCitation5. Indeed, pregabalin and gabapentin have just been reclassified in the United Kingdom such that their prescription will be limited, the rationale including: “pregabalin and gabapentin related mortalities” as well as: “the propensity to cause depression of the central nervous system, resulting in drowsiness, sedation, and respiratory depression”Citation6,Citation7. Additionally, there is an increased risk of drug-drug interactions in often poly-medicated patients (interactions are mainly expected based on pharmacodynamic interactions with other CNS-depressive products (such as oxycodone, ethanol and/or lorazepam), while pharmacokinetic interactions are less likely)Citation8.
In contrast, only one of these guidelines, EFNS, currently recommends lidocaine 700 mg medicated plasters, at the first line, only for PHN and only in the elderlyCitation3. This is perhaps not surprising given that the most recent Cochrane review of topical lidocaine found limited evidence to support its efficacyCitation9. This is partly based on trials, which employed a randomised withdrawal design as requested by regulatory authorities. Studies with this design are excluded from most reviews. Given the likely better safety profile of a topical treatment, a further systematic review to compare lidocaine plasters to pregabalin would seem to be warranted at this time.
This systematic review, therefore, aimed to compare the efficacy and safety of lidocaine 700 mg medicated plaster (LMP) with pregabalin (300 mg or 600 mg daily) across various types of chronic PNP reflecting the different labels in different countries. The label in Europe and the United States is “treatment of neuropathic pain associated with previous herpes zoster infection”; the label in Latin American countries is “treatment of localized neuropathic pain” comprising e.g. DPN, post-surgical pain and other topical, localized forms of peripheral neuropathic pain. In Europe, the clinical trials in DPN and post-surgical neuropathic pain are part of a development program for the label extension towards localized peripheral neuropathic pain.
Research questions
How do LMP and pregabalin (300 mg or 600 mg daily) compare in terms of all relevant risks and enefits in the treatment of PNP?
More specifically, how do they compare in terms of:
Benefits, i.e. efficacy in reducing pain (including health-related quality of life)
Risks, i.e. safety i.e. adverse events (AEs)
Methods
Throughout this review, the methods recommended by the Cochrane Collaboration HandbookCitation10 and the Centre for Reviews and Dissemination (CRD), YorkCitation11 were applied in order to reduce the risk of bias and error.
Inclusion criteria
Population: PNP including post herpetic neuralgia (PHN), diabetic peripheral neuropathy (DPN), postsurgical or posttraumatic pain and PNP of various origins (referred to as mixed PNP in this document).
Intervention: LMP or pregabalin (300 mg or 600 mg daily)
Comparator: Pregabalin (300 mg or 600 mg daily) or no treatment/placebo
Efficacy outcomes including any measure of pain as used in any trial of LMP and any measure of the quality of life as used in any trial of LMP
Safety outcomes/AEs: any AE, any serious AE (SAE), discontinuation due to AE, as well as specific AEs (as studied in the most recent systematic reviews of pregabalinCitation4,Citation5 including weight gain, dizziness, peripheral oedema, and euphoria. Given that LMP is applied to the skin, one other AE was also included, i.e. application site reaction
Study design: RCTs, including withdrawal studies, of at least 20 participants for efficacy data
Population: low back pain, osteoarthritis, complex regional pain syndrome, trigeminal neuralgia, and myofascial pain
Literature searches
The search strategies were developed specifically for each database and included relevant search terms comprising indexed keywords (e.g. medical subject headings MeSH and EMTREE) and free text terms appearing in the title and/or abstract of database records. Searches were not limited by language, publication status (unpublished or published) or date. For full strategies see Supplementary Appendix 1. The following databases were searched from inception to November 2018: MEDLINE (including MEDLINE In-Process, Daily Update & Epub ahead of print), EMBASE, CDSR, CENTRAL, DARE, HTA, KSR Evidence, ClinicalTrials.gov, EU Clinical Trials Register (EUCTR) and WHO International Clinical Trials Registry Platform (ICTRP). Additional grey literature was identified from searches of the FDA, EMA and EudraVigilance websites. Furthermore, references in retrieved articles and systematic reviews were checked for relevant studies.
Methods of trial selection, quality assessment, and data extraction
Trial selection
Two reviewers independently inspected the title and abstract of each reference identified by the search to determine the potential eligibility of each article according to the pre-specified inclusion criteria. For potentially relevant articles, the full publication was obtained, independently inspected by two reviewers, and inclusion criteria applied. Any disagreements were resolved through discussion and consensus.
Assessment of risk of bias
Assessment of risk of bias was based on the Cochrane Collaboration risk of bias checklist and was carried out independently by two reviewers. Any disagreements were resolved by consensusCitation12.
Data collection
For each trial, data were extracted by one reviewer and checked by a second reviewer. Any disagreements were resolved by consensus. Dichotomous data were extracted as the number of individuals with the outcome of interest and the total numbers of individuals analyzed in the intervention and control group. Continuous data were extracted as the mean and standard deviation at follow-up or change from baseline.
Data synthesis
If there were two or more trials considered which were clinically and statistically similar then they were pooled in direct meta-analysis using a random effects model in Review Manager Version 5.3. Statistical heterogeneity was evaluated using the I2 statistic. The random effects model was chosen on the basis that there was likely to be heterogeneity that could not be explained and sensitivity analyses were performed to explore the impact of individual studies in analyses showing high levels of statistical heterogeneity. Dichotomous outcomes (e.g. risk of patients experiencing each type of AE outcome or not) are reported as relative risks (RR) with 95% confidence intervals (CIs). Continuous or ordinal outcomes are reported as mean differences (MDs) with 95% CIs.
For each outcome, a network was created to show how LMP could be compared to pregabalin 300 mg/day or pregabalin 600 mg/day i.e. either directly via one trial or indirectly via a comparator common to more than one trial. Where there was only a direct comparison, no further analysis was required than to use the outcome from that single trial. Where LMP and pregabalin could only be analyzed via indirect comparison e.g. via placebo, then network meta-analysis (NMA) using WinBUGs version 1.4.3 (http://www.mrc-bsu.cam.ac.uk/bugs/winbugs/contents.shtml) was applied using a Bayesian approach consistent with international recommendationsCitation13. NMA combines direct evidence and indirect evidence for particular pairwise comparisons, thereby synthesizing a greater share of the available evidence than traditional meta-analysis. Posterior distribution parameter estimates were obtained from 40,000 simulations after a burn-in period of 40,000 Markov Chain Monte Carlo (MCMC) simulations, using two chains. Non-informative normal priors (mean 0, variance 10,000) were used for treatment effects and a non-informative uniform prior (0, 1) was used for the between study standard deviationCitation14. Convergence and auto-correlation were assessed by monitoring the trace and autocorrelation plots in WinBUGS. Pooled relative risks or mean differences with 95% credible intervals were calculated for each outcome and available treatment comparison. Results from random effects models were presented as the primary result apart from for networks that contained only one or two studies for each comparison. If there were any doubts about the suitability of the model, then results from the simpler fixed effect model were presentedCitation15.
For some studies, mean NRS scores at follow-up were not available and so mean change from baseline was used instead. However, mixing absolute mean and mean change in a meta-analysis of mean difference is in accordance with good practice. As it states in the Cochrane Handbook, Section 9.4.5.2, “There is no statistical reason why studies with change-from-baseline outcomes should not be combined in a meta-analysis with studies with final measurement outcomes…the difference in mean final values will on average be the same as the difference in mean change scores”Citation9.
Given the heterogeneity of follow-up time, a sensitivity analysis on efficacy outcomes was performed, where data was available, based on two follow-up categories: less than 12 weeks of follow-up and at least 12 weeks of follow-up.
Results
Included studies
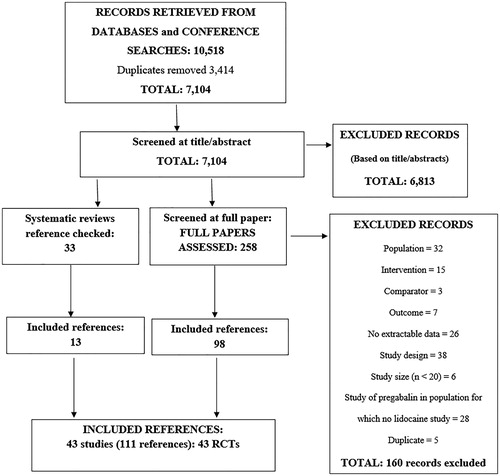
From the searches, after de-duplication, a total of 7,104 records were screened at the title and abstract stage. From these, 258 were re-screened at the full paper stage. From these, a further 160 references were excluded for not meeting the inclusion criteria (See Supplementary Appendix 2). Thirteen additional references were found by reference checking. Therefore, in total 111 references pertaining to 43 RCTs were included. The results of the screening are shown in . A detailed overview of the included RCTs is given in .
Table 1. Study characteristics of 43 randomised controlled trials (see ).
As shown in , most studies were of low risk of bias in most domains. All but one study, by Baron 2009Citation17 were double blind. However, what is not shown in is that five studies were of an “enriched enrolment” design (Galer 1999Citation51, Raskin 2014Citation56, Binder 2009Citation58, Hewitt 2011Citation52, Gilron 2011Citation38,). All enriched enrolment studies were excluded from analyses given that the population in these studies included only those patients who had responded to and tolerated the intervention. Seven identified studies were designated as “cross-over” trials (Bischoff 2013Citation48, Cheville 2009Citation49, Huffman 2015Citation53, Jenkins 2012Citation54, Raskin 2016Citation57, Meier 2003Citation55, Demant 2015Citation50,). Data only for the pre-cross-over period from these studies were included if reported.
Table 2. Cochrane risk of bias assessment of RCTs.
As shown in , DPN was the most frequently reported population (n = 15 studies), followed by PHN (n = 12 studies), postsurgical neuropathic pain (n = 5 studies), mixed PNP (n = 5 studies) (for type of pain see Supplementary Appendix 4), posttraumatic/surgical pain (n = 3 studies) and mixed PHN/DPN i.e. outcomes not presented separately for each population (n = 2 studies); while persistent inguinal post herniorrhaphy pain and post-thoracotomy were only reported in a single study each. Note that Baron 2009Citation17 is counted twice once each for PHN and DPN.
Fourteen trials reported data on the treatment of patients with neuropathic pain using LMP (). The maximum number of patches used varied from one to four. The number of patches was often reported to be at the discretion of the patient according to the size of the painful area. In most of the studies, pregabalin was titrated from 150 mg/day up to a maximum dose (300 mg/day or 600 mg/day) depending on response, tolerability or creatinine clearance function. Five studies, by Sabatowski 2004Citation34, Stacey 2008Citation35, Rosenstock 2004Citation24, Lesser 2004Citation20, and Penide 2012Citation41, reported administering pregabalin at a fixed dose and this was in all cases 300 mg/day. For this reason and for brevity, throughout the manuscript, the dose of pregabalin is expressed as the maximum titrated up to i.e. either 600 mg or 300 mg, as would be expected in clinical practice.
In thirteen trials, concomitant neuropathic pain medications were allowed, based on patients” baseline characteristics or concomitant medication intake before the study period. Twelve trials did not report any information about concomitant medications and the remaining nineteen studies prohibited any type of concomitant medications throughout the entire study. Other characteristics (age, sex, duration of painful condition, mean baseline pain) appeared comparable between trials.
Network Meta-analysis: structure and inputs
For efficacy outcomes, NMA was performed across all follow-up times for each patient population, including: PHN, DPN, post-surgical and post-traumatic pain. For adverse event outcomes, NMA was performed across all patient populations (any PNP) given the sparsity of data and plausible lack of relationship between event risk and PNP type. In total, 26 comparisons were identified for the NMA. This involved five measures of efficacy of LMP versus pregabalin: number of patients with NRS reduction ≥30% from baseline, number of patients with NRS reduction ≥50%, number of patients with NRS reduction 30% or 50%, mean NRS scores and number of patients who discontinued due to lack of efficacy. A summary of all studies required for each of the 26 networks is shown in and a diagram summarising the possible comparisons is presented in .
Figure 2. Network meta-analysis for all potential comparisons of LMP, placebo and pregabalin (300 mg or 600 mg).
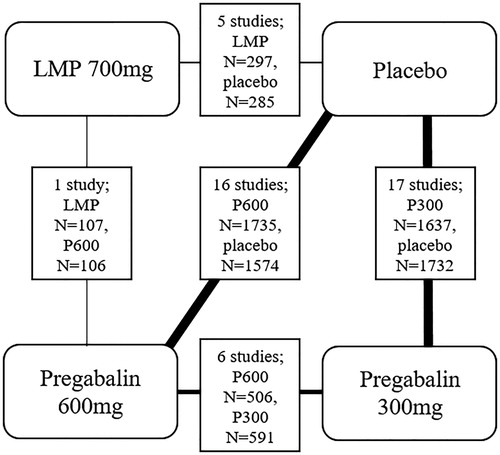
Table 3. Studies included in the NMA for each outcome in each population.
As expected, there was considerable heterogeneity of follow-up time from 2 days to 15 weeks. A sensitivity analysis on efficacy outcomes was therefore performed, where data was available, based on two follow-up categories: less than 12 weeks of follow-up and at least 12 weeks of follow-up. In particular, as shown in , in PHN for mean NRS and number of patients with NRS ≥30% or 50% in the comparison with pregabalin 300 mg, analysis could be performed for mixed follow-up and <12 weeks only. This was also the case in DPN for a number of patients with NRS ≥50%. In post-surgical/traumatic PNP for discontinuation due to lack of efficacy and number of patients with NRS ≥30% or 50% in the comparison with pregabalin 600 mg, analysis could be performed for mixed follow-up and > =12 weeks only and for mean NRS for <12 weeks only and > =12 weeks only.
Only one direct head-to-head comparison of LMP and pregabalin at any dose was available. Baron 2009Citation17 reported a comparison of LMP versus pregabalin 600 mg/day in PHN and DPN. Also, there was no indirect comparison for LMP versus pregabalin 600 mg/day via placebo in PHN and DPN, as denoted by “NA” in the column “LMP vs. placebo” in . Therefore, NMA was not employed to compare LMP with pregabalin 600 mg in PHN or DPN. In all other populations, LMP was compared to pregabalin 600 mg via placebo in the NMA using the studies listed under “LMP vs placebo” in . This included four studies in postsurgical/traumatic pain (Cheville 2009Citation49, Sansone 2017Citation43, Pickering 2017Citation42, Palladini 2019Citation40,) and one study in mixed PNP (Demant 2015Citation50). The studies listed under “pregabalin 600 mg vs placebo” in were also required.
A comparison between LMP and pregabalin 300 mg was achieved only via indirect comparison in the NMA. In PHN or DPN, Baron 2009Citation17, was always used plus the studies listed under pregabalin 600 mg vs pregabalin 300 mg and those listed under both doses of pregabalin vs placebo. In all other populations, at least one of the five studies listed under LMP vs placebo were used plus those listed under both doses of pregabalin vs placebo. In these populations, there were no studies that enabled a comparison between pregabalin 600 mg vs pregabalin 300 mg, as indicated by “NA” in .
It should be noted that for one AE of interest, euphoria, no data were reported for LMP and so no comparison was possible. The risk of application site reaction was similarly not reported for pregabalin 300 mg.
For illustrative purposes, forest plots of all comparisons for all outcomes are also included in Supplementary Appendix 3. In terms of safety, only those forest plots for any AE and any SAE are presented in the Supplementary Appendix.
Network Meta-analysis: outputs
The results of the NMA are shown in , which for ease of readability has the same structure as . Note that, for efficacy, a RR above 1 and a MD above 0 is favorable to LMP. For safety, a RR below 1 is favorable to LMP. In terms of efficacy, what this shows is that for all populations (PHN, DPN, post-traumatic/surgical, mixed LNP), for almost all efficacy outcomes (at least 30/50% reduction in NRS, reduction in NRS) there is no clear difference between LMP and pregabalin 300 mg or 600 mg. A clear difference was identified only in PHN and DPN in favor to LMP with regards to change in EQ-5D. By clear difference we mean that the 95% credible interval (or confidence interval for direct comparison only) does not cross the point of no difference (1 for relative risk and 0 for mean difference). What can also be seen is that these findings are largely insensitive to follow-up. This is indicated by the similarity of the results for LMP versus pregabalin 300 mg in PHN and DPN for mean NRS and for LMP versus pregabalin 600 mg in post-surgical/traumatic PNP for a number of patients with NRS ≥30% or 50%. For example, the MD for mean NRS is -0.76 (-1.64, 0.61) for mixed follow-up versus -0.57 (-1.57, 1.06) for less than 12 weeks only. The finding that there is no clear difference also holds true in post-surgical/traumatic PNP for discontinuation due to lack of efficacy, although the direction of the point estimate is different for > =12 weeks follow-up compared to mixed follow-up.
Table 4. Result of the main NMA (random effects) for each outcome in each population.
In terms of safety, for any local neuropathic pain population, the interpretation of the results varies by outcome and comparison. There was a clear advantage to LMP for the following outcomes: discontinuation due to AE, vs. both 600 mg/day and 300 mg/day and any AE and dizziness vs. 600 mg/day only. No clear difference was found for any other outcomes.
As shown in Supplementary Appendix 3, some meta-analyses seemed to demonstrate statistical heterogeneity as shown by an I2 statistic being quite high. The effect of reducing statistical heterogeneity by excluding studies to decrease I2 has already been described investigated partly via follow-up time. For example, Figure 3 in Supplementary Appendix 3 shows an I2 of 74% for pregabalin 600 mg vs. placebo for NRS reduction ≥30% from in post-surgical/traumatic with mixed follow-up, based on two trials, van Seventer 2010Citation45 and Markman 2018Citation44.
The use of these data leads to a RR (95% CI) of LMP vs. pregabalin 600 mg of 1.00 (0.23, 4.03) (See ). Reducing the heterogeneity by confining to a follow-up of at least 12 weeks only removed one of the trials, van Seventer 2010Citation45 and also negated the statistical heterogeneity to produce a RR (95% CI) of LMP vs. pregabalin 600 mg of 1.17 (0.80, 1.73). Therefore, the statistical heterogeneity had no effect on the direction of effect, little effect on the magnitude and no effect on whether there was a clear difference between the treatments. This same kind of sensitivity analysis was implemented in every case where the I2 exceeded 50%. In all cases, there were the same results i.e. no effect on whether there was a clear difference between the treatments. Indeed, the highest value of I2 was 92% for LMP vs. placebo mean NRS in post-surgical/traumatic with follow-up <12 weeks (See , Supplementary Appendix 3). The clear outlier in this meta-analysis is Sansone 2017Citation43. Its removal resulted in a change in the mean (95% CI) for LMP vs. pregabalin 300 mg and 600 mg from −1.59 (−3.47, 0.32) to −0.40 (−1.90, 0.98) and −0.53 (−2.65, 1.60) to 0.68 (−0.84, 1.95) respectively. Although there is a change in the direction of the point estimate for the comparison with pregabalin 600 mg, as in all other sensitivity analyses, reducing statistical heterogeneity produced no change in whether there was a clear difference between LMP and either dose of pregabalin.
Discussion
This systematic review is to our knowledge the most comprehensive one to date in comparing LMP with pregabalin in PNP. No additional primary studies that compare the effectiveness of LMP with pregabalin for the treatment of patients with neuropathic pain were included in any of the 12 published reviews that we identifiedCitation59–70. Indeed, this review included more RCTs than any one of these previous reviews, including the most recently published RCT by Palladini 2019Citation40.
The review retrieved 7,104 records from which 43 RCTs were included in eight different types of neuropathic pain. Only one RCT, by Baron 2009Citation17, provided a direct comparison between LMP and any dose of pregabalin, that dose being 600 mg/day and only in PHN or DPN. Therefore, an NMA was required to compare LMP with pregabalin 600 mg/day and 300 mg/day for most outcomes in most populations. This involved at least one indirect comparison via either placebo controlled trials or trials comparing pregabalin 600 mg/day with 300 mg/day.
The evidence from the NMA suggests that there is no clear difference (95% credible or confidence interval overlaps point of no difference) in any efficacy outcome (except change in EQ-5D, which was in favor of LMP) in any PNP population between LMP and pregabalin at any dose. This finding held regardless of the follow-up i.e. whether less than or greater than 12 weeks.
The evidence from the NMA also suggests that there is no clear difference in any serious adverse events, somnolence, peripheral oedema, and weight gain. It seems implausible that the rate of serious adverse events, somnolence, peripheral edema or weight gain could be higher for LMP not least due to the very low systemic concentration which was observed in short-term pharmacokinetic trials in PHN patients (52 ng/ml lidocaine) and in healthy subjects (128 ng/ml lidocaine)Citation71. However, based on methodologic conventions used for the NMA, adverse events that were not reported as having not occurred could not be considered as having a risk of zero. Nevertheless, based on the available SPCs, peripheral edema, somnolence, and weight gain belong to the established safety profile of pregabalin, while this is not the case for the lidocaine 700 mg medicated plaster. Indeed, the medical review for the FDA concluded that LMP has a “…fairly benign safety profile”Citation72. In contrast, any adverse event and dizziness vs. 600 mg/day only and discontinuation due to AE, vs. any dose, showed a clear advantage to LMP.
The main strength of the review is its comprehensiveness in terms of included populations, which permits the conclusion that regardless of PNP population there appears to be no clear difference between LMP and pregabalin in pain relieving effect. Also, the ability to pool all of these studies regardless of population permits the conclusion that LMP probably reduces the risk of some AEs, in particular dizziness and discontinuation due to AE. Robustness to variation in follow-up was also demonstrated. The methods of the review also benefited from a sensitive search strategy with no language restriction and a comprehensive range of sources searched.
However, there were some limitations to the review. Some of the evidence was of low quality in terms of study design. In particular, some trials used an enriched enrolment design, which would make the population incomparable to those without it given the inclusion of only those who have already responded to the intervention. However, those trials were excluded from the NMA, as were any results post-crossover from crossover design studies.
Statistical heterogeneity was observed in some of the meta-analyses, as indicated by an I2 statistic being quite high. However, in a sensitivity analysis, where the I2 exceeded 50%, the removal of outlying studies resulted in no change in whether there was a clear difference between LMP and either dose of pregabalin. Also, there was much heterogeneity in terms of follow-up times, although this was to some extent mitigated by applying a threshold of 12 weeks. Some clinical heterogeneity was impossible to remove such as a mixture of types of PNP, although results were very similar across all populations. Moreover, there was heterogeneity in terms of allowance of concomitant medication, but only one study of LMP, by Palladini 2019Citation40, in postsurgical pain, permitted such medication. This study stratified by concomitant medication and showed that LMP was more effective if such medication was excluded. Therefore, the finding of no clear difference in effectiveness between LMP and pregabalin in this population remains plausible. Also, for AEs all studies were pooled regardless of population. However, this was required given that many studies did not fully report AEs and it does seem plausible that there would be little variation in relative effect with PNP population. Additionally, lack of primary data impeded the study of the risk of application site reactions and euphoria or withdrawal symptoms as adverse events of special interest. However, it is difficult to envisage a mechanism by which euphoria, somnolence, peripheral oedema or weight gain would occur with LMP or application site reaction with pregabalin. Finally, lack of data on change in EQ-5D limits the interpretation of results related to change in health-related quality of life.
Comparisons in some types of PNP were impossible due to lack of LMP trials, such as those that are cancer or HIV related. RCTs are therefore needed in these populations.
In conclusion, LMP has been shown to compare favorably with pregabalin across a range of types of PNP. Furthermore, the most recent Cochrane review recommended pregabalin for PNP after comparison with any other active treatment, including gabapentin and drugs also recommended in international guidelinesCitation3,Citation4. It, therefore, seems reasonable to conclude that LMP is recommended as an alternative to those drugs currently recommended in guidelines for PNP. Given a large amount of uncertainty, we would also recommend further high-quality RCTs to compare LMP with pregabalin. These should be in more tightly defined populations in terms of use of concomitant medication and type of PNP e.g. precise type of trauma, given a large amount of clinical heterogeneity. Finally, given that not all AEs were reported in all trials, we would recommend more comprehensive reporting of AEs in all RCTs.
Conclusions
Whilst acknowledging the limitations of the networks meta-analyses, the evidence from RCTs suggests that there is no clear difference between LMP and pregabalin in terms of any measure of pain reduction in any PNP population. However, it was found that there is a clear advantage to LMP with regards to any AE vs. pregabalin 600 mg/day, and discontinuation due to AE vs. both pregabalin 600 mg/day and pregabalin 300 mg/day. These findings support the recommendation of LMP for any PNP as a safe alternative to pregabalin. Given a large amount of uncertainty, we would also recommend further high-quality RCTs to compare LMP with pregabalin.
Transparency
Declaration of funding
The project was funded by Grünenthal GmbH, Germany. T.B. and J.K. had ultimate editorial control of the manuscript.
Declaration of financial/other relationships
T.B., N.A., G.W., S.L.S., C.N., V.H.C., S.R., D.S., and J.K. have disclosed that they are employees of Kleijnen Systematic Reviews Ltd, a company that received funding from Grünenthal GmbH to conduct this study. IS, IB, BB and HL are employees of Grünenthal GmbH. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
T.B. (Health Economist), N.A. (Health Economist), S.R. (Health Economist), D.S. (Health Economist), S.L.S. (Systematic Reviewer), and V.H.C. (Systematic Reviewer) conducted the original systematic review, and contributed to writing and editing this manuscript. G.W. (Statistician) contributed to the meta-analyses and C.N. (Information Specialist) planned the search strategy and ran then searches and both contributed to writing and editing this manuscript. I.S. (Drug Safety), I.B. (Clinical Science) and B.B. (Statistician) contributed to protocol development, selection of AEs, statistical analyses, and contributed to manuscript preparation. J.K. (Director) and H.L. (Health Economist) provided overall project management and contributed to protocol development, and writing and editing the manuscript. All authors read and approved the final manuscript.
Appendix 5
Download MS Word (13.9 KB)Appendix 4
Download MS Word (13 KB)Appendix 3
Download MS Word (785.7 KB)Appendix 2
Download MS Word (31.2 KB)Appendix 1
Download MS Word (26.7 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662.
- Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59.
- Cruccu G, Truini A. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther. 2017;6(Suppl 1):35–42.
- Derry S, Bell RF, Straube S, et al. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):CD007076.
- Onakpoya IJ, Thomas ET, Lee JJ, et al. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ OPEN. 2019;9(1):e023600.
- NHS England. Handling of gabapentin and pregabalin as Schedule 3 Controlled Drugs in health and justice commissioned services: letter from Kate Davies Internet. London: Health & Justice, Armed Forces and Sexual Assault Referral Centres (SARCs); 2019. cited 2019 April 9. Available from: https://www.england.nhs.uk/publication/handling-of-gabapentin-and-pregabalin-as-schedule-3-controlled-drugs-in-health-and-justice-commissioned-services/
- NHS England. Rescheduling of gabapentin and pregabalin as schedule 3 controlled drugs: briefing note Internet. London: NHS England; 2019. cited 2019 April 9. Available from: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Support%20alert/Pregabalin%20and%20Gabapentin%20Briefing%20Note%20-%2012%20Feb%202019%20(002).pdf?ver=2019-02-13-153122-913
- Stanos SP, Galluzzi KE. Topical therapies in the management of chronic pain. Postgrad Med. 2013;125(4 Suppl 1):25–33.
- Derry S, Wiffen PJ, Moore RA, et al. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(7):CD010958.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions Internet. Version 5.1.0 updated March 2011: The Cochrane Collaboration; 2011. cited 2011 March 23. Available from: http://handbook.cochrane.org/.
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s guidance for undertaking reviews in health care. York: University of York; 2009. [cited 2011 March 23]. Available from: http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–437.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials Internet 2011; last updated September 2016. [cited 2016 Dec 8]. Available from: http://www.nicedsu.org.uk
- Song F, Altman DG, Glenny A-M, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326(7387):472.
- Arezzo JC, Rosenstock J, Lamoreaux L, et al. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8(1):33.
- Baron R, Mayoral V, Leijon G, et al. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663–1676.
- Eli Lilly and Company. A study for treatment of pain in patients with diabetic neuropathy. NCT00785577. In: ClinicalTrials.gov Internet. Bethesda (MD): National Library of Medicine (US);2010. [cited 2018 Dec 18]. Available from: https://ClinicalTrials.gov/show/NCT00785577
- Jiang W, Ladd S, Martsberger C, et al. Effects of pregabalin on heart rate variability in patients with painful diabetic neuropathy. J Clin Psychopharmacol. 2011;31(2):207–213.
- Lesser H, Sharma U, LaMoreaux L, et al. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–2110.
- Mu Y, Liu X, Li Q, et al. Efficacy and safety of pregabalin for painful diabetic peripheral neuropathy in a population of Chinese patients: a randomized placebo-controlled trial. J Diabetes. 2018;10(3):256–265.
- Rauck R, Makumi CW, Schwartz S, et al. A randomized, controlled trial of gabapentin enacarbil in subjects with neuropathic pain associated with diabetic peripheral neuropathy. Pain Pract. 2013;13(6):485–496.
- Richter RW, Portenoy R, Sharma U, et al. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253–260.
- Rosenstock J, Tuchman M, LaMoreaux L, et al. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–638.
- Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28(1):109–116.
- Tolle T, Freynhagen R, Versavel M, et al. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12(2):203–213.
- Ziegler D, Duan WR, An G, et al. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain. 2015;156(10):2013–2020.
- Dworkin RH, Corbin AE, Young JP, Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60(8):1274–1283.
- Galer BS, Jensen MP, Ma T, et al. The lidocaine patch 5% effectively treats all neuropathic pain qualities: results of a randomized, double-blind, vehicle-controlled, 3-week efficacy study with use of the neuropathic pain scale. Clin J Pain. 2002;18(5):297–301.
- Lin PL, Fan SZ, Huang CH, et al. Analgesic effect of lidocaine patch 5% in the treatment of acute Herpes zoster. A double-blind and vehicle-controlled study. Reg Anesth Pain Med. 2008;33(4):320–325.
- Liu Q, Chen H, Xi L, et al. A randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of pregabalin for postherpetic neuralgia in a population of Chinese patients. Pain Pract. 2017;17(1):62–69.
- Ogawa S, Suzuki M, Arakawa A, et al. Efficacy and tolerability of pregabalin for postherpetic neuralgia: a multicenter, randomized, double-blind, placebocontrolled clinical trial. J Japan Soc Pain Clinicians. 2010;17:141–152.
- Rowbotham MC, Davies PS, Verkempinck C, et al. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65(1):39–44.
- Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109(1-2):26–35.
- Stacey BR, Barrett JA, Whalen E, et al. Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain. 2008;9(11):1006–1017.
- van Seventer R, Feister HA, Young JP, Jr, et al. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006;22(2):375–384.
- Freynhagen R, Strojek K, Griesing T, et al. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115(3):254–263.
- Gilron I, Wajsbrot D, Therrien F, et al. Pregabalin for peripheral neuropathic pain: a multicenter, enriched enrollment randomized withdrawal placebo-controlled trial. Clin J Pain. 2011;27(3):185–193.
- Grünenthal GmbH. Evaluation of the efficacy, tolerability, and safety of 7 days of treatment with GRT6010 or pregabalin in comparison to placebo in subjects with peripheral neuropathic pain. EudraCT 2011-002092-42. In: EU Clinical Trials Register (EUCTR) Internet. London: European Medicines Agency (EMA); 2011 [cited 2018 Nov 21]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2011-002092-42
- Palladini M, Boesl I, Koenig S, et al. Lidocaine medicated plaster, an additional potential treatment option for localized post-surgical neuropathic pain: efficacy and safety results of a randomized, placebocontrolled trial. Curr Med Res Opin. 2019;35(5):757–766.
- Penide L, Flores-Garnica L, Gomez Agraz JL, et al. Postincitional neuropathic chronic pain after abdominal surgery: comparation efectivity between lidocaine 5% patch vs oral pregabalin. Presented at European Anaesthesiology Congress, EUROANAESTHESIA 2012; 2012 June 9-12; Paris, France. Eur J Anaesthesiol. 2012;29(Suppl 50):202.
- Pickering G, Clermont-Ferrand C. d. Clinical Report: Impact of 5% lidocaine medicated plaster on allodynic symptoms of localized neuropathic pain after knee surgery. A prospective, randomized, placebo controlled study in parallel groups. EudraCT 2015-005601-37 PDF provided by the Company. Clermont-Ferrand: CHU de Clermont-Ferrand; 2017.
- Sansone P, Passavanti MB, Fiorelli A, et al. Efficacy of the topical 5% lidocaine medicated plaster in the treatment of chronic post-thoracotomy neuropathic pain. Pain Manag. 2017;7(3):189–196.
- Markman J, Resnick M, Greenberg S, et al. Efficacy of pregabalin in post-traumatic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 trial. J Neurol. 2018;265(12):2815–2824.
- van Seventer R, Bach FW, Toth CC, et al. Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol. 2010;17(8):1082–1089.
- Guan Y, Ding X, Cheng Y, et al. Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin Ther. 2011;33(2):159–166.
- Moon DE, Lee DI, Lee SC, et al. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin Ther. 2010;32(14):2370–2385.
- Bischoff JM, Petersen M, Uceyler N, et al. Lidocaine patch (5%) in treatment of persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled, crossover trial. Anesthesiology. 2013;119(6):1444–1452.
- Cheville AL, Sloan JA, Northfelt DW, et al. Use of a lidocaine patch in the management of postsurgical neuropathic pain in patients with cancer: a phase III double-blind crossover study (N01CB). Support Care Cancer. 2009;17(4):451–460.
- Demant DT, Lund K, Finnerup NB, et al. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. Pain. 2015;156(11):2234–2244.
- Galer BS, Rowbotham MC, Perander J, et al. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain. 1999;80(3):533–538.
- Hewitt DJ, Ho TW, Galer B, et al. Impact of responder definition on the enriched enrollment randomized withdrawal trial design for establishing proof of concept in neuropathic pain. Pain. 2011;152(3):514–521.
- Huffman C, Stacey BR, Tuchman M, et al. Efficacy and safety of pregabalin in the treatment of patients with painful diabetic peripheral neuropathy and pain on walking. Clin J Pain. 2015;31(11):946–958.
- Jenkins TM, Smart TS, Hackman F, et al. Efficient assessment of efficacy in post-traumatic peripheral neuropathic pain patients: pregabalin in a randomized, placebo-controlled, crossover study. J Pain Res. 2012;5:243–250.
- Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106(1):151–158.
- Raskin P, Huffman C, Toth C, et al. Pregabalin in patients with inadequately treated painful diabetic peripheral neuropathy: a randomized withdrawal trial. Clin J Pain. 2014;30(5):379–390.
- Raskin P, Huffman C, Yurkewicz L, et al. Pregabalin in patients with painful diabetic peripheral neuropathy using an NSAID for other pain conditions: a double-blind crossover study. Clin J Pain. 2016;32(3):203–210.
- Binder A, Bruxelle J, Rogers P, et al. Topical 5% lidocaine (lignocaine) medicated plaster treatment for post-herpetic neuralgia: results of a double-blind, placebo-controlled, multinational efficacy and safety trial. Clin Drug Investig. 2009;29(6):393–408.
- Song D, He A, Xu R, et al. Efficacy of pain relief in different postherpetic neuralgia therapies: a network meta-analysis. Pain Physician. 2018;21(1):19–32.
- Dosenovic S, Jelicic Kadic A, Miljanovic M, et al. Interventions for neuropathic pain: an overview of systematic reviews. Anesth Analg. 2017;125(2):643–652.
- Navez ML, Monella C, Bosl I, et al. 5% lidocaine medicated plaster for the treatment of postherpetic neuralgia: a review of the clinical safety and tolerability. Pain Ther. 2015;4(1):1–15.
- Snedecor SJ, Sudharshan L, Cappelleri JC, et al. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014;14(2):167–184.
- Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161(9):639–649.
- Snedecor SJ, Sudharshan L, Cappelleri JC, et al. Systematic review and meta-analysis of pharmacological therapies for pain associated with postherpetic neuralgia and less common neuropathic conditions. Int J Clin Pract. 2014;68(7):900–918.
- Khadem T, Stevens V. Therapeutic options for the treatment of postherpetic neuralgia: a systematic review. J Pain Pall Care Pharmacother. 2013;27(3):268–283.
- Mick G, Correa-Illanes G. Topical pain management with the 5% lidocaine medicated plaster: a review. Curr Med Res Opin. 2012;28(6):937–951.
- Wolff RF, Bala MM, Westwood M, et al. 5% lidocaine-medicated plaster vs other relevant interventions and placebo for post-herpetic neuralgia (PHN): a systematic review. Acta Neurol Scand. 2011;123(5):295–309.
- Wolff RF, Bala MM, Westwood M, et al. 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): a systematic review. Swiss Med Wkly. 2010;140(21-22):297–306.
- Plested M, Budhia S, Gabriel Z. Pregabalin, the lidocaine plaster and duloxetine in patients with refractory neuropathic pain: a systematic review. BMC Neurol. 2010;10(1):116.
- Khaliq W, Alam S, Puri N. Topical lidocaine for the treatment of postherpetic neuralgia. Cochrane Database Syst Rev. 2007;(2):CD004846.
- Campbell BJ, Rowbotham M, Davies PS, et al. Systemic absorption of topical lidocaine in normal volunteers, patients with post-herpetic neuralgia, and patients with acute herpes zoster. J Pharm Sci. 2002;91(5):1343–1350.
- U.S. Food and Drug administration. Drug approval package: lidoderm (lidocaine) patch (Application No.: 20-612) Internet. Silver Spring: FDA; 2005 [cited 2019 May 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20612.cfm