Abstract
Objectives: To assess characteristics and healthcare costs associated with pharmacologically treated episodes of treatment-resistant depression (TRD) in patients with major depressive disorder (MDD).
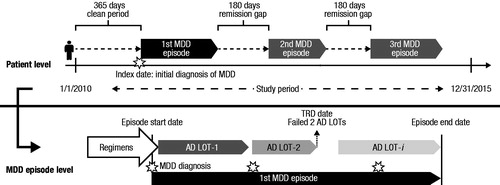
Methods: Patients aged ≥18 years with continuous health plan enrollment for ≥12 months before and after a newly observed MDD diagnosis (observed between 1 January 2010 and 31 December 2015) were included in this retrospective claims-based analysis. A pharmacologically treated episode was defined as beginning at the date of the first MDD diagnosis and ending when a gap of 180 days occurred between MDD diagnoses, or when a gap of 180 days occurred following the end of the antidepressant (AD)/antipsychotic (AP) drug supply. When such a gap occurred, the episode end date was determined to be either the date of the last MDD diagnosis or date of the end of AD/AP drug supply, whichever was later. An episode was considered TRD if ≥3 AD regimens occurred. Episode duration, medication regimens used, and relapse hospitalization were reported for TRD and non-TRD MDD episodes. Total all-cause and per-patient-per-month (PPPM) healthcare costs (in 2016 $) were estimated.
Results: Of 48,440 patients identified with ≥1 AD-treated MDD episode, the mean (SD) age was 39 (15) years, and 62% were female. Of all episodes, 7% were TRD, with a mean duration of 571 (285) days vs. 200 (198) days for non-TRD MDD episodes. Mean total all-cause costs were $19,626 ($44,160) for TRD and $7440 ($25,150) for non-TRD MDD episodes.
Conclusions: Results show TRD episodes are longer and costlier than non-TRD MDD episodes, and that higher costs are driven by episode duration. Longer episodes imply protracted suffering for patients with TRD and increased burden on caregivers. Effective intervention to shorten TRD episodes may lessen disease burden and reduce costs.
Introduction
Major depressive disorder (MDD) is a severe, chronic psychiatric illness associated with a number of general and psychiatric comorbidities. A leading cause of disabilityCitation1, MDD in the US has an estimated 12 month prevalence of 10.4% in adults, more than a third of whom are pharmacologically treatedCitation2. Patients with MDD are at increased risk of suicideCitation3 and collectively suffer decades of potential life lostCitation4–6. The incremental all-cause direct costs from MDD, estimated at $99 billion annuallyCitation7, stress the importance of understanding the course of this disease to achieve timely, effective treatments and reduce economic burden.
Patients with MDD experience relapsing, remitting episodes of illness that, in the clinical setting, are defined symptomatically through various outcome measures. First-line treatment is a combination of psychotherapy and antidepressant (AD) medication, including selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), atypical ADs (e.g. mirtazapine) or norepinephrine dopamine reuptake inhibitors (e.g. bupropion). About a third of patients, however, fail to respond adequately even to multiple varied pharmacological treatmentsCitation8. With only open-ended treatment algorithms to guide clinical practice, such patients come to be considered treatment-resistantCitation8. They tend to have poorer outcomes, more severe symptomsCitation9, longer episodesCitation10, more general and psychiatric comorbid conditionsCitation11–13, poorer quality of lifeCitation14 and higher risk for suicideCitation3,Citation15. Months or years of trying various treatment regimens without finding relief results in increased emotional and financial burden to family and caregiversCitation16–19 and higher cost to payersCitation20. Although no consensus definition exists for treatment-resistant depression (TRD), one that is widely used and recognized this year by the Food and Drug Administration and the Agency for Healthcare Research and Quality is “a failure to achieve an adequate response after application of at least 2 AD treatment trials of adequate dosage and duration”Citation8,Citation21,Citation22.
TRD is not only difficult to manage, but also costly. Both at the episode levelCitation10 and over the course of the illnessCitation23, TRD is significantly more burdensome to patient, caregiver, payer and society than MDD, and direct costs associated with TRD increase significantly with level of resistanceCitation23.
Most previous studies have been conducted at the patient level. An episode-level approach was chosen for this study because of the richness of the data available at the level of the single treatment episode, allowing empirical measures associated with TRD episodes and non-TRD MDD episodes. Thus, this study adds to existing knowledge by assessing the real-world characteristics and economic burden that distinguish TRD from non-TRD MDD episodes among US commercially insured patients. The relapsing, remitting nature of MDD lends itself to examination of treatment patterns at the level of the MDD episode and offers an opportunity to understand the holistic burden of TRD that is distinct from that of non-TRD MDD.
Methods
Study design
This episode-level retrospective cohort study used data extracted from the IBM MarketScan Commercial and Medicare Supplemental Databases. The Commercial Database contains pharmacy and medical (inpatient and outpatient) claims of employees and their dependents, and the Medicare Supplemental Database profiles the healthcare experience of individuals with Medicare supplemental insurance paid for by employers. Both databases provide detailed outcomes measures, including resource utilization and associated costs for individuals covered annually by a geographically diverse group of self-insured employers and private insurance plans across the US. All study data were accessed with protocols compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 regulations. Because this study used only statistically DE-identified patient records, it was exempt from institutional review board approval.
Eligible MDD patients were selected during the study period (1 January 2010–31 December 2015, ). A pharmacologically treated episode was defined as beginning at the date of the first MDD diagnosis and ending when a gap of 180 days occurred between MDD diagnoses, or when a gap of 180 days occurred following the end of the antidepressant (AD) or antipsychotic (AP) drug supply. When such a gap occurred, the episode end date was determined to be either the date of the last MDD diagnosis or date of the end of AD/AP drug supply, whichever was later. Included in the analysis were only pharmacologically treated MDD episodes: that is, only episodes consisting of both an MDD diagnosis and an AD prescription fill, with or without one of the MDD-indicated APs, aripiprazole, brexpiprazole, olanzapine or quetiapine. A treated MDD episode was considered TRD if it had at least three AD regimens, where regimen was defined as a continuous segment of AD medication use (allowing a maximum 60 day gap) with at least 28 days’ supply. The regimen may have included AD polypharmacy and/or augmentation with an AP. The sequence of medication regimens used during an MDD episode defined a line of therapy (LOT).
Study patients
Selected patients had at least one MDD diagnosis (International Classification of Diseases, 9th Revision [ICD-9] code beginning with 296.2, 296.3; or International Classification of Diseases, 10th Revision [ICD-10] code starting with F32, F33) during the study period. The index date was the date of the newly observed MDD diagnosis (i.e. the first medical claim of MDD during the study period). Patients were to be at least 18 years old as of the index date and have at least 12 months of continuous health plan enrollment prior to and following the index date. Patients were excluded if they had an MDD diagnosis or used an AD/AP medication in the 12 months prior to the index date, or had a diagnosis of psychosis, schizophrenia, bipolar disorder, dementia, or Tourette syndrome during the study period. Patients whose episode either persisted beyond the study period or had only one diagnosis of MDD were excluded. This ensured that our study sample contained only completed episodes. The study cohorts were classified as TRD or non-TRD MDD based on whether patients’ episodes qualified as TRD.
Study measures
The main study outcomes were episode duration and all-cause direct healthcare costs associated with the newly observed pharmacologically treated MDD episode (referred to throughout as the “newly observed treatment episode”). Episode duration was examined because, compared to non-TRD MDD, TRD is thought to be associated with longer episodes, and thus to have a more protracted illness burden. Because MDD is associated with a number of co-occurring general medical and psychiatric conditions, total all-cause healthcare costs were examined as a more inclusive measure of cost burden than depression-only costs. Total all-cause costs were defined as the sum of insurer payments (including coordination of benefits) and patient-paid cost sharing (including co-payment, deductible and co-insurance) incurred from fully adjudicated claims of prescription and medical services associated with any condition. All-cause medical services included the healthcare resource utilization associated with all medical conditions related to inpatient admissions, emergency room (ER) visits, physician office visits and other outpatient services. Because episode length varies among patients, all-cause costs were also reported as per patient per month (PPPM). All dollar estimates were inflated to 2016 US dollars using the medical care component of the Consumer Price Index (CPI).
Characteristics of the newly observed treatment episode examined include the following: percentage of episodes considered TRD; episode duration (days between episode start and end); episode severity proxy (based on the ICD-9 and ICD-10 diagnosis code observed on the index date, and classified as mild [ICD-9 296.21, 296.31 or ICD-10 F32.0, F33.0] or moderate to severe [ICD-9 296.22–296.24, 296.32–296.34 or ICD-10 F32.1–F32.3, F33.1–F33.3]); occurrence of relapse hospitalization (hospitalization with a primary diagnosis of MDD or suicidal ideation); and number of LOTs.
Patient demographics such as age, gender, geographic region (US census division), and type of insurance as of the index date were described. The composite score, Quan–Charlson Comorbidity Index (QCI), was used as a measure of morbidity during the 12 months prior to the index dateCitation24.
Statistical analyses
Descriptive statistics were calculated for study measures and outcomes. Mean and standard deviation (SD) were calculated for all continuous variables; frequencies and percentages were reported for categorical variables. All statistical analyses were conducted using SAS Enterprise Guide 7 (SAS Institute Inc., Cary, NC).
Results
Patient attrition and demographic characteristics
shows enrolled patient attrition. Of 98,809 MDD patients with at least one completed MDD episode, 48,440 (49%) received AD/AP treatment in their newly observed treatment episode. Mean (SD) age was 39 (15) years; 62% were female; 55% were covered by a PPO health plan ().
Figure 2. Sample selection. Abbreviations. AD/AP, Anti-depressant/MDD-indicated anti-psychotic; MDD, Major depressive disorder; TRD, Treatment-resistant depression. (a) Exclusionary diagnoses were psychosis, schizophrenia, bipolar disorder, dementia and Tourette syndrome. (b) All included episodes must have begun and ended during the study period and, in addition to a first eligible MDD diagnosis, must have had a subsequent MDD diagnosis or AD/AP medication claim.
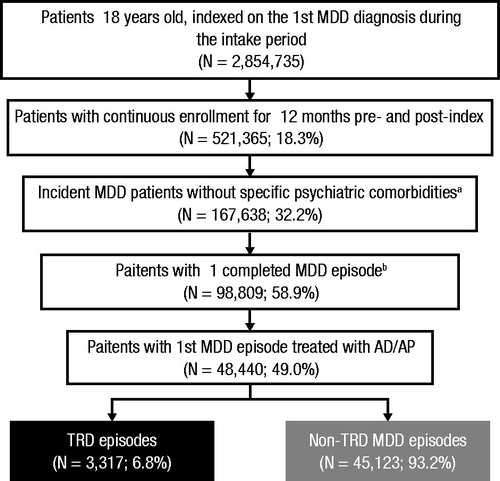
Table 1. Patient characteristics of MDD patients with at least one MDD episode.
Characteristics of the newly observed treatment episode
Mean (SD) newly observed treatment episode duration was 226 (225) days (). AD/AP therapy began an average of 24 (60) days after initial MDD diagnosis, and an average of 1.4 (0.7) LOTs were used. Slightly more than half of patients (52.0%) were diagnosed with moderate-to-severe MDD at index, and 9.9% experienced a relapse hospitalization. The most common first-line AD/AP regimens were monotherapy with SSRIs (63.0%), bupropion (10.5%) and SNRIs (7.0%).
Figure 3. Average duration and healthcare costs of newly observed treatment: MDD episodes. Abbreviations. ER, Emergency room; MDD, Major depressive disorder; SD, Standard deviation; TRD, Treatment-resistant depression.
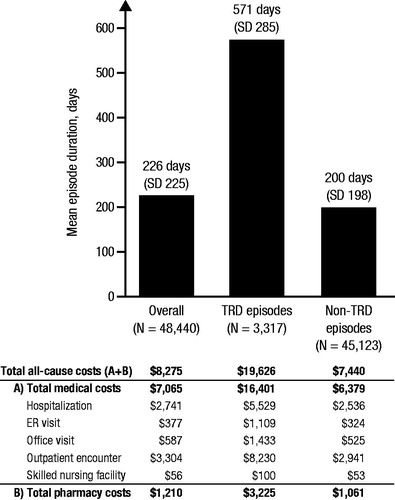
Of the 48,440 newly observed treatment episodes, 3317 (6.8%) met the criteria for TRD and were included in the TRD cohort. TRD episodes averaged 571 days’ duration and 3.5 (0.8) LOTs; 57.6% of patients with newly observed treatment episodes of TRD were diagnosed with moderate-to-severe MDD at index, and 13.7% of episodes included a relapse hospitalization.
Non-TRD MDD episodes averaged 200 days’ duration and 1.2 (0.4) LOTs; 51.8% of patients with newly observed treatment episodes of non-TRD MDD were diagnosed with moderate-to-severe MDD at index, and 9.6% of episodes included a relapse hospitalization. Mean (SD) Quan–Charlson comorbidity score was the same (0.3 [0.9], ) for both TRD and non-TRD MDD cohorts, indicating similar baseline physical comorbidity across the two cohorts.
All-cause health resource utilization of newly observed treatment episodes
Across all newly observed treatment episodes, 14.7% included an all-cause hospitalization and 14.7% had at least one ER visit. Most episodes included a physician office visit (88.4%) or other outpatient services (91.3%). Among TRD episodes, 24.3% included an all-cause hospitalization and 32.6% had at least one ER visit. Nearly all episodes included a physician office visit (97.6%) or other outpatient services (99.1%). Among non-TRD MDD episodes, 14.0% included an all-cause hospitalization and 13.4% had at least one ER visit. Most episodes had physician office visits (87.7%) or other outpatient services (90.8%).
All-cause healthcare costs of the newly observed treatment episode
presents all-cause healthcare costs for all newly observed treatment episodes and by TRD status. On average within newly observed treatment episodes, total all-cause healthcare costs were $8275 (±$27,059; median: $2132; quartile range: $6113), 85.4% of which were medical and 14.6% were pharmacy. Medical costs were mainly attributable to other outpatient services (39.9%) and inpatient hospitalization (33.1%), followed by physician office visits (7.1%), ER visits (4.6%) and long-term care (0.7%). Total all-cause PPPM costs averaged $1101 (±$3395; median: $420; quartile range: $725).
TRD episodes had a mean (SD) total all-cause PPPM cost of $1031 (±$2195; median: $487; quartile range: $776); 83.6% were medical and 16.4% were pharmacy. Medical costs were mainly attributable to other outpatient encounters (41.9%) and inpatient hospitalization (28.2%), followed by physician office visits (7.3%), ER visits (5.7%) and long-term care (0.5%).
Non-TRD MDD episodes had a mean (SD) total all-cause PPPM cost of $1115 (±$3596; median: $406; quartile range: $709); 85.7% were medical and 14.3% were pharmacy. Medical costs were mainly attributable to other outpatient encounters (39.5%) and inpatient hospitalization (34.1%), followed by physician office visits (7.1%), ER visits (4.3%) and long-term care (0.7%).
Discussion
This study, based on health claims data from 2010 to 2015, provides real-world insights at the episode level on the characteristics of pharmacologically treated MDD episodes and the distinguishing economic burden of treatment resistance. MDD and TRD are episodic in nature. Although previous real-world studies have addressed the burdens associated with MDD and TRD, this study adds to existing knowledge by focusing specifically on the characteristics and burden (including hospitalization as a proxy measure of relapse) of a newly observed treatment episode.
This study aimed to further understand the natural course of MDD and TRD episodes through the empirical measurement of select episode features. Although adjustment would better quantify the incremental burden potentially causally attributable to TRD, such work has been done in prior studies of TRD, whereas these unadjusted results represent a more novel approach. For many stakeholders, unadjusted results can better inform decision making compared with adjusted results, including: (1) a healthcare provider communicating a prognosis on treatment and episode duration to a patient in an MDD or TRD episode; (2) a population-health decision maker estimating MDD or TRD episode churn and case mix in a population; or (3) an economic modeler determining an appropriate time horizon for individual treatments and episode length in an economic model. In these cases and others, unadjusted results are preferable to adjusted results.
The literature supports that comorbid conditions are particularly prevalent among patients with MDDCitation7,Citation25. An ad hoc patient-level analysis by Sheehan et al. showed that patients with TRD were more frequently diagnosed at baseline with co-occurring anxiety than patients with non-TRD MDD (13.0% vs. 11.7%, respectively)Citation26. Due to stigma surrounding the disease, patients may report somatic complaints like pain, fatigue, and sleep and appetite disturbances rather than psychiatric complaints. Therefore, as a more complete reflection of illness burden, both to patients and to payers, we measured all-cause costs rather than costs related to depression only.
Our descriptive analysis found that TRD episodes were nearly three times longer on average than non-TRD MDD episodes and involved more all-cause hospitalizations (24%, TRD; 14%, non-TRD MDD). While PPPM costs were similar for TRD and non-TRD episodes ($1031 and $1115, respectively), total all-cause costs for TRD episodes were 3.5-fold higher on average than non-TRD MDD episodes, due to their longer duration (571 days, TRD; 200 days, non-TRD MDD). These findings add to existing evidence. Ivanova et al.Citation27 reported significantly higher average direct 2 year costs for “TRD-likely” employees ($22,784 in 2010 USD) compared with MDD controls ($11,733); and Kubitz et al.Citation10, who estimated costs across multiple episodes, found TRD costs were three to six times higher, depending on resource type. Mean episode duration for TRD episodes from our study was shorter than that from Kubitz et al. (571 days vs. 1004 days) which could be due to their earlier study period (pre- vs. post-2010) and our longer clean period (180 day vs. 120 day). In a recently published article, Sussman et al.Citation28 set the index date for TRD at the initiation of the third treatment course as opposed to the first treatment course, which shifts the focus of the comparison to later in the episode. Sussman et al. found smaller cost differences between TRD and non-TRD MDD compared with prior research using index dates earlier in the episode. In our episode-based analysis, patients with TRD are followed for a longer duration than those with non-TRD MDD because TRD episodes are longer. In accordance with Sussman’s findings, our episode-based approach led to smaller differences in PPPM between TRD and non-TRD MDD, even while the total cost of the TRD episode is higher, because costs may decrease later in an episode.
Prevalence estimates for TRD vary broadly due to differences in study design, TRD definition and methodology. Our study found that 6.8% of newly observed treatment episodes were treatment-resistant. Although lower than prevalence estimates from clinical studies, 6.8% is compatible with other recent claims-based estimates. Kubitz et al.Citation10, using a US commercial database and 15 year study period, reported that 6.6% of treated MDD episodes met the criteria for TRD and, after adjusting for mean episode duration, 13.6% of episodes were treatment-resistant. The Kubitz study differed from ours in its longer study period, shorter clean period requirement and multi-episode analysis. Amos et al.Citation23, in a patient-level analysis, reported 16% of MDD patients as treatment resistant. The Amos study did not require episode termination during follow-up, used a slightly longer study period and included a broader list of AD medications (e.g. lithium, psychostimulants and thyroid hormone) to meet TRD classification.
It should be noted that findings from clinical trials expectedly differ from claims-based analyses, due mainly to inherent design and methodological differences. Clinical trial designs, for example, rely on comparatively homogeneous study populations and well defined operational methods. Strict inclusion and exclusion criteria result in an ideal study population, enriched with the disease of interest, but not necessarily reflective of the real world. In addition, clinical trial protocols usually require regularly scheduled, symptom-based assessments of treatment response, enriching the resultant data with an empirically measured outcome of interest. However, in the clinical setting, if clinicians do not promptly adjust treatment when confronted with inadequate response, the real-world incidence of TRD will appear lower than in trial settings.
Lacking information from clinical assessments of treatment response, claims-based analyses rely instead on indirect translation of clinical decisions into proxy measures, such as diagnosis codes and prescription fills. To better define the treated MDD population, this study used both medical and pharmacy claims, which increases the specificity of patient identification. Our study used a useful, but arbitrary and possibly overly restrictive, 365 day pre-index “clean period” during which there was no relevant diagnosis or prescription codes. Our investigation also required a long, 180 day clean period between episodes, to account for the depletion of drug supply with an additional 2 months, serving as a conservative proxy for remission. Finally, the inclusion of only completed newly observed treatment episodes allowed the cleanest and most complete data set. These design aspects, while restrictive, are rigorous and conservative in the identification of treatment non-response; however, they also result in a prevalence estimate that is lower than reported elsewhere.
MDD is often underdiagnosedCitation29. When present, it often does not respond adequately to currently available standard treatments, which generally start with SSRI monotherapy. The STAR*D study showed a significant drop in the likelihood of achieving remission after two failed LOTs. Correspondingly, our study found that although SSRIs continued to be the drug class used most often across multiple LOTs, their use dropped with subsequent LOTs. The degree of inadequate response to currently available treatments points to a significant unmet treatment need.
Taken together, findings from this episode-level analysis add detail and empirical evidence that TRD imposes substantial burdens, distinct from non-TRD MDD, on patients and payers.
Limitations
Interpretation of results should consider the following study limitations. Administrative claims data are collected for facilitating payment for healthcare services. As such, diagnoses may be incomplete or inaccurate, leading to potential misclassification bias. For MDD specifically, stigma may contribute to an underestimation of prevalence. Similarly, generic prescriptions paid out of pocket are not captured in claims databases, potentially contributing to underestimation of both drug utilization and, in the case of our study design, episode duration. Prescription dispensing records are not necessarily reflective of actual medication taken, potentially leading to misinformation bias. Psychological therapies are commonly utilized in the treatment of TRD. However, they are generally underreported in administrative claims databases due to the mental/behavioral health “carve-out” billing mechanism, where mental health services are contracted directly with mental health organizations and billed for separately from remaining healthcare benefits. Therefore, this study did not report the utilization and costs of psychotherapies. This study employed an empirical clean period length and maximum permissible gap, which may impact the identification of MDD episodes and regimen LOTs. Patients with only one MDD diagnosis and episodes without an AD/AP pharmacy claim were excluded from analysis as a measure to increase the specificity of sample selection. Such cases were considered to be invalid as they could potentially arise from administrative errors. Although this approach improved our confidence in accurately representing the TRD population, it also resulted in a smaller overall sample size and higher estimated burden in the control group. Costs represented in the claims data are limited to the paid amounts of adjudicated claims to individual hospitals and providers; thus, indirect costs, such as those associated with caregiver burden, were not considered. This study was composed of patients covered by commercial or Medicare supplemental insurance; therefore, results are not generalizable to other populations. By design these results do not generalize to patients with episodes that extended beyond the follow-up period, as only patients with completed treatment episodes were eligible.
Conclusions
This study examines, in a focused real-world setting using the newly observed treatment episode, the characteristics of treated MDD episodes and the distinguishing economic burden of treatment resistance. TRD is associated with longer, and thus costlier, episodes than non-TRD MDD. The results underscore the importance of understanding what distinguishes TRD from MDD to facilitate its early identification and reduce healthcare costs.
Transparency
Declaration of funding
This study was supported by Janssen Scientific Affairs LLC.
Author contributions
Q.C. and B.W. contributed to the concept and design of the study, acquisition of the data, analysis and interpretation of the data, and statistical analysis. J.J.S. and C.B. contributed to the concept and design of the study, and analysis and interpretation of the data; C.B. also contributed to development of the statistical analysis plan. L.A. and N.C. contributed to analysis and interpretation of the data. All authors contributed to drafting and critical revision of the manuscript. All authors approved the final version and agree to be accountable for all aspects of the work.
Declaration of financial/other relationships
QC, JJS, BW, NC and CB have disclosed that they are employees of Janssen Scientific Affairs LLC. LA was an employee of Janssen Scientific Affairs LLC at the time of study execution.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
These data were previously presented, in part, in a poster at the AMCP Managed Care & Specialty Pharmacy Annual Meeting 2018; 2018 Apr 23–26; Boston, MA. Poster F29.
Data availability statement
Data for this study were available to the authors via third-party license from IBM MarketScan Research Databases, a commercial data provider in the US, and Janssen Pharmaceuticals, who has a license for analysis of the de-identified IBM MarketScan Commercial and Medicare Supplemental databases. As such, the authors cannot provide the raw data; however, other researchers may access the data by purchase through IBM, and the inclusion criteria specified in the methods would allow them to identify the same cohort of patients. Interested individuals may visit https://www.ibm.com/watson/health/ for more information on accessing IBM MarketScan Research Databases.
Acknowledgements
We would like to acknowledge Christopher Pericone PhD, Associate Director Scientific Writing at Janssen Pharmaceuticals, for his editorial assistance.
References
- World Health Organization [Internet]. Media center fact sheet. Depression [updated 2017 Feb; cited 2017 Aug 25]. Available from: http://www.who.int/mediacentre/factsheets/fs369/en
- Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 2018;75(4):336–346.
- Coryell W, Young EA. Clinical predictors of suicide in primary major depressive disorder. J Clin Psychiat. 2005;66(4):412–417.
- Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing chronic disease: Public health research, practice and policy. Prev Chronic Dis. 2006;3(2):A42.
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disorders. 2002;2(3):227–236.
- Vos T, Flaxman MN, Lozano R, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiat. 2015;76(02):155–162.
- Rush J, Trivedi M, Wisniewski S, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiat. 2006;163(11):1905–1917.
- Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharm. 1999;9(1–2):83–91.
- Kubitz N, Mehra M, Potluri RC, et al. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial claims database. PLoS One. 2013;8(10):e76882.
- Gruber AJ, Hudson JI, Pope HG Jr. The management of treatment-resistant depression in disorders on the interface of psychiatry and medicine. Fibromyalgia, chronic fatigue syndrome, migraine, irritable bowel syndrome, atypical facial pain, and premenstrual dysphoric disorder. Psychiatr Clin North Am. 1996;19(2):351–369.
- Franco-Bronson K. The management of treatment-resistant depression in the medically ill. Psychiatr Clin North Am. 1996;19(2):329–350.
- Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adher. 2012;6:369–388.
- Russell JM, Hawkins K, Ozminkowski RJ, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65(3):341–347.
- Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression. PS. 2014;65(8):977–987.
- McCusker J, Latimer E, Cole M, et al. Major depression among medically ill elders contributes to sustained poor mental health in their informal caregivers. Age Ageing. 2007;36(4):400–406.
- Rane LJ, Fekadu A, Papadopoulos AS, et al. Psychological and physiological effects of caring for patients with treatment-resistant depression. Psychol Med. 2012;42(9):1825–1833.
- Chang CC, Yen CF, Jang FL, et al. Comparing affiliate stigma between family caregivers of people with different severe mental illness in Taiwan. J Nerv Ment Dis. 2017;205(7):542–549.
- Albert SM, Schulz R, Colombi A [Internet]. The MetLife Study of working caregivers and employer health care costs: case study. New insights and innovations for reducing health care costs for employers. 2010 [cited 2018 Apr 5]. Available from: http://www.caregiving.org/data/Caregiver_Costs_Study_Web_FINAL_2-12-10.pdf
- Olchanski N, McInnis MM, Halseth M, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35(4):512–522.
- Gaynes BN, Asher G, Gartlehner G, et al [Internet]. Definition of treatment-resistant depression in the Medicare population. Technology Assessment Program. Rockville, MD: Agency for Healthcare Research and Quality; 2018 [cited 2018 May 18]. Available from: https://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id105TA.pdf
- US Food and Drug Administration [Internet]. Major depressive disorder: developing drugs for treatment. Guidance for industry. 2018 [cited 2018 Jul 17]. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM611259.pdf
- Amos T, Tandon N, Lefebvre P, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry. 2018;79(2):pii: 17m11725.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- Corey-Lisle PK, Birnbaum HG, Greenberg PE, et al. Identification of a claims data “signature” and economic consequences for treatment-resistant depression. J Clin Psychiatry. 2002;63(8):717–726.
- Sheehan J, Cai Q, Wu B, et al. Real-world economic burden associated with treatment-resistant depression. Poster presented at: ASHP Summer Meetings and Exhibition; 2018 Jun 2–6; Denver, CO.
- Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26(10):2475–2484.
- Sussman M, O’sullivan AK, Shah A, et al. Economic burden of treatment-resistant depression on the U.S. health care system. JMCP. 2019;25:823–835.
- Sheehan DV. Depression: underdiagnosed, undertreated, underappreciated. Manag Care. 2004;13(6 Suppl Depression):6–8.