Abstract
Objective: Bilastine is a potent and highly selective H1-antihistamine approved for the treatment of allergic rhinoconjunctivitis and urticaria. This article summarizes available data on the use of bilastine in the treatment of allergic disorders in different age groups, including younger and older adults, and school-age children and adolescents.
Methods: A PubMed literature search (“bilastine”) was conducted on 25 February 2019. Additional literature known to the authors and identified from the reference lists of cited publications was included.
Results: Bilastine is administered orally at a dose of 20 mg once daily in adults and adolescents aged ≥12 years and 10 mg once daily in children aged 6 to <12 years. Clinical trials have demonstrated its efficacy at improving nasal and ocular symptoms in patients with allergic rhinitis, and wheals and itching in patients with urticaria. It has a rapid onset of action and long duration of action. Bilastine does not undergo significant metabolism and does not interact with the CYP450 system, which limits its potential for drug-drug interactions. No dosage adjustments are required in patients with renal or hepatic impairment, or in the elderly. Bilastine is generally well tolerated, even when administered at above-standard doses. It does not exhibit anticholinergic effects or cardiotoxic effects, shows no central nervous system penetration and has minimal sedative properties. It has been shown to improve health-related quality of life.
Conclusions: Bilastine is a suitable option for the treatment of patients with allergic rhinoconjunctivitis or urticaria across age groups from school-age children to elderly patients.
Introduction
Allergic diseases such as allergic rhinoconjunctivitis and urticaria are common worldwide, and the prevalence is increasing, particularly amongst childrenCitation1,Citation2. Allergies impose a substantial socioeconomic burden on patients, their families, and societyCitation1. These disorders have a negative impact on patients’ physical, psychological, social, educational and work functioning, with financial implications for healthcare systems and also for society through lost work productivityCitation1,Citation3,Citation4.
Given the role of histamine in allergic responses, many allergic disorders, including allergic rhinoconjunctivitis and urticaria, are treated with H1-antihistaminesCitation5–7. First-generation H1-antihistamines have pronounced anticholinergic effects, sedative effects and interactions with alcohol and numerous drugsCitation5,Citation7. In contrast, modern second-generation H1-antihistamines cause minimal or no sedation, are free of anticholinergic effects, and are recommended as first-line therapeutic options for patients with allergic rhinitis or urticariaCitation5–7.
Bilastine is a selective, second-generation H1-antihistamine. It was first approved in the European Union in 2010 for the symptomatic treatment of allergic rhinoconjunctivitis (seasonal and perennial) and urticaria in patients aged 12 years or older, and is now available in approximately 100 countries worldwideCitation8. More recently, it has been approved in Europe for use in children aged 6 to <12 yearsCitation8. This narrative review summarizes available data on the use of bilastine in the treatment of allergic disorders in different age groups, including younger and older adults, and school-age children and adolescents.
Search methodology
A search of PubMed up to 25 February 2019 was undertaken using the search term “bilastine” to identify relevant publications about the drug. Preclinical and clinical publications were assessed for inclusion, with priority given to clinical papers, particularly randomized controlled trials when available.
Bilastine: pharmacological profile
Bilastine is a second-generation H1-antihistamine. Although the efficacy of second-generation H1-antihistamines is generally similar, pharmacokinetic properties and the potential for drug-drug and food-drug interactions differ between individual drugsCitation9. Bilastine has high specificity for H1-receptors and negligible affinity for other receptors, and demonstrates antihistamine and antiallergic propertiesCitation10,Citation11. Bilastine has a rapid onset of action and a long duration of actionCitation12–15. It has been shown to have a long residence time at the H1-receptor, resulting in prolonged receptor antagonism, with 60–70% antagonism evident 24 hours after dosingCitation8.
Bilastine is administered orally once daily. It is rapidly absorbed after oral administration, achieving maximum plasma concentrations after 1–1.5 hoursCitation12,Citation15,Citation16. Concurrent food intake reduces the bioavailability of bilastine. In a recent study, food intake reduced histamine-induced wheal significantly less at 0.5 and 1 hour after bilastine administration as compared to drug intake under fasting, but not thereafter. Once steady-state was reached at 4 days, food had no significant effect on the wheal response at any timeCitation17. Mean oral bioavailability is about 60% and it is 84–90% bound to plasma proteinsCitation12,Citation16,Citation18. It does not undergo significant hepatic metabolism and approximately 95% is excreted unchanged in either the faeces (67%) or urine (33%)Citation19. Bilastine has a mean elimination half-life of approximately 12–14.5 hoursCitation8,Citation12,Citation15,Citation17. No dosage adjustments are required in patients with renal or hepatic impairment or in elderly peopleCitation18,Citation20. Bilastine does not interact significantly with the cytochrome P450 (CYP) enzyme systemCitation21. This, and the lack of hepatic metabolism, limits the potential for drug–drug interactions. Bilastine is a substrate for P-glycoproteinCitation22, which restricts its passage across the blood–brain barrier, limiting the likelihood of central nervous system (CNS) effects such as sedationCitation23.
Adults with allergic rhinitis
Allergic rhinitis affects 10–40% of individuals worldwideCitation24, and it has a substantial negative effect on patients’ quality of life (QoL), sleep and daily activitiesCitation1,Citation24. Patients with allergic rhinitis require a fast-acting, effective, and non-sedating treatment, and a modern second-generation oral H1-antihistamine such as bilastine meets these criteria. Patients often do not take their antihistamine medication in accordance with allergic rhinitis guidelines; instead, they tend to treat themselves only when they have symptoms and to stop when they feel their symptoms are controlledCitation25,Citation26. Moreover, adherence tends to be worse in people taking a higher number of medications. Compliance may be better if patients only have to take one tablet daily. The practical message for physicians is to “keep it simple”. Antihistamines that can be administered once daily and have a rapid onset of action, such as bilastineCitation12,Citation13,Citation15,Citation18, are a practical option for patients with allergic rhinitis.
Patients with allergic rhinitis experience nasal symptoms including congestion, itching, rhinorrhoea, and sneezing. Those who also have allergic conjunctivitis experience redness, tears and itching of the eyesCitation27. The goal of treatment in allergic rhinoconjunctivitis is symptom relief. The efficacy of bilastine 20 mg once daily has been demonstrated in several clinical trials in adults and adolescents with seasonal allergic rhinitis (SAR) or perennial allergic rhinitis (PAR). Studies conducted in Europe generally enrolled patients aged 12–70 years, whereas studies in Japan usually included adults aged 18–74 years.
Two randomized, controlled, crossover studies using environmental exposure chambers evaluated the effect of bilastine during 2–3 days of allergen exposure in subjects with allergic rhinitisCitation28,Citation29. Bilastine had a rapid onset and long duration of action. In European subjects exposed to grass pollen, the onset of action for bilastine 20 mg or cetirizine 10 mg was similar and both had a longer duration of action than fexofenadine 120 mgCitation28; in Japanese subjects exposed to Japanese cedar pollen, bilastine 20 mg had a faster onset of action than fexofenadine 60 mgCitation29. All active drugs were more effective than placebo.
Four randomized controlled clinical trials evaluated the efficacy of 2 or 4 weeks of treatment with once-daily bilastine 20 mg in adults/adolescents with SAR or PAR (). The primary efficacy endpoint was the area under the curve for the total symptom score (TSS; i.e. nasal and non-nasal symptoms) over the treatment period or the mean change from baseline in total nasal symptom score (TNSS). Overall, these studies indicated that bilastine 20 mg once daily was more effective than placebo at reducing symptoms in patients with SAR or PAR, and as effective as desloratadine 5 mg once daily, cetirizine 10 mg once daily or fexofenadine 60 mg twice dailyCitation30–33. Pooled analyses of seven clinical trials confirmed that bilastine was effective at controlling the nasal obstruction and ocular symptoms of allergic rhinoconjunctivitisCitation34,Citation35.
Table 1. Bilastine in adults/adolescents with seasonal or perennial allergic rhinitis: double-blind randomized controlled trials of ≥2 weeks in duration.
Allergic rhinitis has a marked negative impact on QoL, causing limitations in many areas of daily activity, including concentration, productivity, sleep and sexual functionCitation3,Citation36. Randomized controlled studies showed that bilastine 20 mg once daily was significantly more effective than placebo, and as effective as loratadine 10 mg or desloratadine 5 mg once daily, at improving health-related QoL in patients with allergic rhinitis, as assessed using the Rhinoconjunctivitis Quality of Life QuestionnaireCitation30,Citation37,Citation38.
Long-term efficacy is important for antihistamine medications, particularly for patients with PAR. A single-arm study, in which 64 patients with PAR were treated for up to 52 weeks, confirmed that the efficacy of bilastine 20 mg once daily, including reductions in TSS, TNSS and total ocular symptoms score (TOSS), and an improvement in QoL, was maintained for up to 1 yearCitation39.
The overall incidence of adverse events with bilastine in trials in allergic rhinitis was generally similar to that seen with placeboCitation30–33. The most common treatment-emergent adverse events (TEAEs) included headache, somnolence and fatigue, with an incidence that was similar to that seen with placebo. Bilastine 20 mg once daily was found to be associated with significantly less somnolence (1.8 vs. 7.5%, p < .001) and fatigue (0.4 vs. 4.8%, p = .02) than cetirizine 10 mg once dailyCitation31. A lack of sedation and adverse cognitive effects is an important feature for an antihistamine, particularly since allergic rhinitis itself can have a sedative effect and impair psychomotor functions such as driving abilityCitation40,Citation41. Antihistamine treatment has been shown to improve vigilance and partially counteract the negative effect of allergic rhinitis on driving abilityCitation40,Citation41. Bilastine 20 mg once daily was shown to be safe and well tolerated over a 1-year treatment period in an open-label extension phase of a multicentre, randomized, placebo-controlled, double-blind, parallel-group study in 513 mainly Caucasian patientsCitation32 and 55 Japanese patients diagnosed with PAR who received continuous bilastine 20 mg once daily for 52 weeksCitation39.
Adults with urticaria
Urticaria has a lifetime prevalence of more than 20%Citation1. Chronic urticaria, with an estimated prevalence of 0.6–1.5%, has a substantial negative effect on patients’ QoL and job performance, with work productivity impaired by 10–30%Citation1,Citation7. Urticaria is a mast-cell driven disease. The release of histamine from mast cells produces the characteristic symptoms and signs of urticaria – wheals, flare and itch. Antihistamines are inverse agonists of the histamine receptor and can counteract these histamine-driven features. The goal of treatment for urticaria is to achieve complete relief of symptomsCitation7. Second-generation H1-antihistamines are recommended as first-line therapy for urticaria. If control is inadequate after 2–4 weeks, the dose can be increased (by up to four-fold), with other treatments added only if antihistamine up-dosing still provides inadequate controlCitation7.
The ideal antihistamine should be effective at relieving symptoms, have a rapid onset of action and long duration of action, preferably be administered once daily, and not cause unwanted effects such as drowsiness. Bilastine fits this profile for the treatment of patients with urticaria.
Studies in healthy adult volunteers showed that bilastine suppressed the wheal and flare response compared with placebo and that it had a rapid onset of action, with a high level of inhibition evident after 0.5–2 hoursCitation14,Citation15,Citation42. Bilastine had a faster onset of action than cetirizine, desloratadine and rupatadineCitation13,Citation14. At 1.5 hours, both wheals and flares were inhibited by >70% in 11 of 12 recipients of bilastine compared with 3 of 11 recipients of cetirizine (p = .003); differences were not significant at later timepointsCitation13. In the study by Antonijoan et al., the onset of wheal inhibition was 1 hour for bilastine 20 mg and 4 hours for desloratadine 5 mg or rupatadine 10 mg whereas, for flare area inhibition, bilastine 20 mg had an onset of action at 30 minutes, while desloratadine and rupatadine needed 4 hours to show a significant difference compared with placebo. Bilastine also reduced itching significantly whereas this was not seen with desloratadine or rupatadineCitation14.
Bilastine has been evaluated in several randomized controlled clinical trials in adults with urticaria (). Studies conducted in Europe generally enrolled patients aged 12–70 years, whereas studies in Japan enrolled adults aged 18–74 years. Bilastine 20 mg once daily was significantly more effective than placebo at reducing the symptoms of chronic spontaneous urticaria (assessed using TSS)Citation43,Citation44, with similar efficacy to levocetirizine 5 mg once dailyCitation43. Bilastine 20 mg once daily was also significantly more effective than placebo at controlling symptoms in patients with cold contact urticariaCitation45. Bilastine had a rapid onset of action, with significant improvements in TSS seen after 1 day of treatment in patients with chronic spontaneous urticariaCitation43,Citation44. Bilastine 20 mg also provided significant improvements in individual symptoms, including wheal, flare and itchingCitation43–45.
Table 2. Bilastine in adults with chronic spontaneous urticaria or cold contact urticaria: double-blind randomized controlled trials of ≥1 week in duration.
Chronic urticaria has a considerable adverse effect on patients’ QoLCitation4,Citation46. Bilastine 20 mg once daily has been shown to improve QoL in patients with chronic spontaneous urticariaCitation38,Citation43,Citation44. In the largest randomized trial, mean Dermatology Life Quality Index (DLQI) Global score decreased significantly versus placebo after 4 weeks (–9.45 vs. –5.93, p < .001)Citation43.
Consistent with the results of studies in allergic rhinitis, bilastine 20 mg was well tolerated in adult patients with urticaria with an overall incidence of adverse events similar to that seen with placeboCitation43,Citation44.
A 1-year noncomparative study involving 198 adults with chronic spontaneous urticaria (n = 56) or pruritus associated with other skin diseases (such as eczema/dermatitis, prurigo or cutaneous pruritus) showed that the efficacy of bilastine 20 mg once daily was maintained, and the drug remained well tolerated over the long termCitation47. TSS, rash and itch scores, and DLQI score improved significantly (p < .001) compared with baseline from week 2 and remained the same thereafter. Bilastine-related adverse events (all mild to moderate in severity) occurred in 2.5% of patients during the 1-year study period; somnolence was reported for 1% of patients.
The EAACI/GA2LEN/EDF/WAO guidelines recommend second-generation H1-antihistamines at licensed doses as first-line therapy for patients with urticaria and suggest increasing the dose by two- to four-fold in patients who are unresponsive to the licensed doseCitation7. The efficacy and safety of a bilastine updosing strategy has been demonstrated in two studies. In a randomized, crossover trial in patients with cold contact urticaria, the standard dose of 20 mg was effective at reducing the critical temperature threshold at which symptoms occurred, and efficacy was increased further when the dose was increased to 40 mg and 80 mg, such that 60% of patients were symptom-free when treated with the highest dose ()Citation45.
A small study in a real-world setting evaluated bilastine updosing in 29 patients with moderate-to-severe chronic spontaneous urticaria that had not responded adequately to other H1-antihistamines at licensed dosesCitation48. All patients initially received bilastine 20 mg once daily for 2 weeks, after which any non-responders (defined as 7-day Urticaria Activity Score [UAS7] > 3) were updosed to bilastine 40 mg once daily for another 2 weeks, with any continuing non-responders subsequently updosed to bilastine 80 mg once daily for 2 weeks. After 2 weeks on bilastine 20 mg (n = 29), mean UAS7 decreased by 37% compared with baseline (p < .001). The pruritus and wheal components decreased by 44 and 29%, respectively. Updosing to bilastine 40 mg (n = 23) resulted in a further significant reduction (23%) in mean UAS7 (p = .007), with reductions in the pruritus and wheal components of 24 and 17%, respectively. Further updosing to bilastine 80 mg (n = 21) produced a modest reduction in UAS7 (7%) which was not statistically significant, with reductions in the pruritus and wheal components of 0 and 12%. The small difference seen between bilastine 40 mg and 80 mg is probably explained by the fact that bilastine is particularly effective against pruritus, with most of the inhibition of this symptom occurring at a dose of 20 mg ()Citation45,Citation48.
Figure 1. Differential effect of bilastine against the pruritus and wheal components of the 7-day urticaria activity score in patients with chronic spontaneous urticaria in a real-world study evaluating a bilastine updosing strategy in patients with an inadequate response to other H1-antihistamines. Data from Weller et al. 2018Citation48.
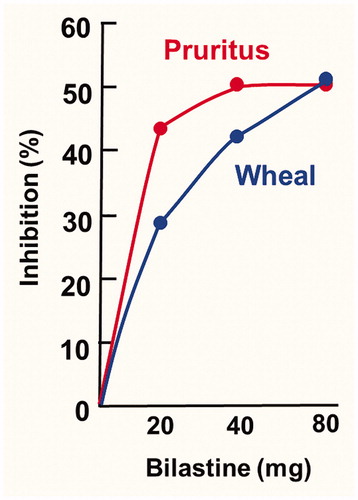
In both updosing studies, bilastine was well tolerated at doses up to 80 mg once daily, with no evidence of increased sedation at higher dosesCitation45,Citation48.
Safety in adults
Bilastine is generally well toleratedCitation49. In clinical trials involving adult/adolescent patients with allergic rhinoconjunctivitis or chronic idiopathic urticaria the incidence of adverse events was similar for bilastine (12.7%) and placebo (12.8%)Citation18. The most common adverse drug reactions reported for bilastine 20 mg were headache, somnolence, dizziness and fatigue, which occurred at a similar frequency in placebo recipientsCitation18. Bilastine remained well tolerated in the long-termCitation32,Citation33,Citation47.
Bilastine is highly specific for the H1-receptor, limiting the risk of adverse events caused by interactions with other receptors, such as anticholinergic effectsCitation5,Citation10. Bilastine has minimal H1-receptor occupancy (H1RO) in the CNS, similar to placebo (in comparison, hydroxyzine 25 mg [positive control] was associated with significant brain H1RO, resulting in sedation)Citation23. Furthermore, an indirect comparison of published data showed that the cerebral H1RO for bilastine 20 mg is one of the lowest among first- and second-generation H1-antihistaminesCitation50, resulting in the classification of bilastine as a non-brain-penetrating antihistamineCitation51. Bilastine also has minimal adverse effects on psychomotor performance or subjective assessment of drowsinessCitation52 and does not augment the CNS effects of alcohol or lorazepamCitation18,Citation53. Bilastine remains non-sedating even at a dose of 80 mg once dailyCitation45,Citation48. Driving ability was not altered by bilastine 20 mg, or even at a double dose (40 mg), in healthy volunteersCitation54 and bilastine 20 mg had no effect on driving performance in patients with allergic rhinitis and/or chronic urticaria using a Formula One-high speed simulator-driving testCitation55. In the setting of simulated flight-associated hypobaric hypoxia, bilastine did not affect vigilance or cognitive performanceCitation56,Citation57. In the most recent of these studies, a randomized, double-blind, comparative, placebo-controlled crossover studyCitation56, bilastine 20 mg did not impair any of the tested abilities, either at ground level or under hypobaric hypoxia. In contrast, cetirizine 10 mg increased the number of errors at ground level and, at the simulated altitude (4000 m), additional impairment was observed in cetirizine 10 mg recipients in a distributive attention testCitation56. Bilastine has no significant effect on QTc intervalCitation58,Citation59, and there is no evidence of cardiotoxicity even when updosed up to four times the standard licensed doseCitation60. Based on the European Medicines Agency (EMA) periodic safety update report of post-marketing activity, a cumulative review of hypersensitivity reactions was performed for bilastine. Causality was assessed as possible in most of the cases, and as probable in a few cases. While acknowledging that the benefit-risk balance for bilastine remains unchanged, the EMA Pharmacovigilance Risk Assessment Committee concluded that hypersensitivity reactions (frequency not known) should be included in the bilastine product informationCitation61.
Elderly patients
The number of people aged ≥65 years is increasing and by 2030 this age group will represent about 20% of the total world populationCitation62. The prevalence of allergic diseases is currently estimated to be approximately 5–10% in the elderly and is likely to rise due to the increasing number of people with allergiesCitation62–64. A decline in immune function and age-related changes in tissue structures have an impact on allergies in the elderly, while comorbidities, polypharmacy and adverse drug reactions can complicate the pictureCitation65.
The most common allergic diseases in elderly people include allergic rhinitis (approximately 5–11%) and chronic urticaria (prevalence in elderly uncertain but general prevalence around 0.5–1%)Citation46. In elderly individuals, allergic rhinitis often occurs in association with other types of chronic non-allergic rhinitis (e.g. atrophic rhinitis, vasomotor rhinitis, drug-related rhinitis)Citation66. Chronic urticaria in the elderly is often associated with the general consequences of skin aging, such as atrophy of the epidermis and dermis, progressive deterioration of skin structural integrity and function, impaired skin barrier function and immune response, vascular impairment, and a build-up of reactive oxygen speciesCitation67. Urticaria in the elderly may be induced by systemic diseases or may be drug-inducedCitation68.
Comorbidities and the use of polypharmacy are common amongst the elderlyCitation69,Citation70. Polypharmacy is associated with increased risks of drug–drug interactions and adverse drug reactionsCitation70. The aging process itself can also interfere with the pharmacokinetics and pharmacodynamics of medications. For example, reductions occur in liver blood flow, liver volume and renal glomerular filtration rate, which can result in impaired hepatic and/or renal drug clearanceCitation71,Citation72. Older individuals may also have reduced cognitive performance, particularly executive function, and memory dysfunctionCitation73. They have an increased risk of developing drug-induced delirium, a condition which can, in part, be related to a reduction in cholinergic functionCitation74. Therefore, in elderly patients, it is important to avoid drugs which can cause confusion or sedation/drowsiness, as well as those which have effects on the cholinergic system, such as first-generation and some second-generation H1-antihistamines. Although first-generation H1-antihistamines are efficacious, they are associated with numerous important adverse effects, caused by interactions with CNS H1-receptors, muscarinic receptors, serotonin receptors, α-adrenergic receptors and cardiac ion channelsCitation5. Consequently, although first-generation H1-antihistamines are not recommended for use in any patients with allergic disease, they can be particularly hazardous in seniors (and children).
Non-sedating, second-generation H1-antihistamines, such as bilastine, should be used in elderly patients with allergic disorders. As well as being efficacious, bilastine is safe and well tolerated even at supratherapeutic dosages and has additional features which make it suitable for use in this age group. As discussed elsewhere in this article, bilastine does not cause sedative effects or affect cognitive performance and does not potentiate the effects of alcohol or benzodiazepinesCitation18,Citation45,Citation48,Citation52,Citation53. It does not have anticholinergic effectsCitation63 or exhibit cardiotoxic effectsCitation58,Citation59. Since bilastine does not undergo significant metabolism, dose adjustments are not needed in elderly patients or those with renal or hepatic impairmentCitation18,Citation20. Finally, bilastine has limited propensity for drug–drug interactionsCitation19,Citation21.
Clinical trials with bilastine in adults allowed patients up to the age of 70 or 74 years to be enrolled. However, relatively few participants were aged ≥65 years. In clinical trials performed as part of the European development programme for bilastine, only 69 patients were aged ≥65 years, of whom 34 received bilastineCitation75. Consequently, a prospective observational study in a real-world practice setting was undertaken to evaluate the safety profile in 146 patients aged ≥65 years with allergic rhinoconjunctivitis and/or urticaria who were prescribed bilastine 20 mg once daily and followed up for 3 monthsCitation75. The mean ± SD age of participants was 74.8 ± 6.6 years; 74% had allergic rhinoconjunctivitis, 19% had urticaria and 7% had both disorders. At inclusion, 61 patients (41.8%) reported at least one previous medical condition and the most common comorbidities were hypertension (66.4%), osteoarthritis (43.8%), dyslipidaemia (29.5%), hypercholesterolaemia (24.0%) and depression (19.2%). The mean ± SD duration of treatment was 35.8 ± 29.7 days.
During the study period, 74 patients (50.7%) reported 129 non-serious TEAEs during the study period () and, of these, only eight TEAEs (in seven patients, 4.8%) were judged to be at least possibly related to bilastine. Mild/moderate somnolence was the only bilastine-related TEAE reported by more than one patient (four patients, 2.7%). The monthly and quarterly incidence rates for TEAEs were 0.29 (95% confidence interval 0.229–0.367) and 0.88 (95% CI 0.688–1.100), respectively. No serious TEAEs considered to be related to bilastine occurred. Overall, this study showed that bilastine 20 mg once daily had a favourable safety profile, with a low incidence of TEAEs, in elderly patients with allergic rhinoconjunctivitis and/or urticaria. Moreover, the safety profile in elderly patients was not different from that in placebo-controlled clinical trials in which adverse event rates reported for bilastine were similar to those for placeboCitation75.
Table 3. Tolerability of bilastine in elderly patients: treatment-emergent adverse events with an incidence ≥2% reported in elderly patients (≥65 years) with allergic rhinoconjunctivitis and/or urticaria treated with bilastine 20 mg once daily for a mean of 36 days.
School-age children
Rhinoconjunctivitis is common in school-age children and adolescents, with an average global prevalence of 8.5% in children aged 6–7 years and 14.6% in those aged 13–14 yearsCitation76. The prevalence of rhinoconjunctivitis appears to be increasing, particularly among older childrenCitation2. The prevalence of acute urticaria in children is 1–14%, and that of persistent or chronic urticaria is 0.1–1.8%Citation77,Citation78. Allergic rhinitis and urticaria both have an adverse effect on QoL in children and adolescents, causing emotional and practical problems, limitations in daily activities and sleep problemsCitation79–82. Of importance in this age group, allergic rhinitis and urticaria can have a negative impact on school attendance and academic achievementCitation81,Citation83,Citation84.
Clinical trials in perennial and seasonal allergic rhinoconjunctivitis or urticaria performed as part of the main bilastine clinical development programme enrolled 198 patients aged 12 to 18 years, of whom 81 received bilastine 20 mg, including 68 who received it for 12 monthsCitation52. Bilastine 20 mg once daily was determined to be effective and safe in this age groupCitation52, and the original approval for bilastine covered adults and adolescents aged ≥12 yearsCitation18.
More recently, bilastine was approved widely for use in children aged 6–11 years (who weigh ≥20 kg)Citation85,Citation86. This approval was based on data from studies included in the bilastine Paediatric Investigation Plan, approved by the EMA Paediatric Committee, which included a paediatric pharmacokinetic studyCitation87,Citation88 and an international, double-blind, randomized, placebo-controlled study evaluating safety and tolerabilityCitation89, both performed in children aged >2 years to <12 years.
A model-informed development approach was used to select the paediatric dose and design the sampling schedule for the pharmacokinetic studyCitation87,Citation88. The multicentre, adaptive, open-label, repeated-administration pharmacokinetic study enrolled children in two age groups, 6 to <12 years (n = 24) and 2 to <6 years (n = 7). Participants received bilastine 10 mg, as an orodispersible tablet, once daily for 6 days. Children in the older age group were enrolled first, and younger children only after an interim analysis had confirmed that pharmacokinetic profiles were as predicted, that there were no safety concerns, and that the dose was appropriate. Overall, the study established that in children aged 2 to <12 years a 10 mg dose of bilastine provided equivalent systemic exposure and similar pharmacodynamic outcomes to a 20 mg dose in adultsCitation88.
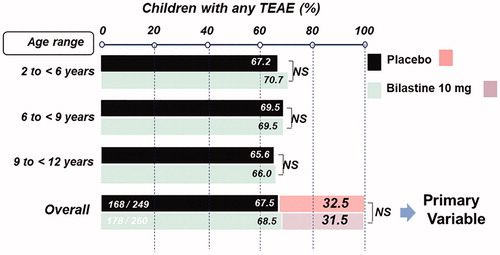
To evaluate safety in children, 506 patients aged 2 to ≤12 years with symptomatic allergic rhinoconjunctivitis or chronic urticaria were randomized to treatment with either bilastine 10 mg orodispersible tablet or placebo for 12 weeksCitation89. Patients were stratified into three age groups: 2 to <6 years (20% of patients), 6 to <9 years (40%) and 9 to <12 years (40%). The primary outcome was the proportion of children in each group without TEAEs during the study. After 12 weeks of treatment, there was no statistically significant difference between bilastine and placebo in the proportion of children without TEAEs (31.5 versus 32.5%; treatment difference 0.99%, 95% CI –9.10 to 7.10; ). The upper limit of the 95% CI for the difference was less than 10%, meeting the predefined criterion for non-inferiority. There were also no differences between bilastine and placebo for secondary endpoints, including the incidence of TEAEs () or related-TEAEs, either overall or within the different age groups. In addition, bilastine demonstrated similar outcomes to placebo in the four domains of the Paediatric Sleep Questionnaire (sleep-related breathing disorder, daytime sleepiness, snoring and inattention). Overall, this study showed that bilastine 10 mg had a tolerability profile similar to that of placebo in children aged 2 to ≤12 yearsCitation89.
Figure 2. Tolerability of bilastine 10 mg once daily in children aged 2 to <12 years with allergic rhinitis or chronic urticaria: results of an international, double-blind, randomized, placebo-controlled clinical trial. Reproduced with permission from Novák et al.Citation89.
Discussion
Oral second-generation H1-antihistamines, such as bilastine, are recommended as first-line medication for the treatment of allergic rhinitis and chronic urticaria in adults and childrenCitation6,Citation7,Citation90,Citation91. The EAACI/ARIA guidelines group have defined the features that an ideal oral H1-antihistamine should possessCitation92. Bilastine has a high number of the specified attributes and meets the EAACI/ARIA criteria for medications for the treatment or allergic rhinitisCitation93.
Bilastine is a potent and highly selective oral H1-antihistamine. It is efficacious in the treatment of allergic rhinoconjunctivitis and chronic urticaria in adults (at a dose of 20 mg once daily) and in children aged 2–11 years (at a dose of 10 mg once daily), is generally well tolerated, even at supratherapeutic doses, and improves patients’ QoL. Bilastine displays only limited penetration across the blood-brain barrier, does not cause somnolence or affect cognitive performance or the ability to drive, and does not potentiate the effects of alcohol. It does not exhibit anticholinergic effects or cardiotoxic effects. No dosage adjustments are required in patients with renal or hepatic impairment, or in the elderly. In patients with moderate or severe renal impairment, coadministration of bilastine with P-glycoprotein inhibitors (e.g. ketoconazole, erythromycin, cyclosporine, ritonavir or diltiazem) may increase plasma levels of bilastine. Bilastine does not undergo significant metabolism and does not interact with the CYP system, which limits its potential for drug–drug interactions.
Allergic disorders affect people of all ages and can have a substantial adverse effect on an individual’s QoL and their work or educational performanceCitation3,Citation4,Citation36,Citation46,Citation79–82. Bilastine is effective and well tolerated in different age groups, from school-age children through to elderly adults. The dose in elderly patients is the same as for younger adults (20 mg tablet once daily)Citation18. The dose for adolescents aged ≥12 years is also 20 mg once dailyCitation18. In children aged 6–11 years with a bodyweight of ≥20 kg, the dose is 10 mg once dailyCitation85,Citation86. In addition to the standard 20 mg tablet, two paediatric formulations are available – a 10 mg orodispersible tablet and a 2.5 mg/mL oral solutionCitation85,Citation86.
The EAACI/GA2LEN/EDF/WAO guidelines for the treatment of chronic urticaria suggest that doses of second-generation H1-antihistamines can be increased by up to four-fold above the licensed dose in patients who have failed to respond to a standard doseCitation7. Bilastine has been shown to be safe and to provide additional efficacy at doses of up to 80 mg once daily (four times the standard adult dose) in patients with chronic urticaria. It has also been shown to be effective in patients who have failed to respond to other second-generation H1-antihistamines.
Studies that compared bilastine with other oral second-generation H1-antihistamines showed that bilastine was as effective as desloratadine, cetirizine and fexofenadine at reducing symptoms in patients with allergic rhinitis, and as effective as loratadine or desloratadine at improving health-related QoL. Bilastine was as effective as levocetirizine at improving symptoms in patients with chronic spontaneous urticaria. It was also shown that bilastine was associated with significantly less somnolence and fatigue than cetirizine.
In conclusion, bilastine displays good efficacy with a rapid onset of action and long duration of action and good tolerability with minimal sedative properties and a low propensity for drug-drug interactions. Bilastine is an attractive option for the treatment of patients with allergic rhinoconjunctivitis or urticaria across age groups from school-age children through to the elderly.
Transparency
Declaration of funding
Writing assistance was funded by FAES FARMA and Menarini.
Declaration of financial/other relationships
Martin Church has been a speaker or consultant for Almirall, FAES FARMA, Menarini, Moxie, MSD, Novartis, UCB Pharma, Sanofi-Aventis and Uriach.
Marysia Recto has participated in advisory boards for A. Menarini and Abbott Nutrition. She has been a speaker for FAES FARMA, A. Menarini, Abbott Nutrition, Nestle Nutrition, Galderma, Leo Pharma, Mylan, Astra Zeneca, Novartis, Glenmark, Natrapharm and Kalbe Nutrition.
Zoltán Novák has participated in advisory boards for AbbVie, AstraZeneca, Berlin-Chemie/A. Menarini, FAES FARMA GlaxoSmithKline, MEDA Pharma, Novartis, Nusivan, Orion Pharma, Sandoz Genzyme. He has been a speaker for the above companies and Boehringer, Chiesi, Ewopharma, Sandoz. The authors have no ethical conflicts to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed extensively to the work presented in this paper. All authors have participated in drafting and/or reviewing the manuscript and have approved its submission.
Acknowledgements
Medical writing/editorial assistance was provided, under the guidance of the authors, by David P. Figgitt PhD, ISMPP CMPP™, and Kathy Croom, Content Ed Net, with funding from FAES FARMA.
References
- Pawankar R, Canonica GW, Holgate ST, et al. World Allergy Organization (WAO) white book on allergy: update 2013. Milwaukee, USA: World Allergy Organization; 2013. p. 11–13; 27–31, 60–63.
- Björkstén B, Clayton T, Ellwood P, ISAAC Phase III Study Group, et al. Worldwide time trends for symptoms of rhinitis and conjunctivitis: phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19(2):110–124.
- Camelo-Nunes IC, Solé D. Allergic rhinitis: indicators of quality of life. J Bras Pneumol. 2010;36(1):124–133.
- Maurer M, Abuzakouk M, Bérard F, et al. The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy. 2017;72(12):2005–2016.
- Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139–1150.e4.
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–476.
- Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414.
- Bosma R, van den Bor J, Vischer HF, et al. The long duration of action of the second generation antihistamine bilastine coincides with its long residence time at the histamine H(1) receptor. Eur J Pharmacol. 2018;5(838):107–111.
- Paśko P, Rodacki T, Domagała-Rodacka R, et al. Second generation H1 – antihistamines interaction with food and alcohol-A systematic review. Biomed Pharmacother. 2017;93:27–39.
- Corcóstegui R, Labeaga L, Innerárity A, et al. Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: receptor selectivity and in vitro antihistaminic activity. Drugs R D. 2005;6(6):371–384.
- Corcóstegui R, Labeaga L, Innerárity A, et al. In vivo pharmacological characterisation of bilastine, a potent and selective histamine H1 receptor antagonist. Drugs R D. 2006;7(4):219–231.
- Jauregizar N, de la Fuente L, Lucero ML, et al. Pharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) effect of bilastine. Clin Pharmacokinet. 2009;48(8):543–554.
- Church MK. Comparative inhibition by bilastine and cetirizine of histamine-induced wheal and flare responses in humans. Inflamm Res. 2011;60(12):1107–1112.
- Antonijoan R, Coimbra J, García-Gea C, et al. Comparative efficacy of bilastine, desloratadine and rupatadine in the suppression of wheal and flare response induced by intradermal histamine in healthy volunteers. Curr Med Res Opin. 2017;33(1):129–136.
- Togawa M, Yamaya H, Rodríguez M, et al. Pharmacokinetics, pharmacodynamics and population pharmacokinetic/pharmacodynamic modelling of bilastine, a second-generation antihistamine, in healthy Japanese subjects. Clin Drug Investig. 2016;36(12):1011–1021.
- Sádaba B, Gómez-Guiu A, Azanza JR, et al. Oral availability of bilastine. Clin Drug Investig. 2013;33(5):375–381.
- Coimbra J, Campo C, Labeaga L, et al. Lack of clinical relevance of bilastine-food pharmacokinetic interaction assessed by inhibition of histamine-induced wheal and flare response in healthy volunteers. Skin Allergy Meeting; 2019 April 4–6; Munich, Germany.
- Bilastine 20 mg tablets: summary of product characteristics. 2018; [cited 2019 Mar 3]. Available from: http://www.gov.uk/pil-spc.
- Sologuren A, Lucero ML, Valiente R, et al. Human mass balance with [14C]-bilastine following oral administration to healthy volunteers. Basic Clin Pharmacol Toxicol. 2009;105(1):106–107.
- Lasseter KC, Sologuren A, La Noce A, et al. Evaluation of the single-dose pharmacokinetics of bilastine in subjects with various degrees of renal insufficiency. Clin Drug Investig. 2013;33(9):665–673.
- Lucero ML, Gonzalo A, Mumford R, et al. An overview of bilastine metabolism during preclinical investigations. Drug Chem Toxicol. 2012;35(Supp1):18–24.
- Lucero ML, Gonzalo A, Ganza A, et al. Interactions of bilastine, a new oral H1 antihistamine, with human transporter systems. Drug Chem Toxicol. 2012;35(Supp1):8–17.
- Farré M, Pérez-Mañá C, Papaseit E, et al. Bilastine vs. hydroxyzine: occupation of brain histamine H1 -receptors evaluated by positron emission tomography in healthy volunteers. Br J Clin Pharmacol. 2014;78(5):970–980.
- Brożek JL, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958.
- Menditto E, Costa E, Midão L, et al. Adherence to treatment in allergic rhinitis using mobile technology. The MASK study. Clin Exp Allergy. 2019;49(4):442–460.
- Bousquet J, Devillier P, Arnavielhe S, et al. Treatment of allergic rhinitis using mobile technology with real-world data: the MASK observational pilot study. Allergy. 2018;73(9):1763–1774.
- Small P, Keith PK, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14(S2):51.
- Horak F, Zieglmayer P, Zieglmayer R, et al. The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. Inflamm Res. 2010;59(5):391–398.
- Hashiguchi K, Wakabayashi KI, Togawa M, et al. Therapeutic effect of bilastine in Japanese cedar pollinosis using an artificial exposure chamber (OHIO Chamber). Allergol Int. 2017;66(1):123–131.
- Bachert C, Kuna P, Sanquer F, et al. Comparison of the efficacy and safety of bilastine 20 mg vs desloratadine 5 mg in seasonal allergic rhinitis patients. Allergy. 2009;64(1):158–165.
- Kuna P, Bachert C, Nowacki Z, et al. Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo for the symptomatic treatment of seasonal allergic rhinitis: a randomized, double-blind, parallel-group study. Clin Exp Allergy. 2009;39(9):1338–1347.
- Sastre J, Mullol J, Valero A, Bilastine Study Group, et al. Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo in the treatment of perennial allergic rhinitis. Curr Med Res Opin. 2012;28(1):121–130.
- Okubo K, Gotoh M, Asako M, et al. Efficacy and safety of bilastine in Japanese patients with perennial allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase III study. Allergol Int. 2017;66(1):97–105.
- Bartra J, Mullol J, Montoro J, et al. Effect of bilastine upon the ocular symptoms of allergic rhinoconjunctivitis. J Investig Allergol Clin Immunol. 2011;21(3):24–33.
- Dávila I, Sastre J, Mullol J, et al. Effect of bilastine upon nasal obstruction. J Investig Allergol Clin Immunol. 2011;21(3):2–8.
- Kirmaz C, Aydemir O, Bayrak P, et al. Sexual dysfunction in patients with allergic rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2005;95(6):525–529.
- Locks RB, Dos Santos K, da Silva J. Quality of life in patients with allergic rhinitis: a clinical trial comparing the use of bilastine versus loratadine. Clin Otolaryngol. 2017;42(2):218–224.
- Jáuregui I, Bartra J, del Cuvillo A, et al. Bilastine and quality of life. J Investig Allergol Clin Immunol. 2011;21(3):16–23.
- Okubo K, Gotoh M, Togawa M, et al. Long-term safety and efficacy of bilastine following up to 12 weeks or 52 weeks of treatment in Japanese patients with allergic rhinitis: results of an open-label trial. Auris Nasus Larynx. 2017;44(3):294–301.
- Spaeth J, Klimek L, Mösges R. Sedation in allergic rhinitis is caused by the condition and not by antihistamine treatment. Allergy. 1996;51(12):893–906.
- Vuurman EF, Vuurman LL, Lutgens I, et al. Allergic rhinitis is a risk factor for traffic safety. Allergy. 2014;69(7):906–912.
- García-Gea C, Martínez-Colomer J, Antonijoan RM, et al. Comparison of peripheral and central effects of single and repeated oral dose administrations of bilastine, a new H1 antihistamine: a dose-range study in healthy volunteers with hydroxyzine and placebo as control treatments. J Clin Psychopharmacol. 2008;28(6):675–685.
- Zuberbier T, Oanta A, Bogacka E, et al. Comparison of the efficacy and safety of bilastine 20 mg vs. levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: a multi-centre, double-blind, randomized, placebo-controlled study. Allergy. 2010;65(4):516–528.
- Hide M, Yagami A, Togawa M, et al. Efficacy and safety of bilastine in Japanese patients with chronic spontaneous urticaria: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II/III study. Allergol Int. 2017;66(2):317–325.
- Krause K, Spohr A, Zuberbier T, et al. Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy. 2013;68(7):921–928.
- Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66(3):317–330.
- Yagami A, Furue M, Togawa M, et al. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44(4):375–385.
- Weller K, Church MK, Hawro T, et al. Updosing of bilastine is effective in moderate to severe chronic spontaneous urticaria: a real-life study. Allergy. 2018;73(10):2073–2075.
- Scaglione F. Safety profile of bilastine: 2nd generation H1-antihistamines. Eur Rev Med Pharmacol Sci. 2012;16(14):1999–2005.
- Jáuregui I, Ramaekers JG, Yanai K, et al. Bilastine: a new antihistamine with an optimal benefit-to-risk ratio for safety during driving. Expert Opin Drug Saf. 2016;15(1):89–98.
- Kawauchi H, Yanai K, Wang DY, et al. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. IJMS. 2019;20(1)pii:E:213.
- Montoro J, Mullol J, Dávila I, et al. Bilastine and the central nervous system. J Investig Allergol Clin Immunol. 2011;21(3):9–15.
- García-Gea C, Martínez J, Ballester MR, et al. Psychomotor and subjective effects of bilastine, hydroxyzine, and cetirizine, in combination with alcohol: a randomized, double-blind, crossover, and positive-controlled and placebo-controlled Phase I clinical trials. Hum Psychopharmacol Clin Exp. 2014;29(2):120–132.
- Conen S, Theunissen EL, Van Oers AC, et al. Acute and subchronic effects of bilastine (20 and 40 mg) and hydroxyzine (50 mg) on actual driving performance in healthy volunteers. J Psychopharmacol. 2011;25(11):1517–1523.
- Demonte A, Guanti MB, Liberati S, et al. Bilastine safety in drivers who need antihistamines: new evidence from high-speed simulator driving test on allergic patients. Eur Rev Med Pharmacol Sci. 2018;22(3):820. 8.
- Reményi Á, Grósz A, Szabó SA, et al. Comparative study of the effect of bilastine and cetirizine on cognitive functions at ground level and at an altitude of 4,000 m simulated in hypobaric chamber: a randomized, double-blind, placebo-controlled, cross-over study. Expert Opin Drug Saf. 2018;17(9):859–868.
- Valk PJ, Simons R, Jetten AM, et al. Cognitive performance effects of bilastine 20 mg during 6 hours at 8000 ft Cabin Altitude. Aerosp Med Hum Perform. 2016;87(7):622–627.
- Graff C, Struijk JJ, Kanters JK, et al. Effects of bilastine on T-wave morphology and the QTc interval: a randomized, double-blind, placebo-controlled, thorough QTc study. Clin Drug Investig. 2012;32(5):339–351.
- Tyl B, Kabbaj M, Azzam S, et al. Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: results of a thorough QT study (TQTS) with QT-concentration analysis. J Clin Pharmacol. 2012;52(6):893–903.
- Cataldi M, Maurer M, Taglialatela M, et al. Cardiac safety of second-generation H1-antihistamines when updosed in chronic spontaneous urticaria. Clin Exp Allergy. 2019.
- European Medicines Agency CMDh. Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s). 2017; [cited 10 Sep 2019]. Available from: https://www.ema.europa.eu/en/documents/psusa/bilastine-cmdh-scientific-conclusions-grounds-variation-amendments-product-information-timetable/00003163/201703_en.pdf.
- Yáñez A, Cho SH, Soriano JB, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J. 2014;7(1):8.
- Bozek A. Pharmacological management of allergic rhinitis in the elderly. Drugs Aging. 2017;34(1):21–28.
- De Martinis M, Sirufo MM, Ginaldi L. Allergy and aging: an old/new emerging health issue. A&D. 2017;8(2):162–175.
- Milgrom H, Huang H. Allergic disorders at a venerable age: a mini-review. Gerontology. 2014;60(2):99–107.
- Pinto JM, Jeswani S. Rhinitis in the geriatric population. Allergy Asthma Clin Immunol. 2010;6(1):10.
- Farage MA, Miller KW, Elsner P, et al. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res. 2008;20(3):195–200.
- Ventura MT, Scichilone N, Gelardi M, et al. Management of allergic disease in the elderly: key considerations, recommendations and emerging therapies. Expert Rev Clin Immunol. 2015;11(11):1219–1228.
- Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43.
- Morin L, Johnell K, Laroche ML, et al. The epidemiology of polypharmacy in older adults: register-based prospective cohort study. CLEP. 2018;10:289–298.
- Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14.
- Ellam T, Twohig H, Khwaja A. Chronic kidney disease in elderly people: disease or disease label? BMJ. 2016;352:h6559.
- Eshkoor SA, Hamid TA, Mun CY, et al. Mild cognitive impairment and its management in older people. Clin Interv Aging. 2015;10:687–693.
- Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15(1):15–28.
- Sologuren A, Viñas R, Cordón E, et al. Open-label safety assessment of bilastine in elderly patients with allergic rhinoconjunctivitis and/or urticaria. Allergy Asthma Proc. 2018;39(4):299–304.
- Aït-Khaled N, Pearce N, Anderson HR, ISAAC Phase Three Study Group, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Allergy. 2009;64(1):123–148.
- Kudryavtseva AV, Neskorodova KA, Staubach P. Urticaria in children and adolescents: an updated review of the pathogenesis and management. Pediatr Allergy Immunol. 2019;30(1):17–24.
- Lee SJ, Ha EK, Jee HM, et al. Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy Asthma Immunol Res. 2017;9(3):212–219.
- Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol. 1994;93(2):413–423.
- Keil T, Bockelbrink A, Reich A, et al. The natural history of allergic rhinitis in childhood. Pediatr Allergy Immunol. 2010;21(6):962–969.
- Blaiss MS, Hammerby E, Robinson S, et al. The burden of allergic rhinitis and allergic rhinoconjunctivitis on adolescents: a literature review. Ann Allergy Asthma Immunol. 2018;121(1):43–52.e3.
- Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155(1):145–151.
- Walker S, Khan-Wasti S, Fletcher M, et al. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007;120(2):381–387.
- Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergológica 2005. J Investig Allergol Clin Immunol. 2009;19(2):21–26.
- Bilastine 10 mg orodispersible tablets: summary of product characteristics; 2018 [cited 2019 Mar 3]. Available from: http://www.gov.uk/pil-spc.
- Bilastine 2.5 mg/ml oral solution: summary of product characteristics; 2018 [cited 2019 Mar 3]. Available from: http://www.gov.uk/pil-spc.
- Vozmediano V, Sologuren A, Lukas JC, et al. Model informed pediatric development applied to bilastine: ontogenic pk model development, dose selection for first time in children and PK study design. Pharm Res. 2017;34(12):2720–2734.
- Vozmediano V, Lukas JC, Encinas E, et al. Model-informed pediatric development applied to bilastine: analysis of the clinical PK data and confirmation of the dose selected for the target population. Eur J Pharm Sci. 2019;128:180–192.
- Novák Z, Yáñez A, Kiss I, et al. Bilastine Paediatric Safety Study Group”. Safety and tolerability of bilastine 10 mg administered for 12 weeks in children with allergic diseases. Pediatr Allergy Immunol. 2016;27(5):493–498.
- Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68(9):1102–1116.
- Fitzsimons R, van der Poel LA, Thornhill W, et al. Antihistamine use in children. Arch Dis Child Educ Pract Ed. 2015;100(3):122–131.
- Bousquet J, Van Cauwenberge P, Bachert C, et al. Requirements for medications commonly used in the treatment of allergic rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and its Impact on Asthma (ARIA). Allergy. 2003;58(3):192–197.
- Bousquet J, Ansótegui I, Canonica GW, et al. Establishing the place in therapy of bilastine in the treatment of allergic rhinitis according to ARIA: evidence review. Curr Med Res Opin. 2012;28(1):131–139.
