Abstract
Objective: To describe the timeline to diagnosis for children with central precocious puberty (CPP) and evaluate their psychosocial and health-related quality of life (HRQoL).
Methods: A cross-sectional survey was used to prospectively collect data from caregivers, recruited via the MAGIC Foundation, of children with CPP. The control (non-CPP) group was recruited from a national panel of parents/caregivers. After completing a screening survey, respondents completed a burden of illness survey. Respondents in both groups completed the Pediatric Quality of Life Inventory (PedsQL) and Patient-Reported Outcomes Measurement Information System (PROMIS) peer relationship instruments.
Results: Responses from 142 caregivers of children with and 300 without CPP were assessed. Mean time to treatment after a child’s visit to the pediatric endocrinologist was 220 days and time from onset of symptoms to initiating treatment was approximately 2 years. Responses to HRQoL inventories were all lower in children with CPP versus non-CPP. Adjusted mean (± standard error) PedsQL total (65.3 ± 1.8 versus 75.7 ± 1.2), Psychosocial Health Summary (62.4 ± 1.8 versus 73.4 ± 1.2), and Physical Health Summary (70.7 ± 2.2 versus 79.9 ± 1.5) scores were significantly lower (p < .01) in CPP versus non-CPP group. PROMIS peer relationship T score (± standard error) was numerically lower for the CPP versus non-CPP group (45.4 ± 1.0 versus 47.4 ± 0.7, p = .11).
Conclusions: In clinical practice, there is a longer than expected delay between CPP symptom onset and referral to an endocrinologist and ultimate treatment. Children with CPP experience a substantial disease burden with a significant impact on emotional, social, and physical functioning compared with children without CPP.
Introduction
Central precocious puberty (CPP) is a rare disease with an incidence of 1 in 5000–10,000 childrenCitation1, characterized by early onset of puberty (before age 8 years in girls and 9 years in boys)Citation2 and is caused by premature activation of the hypothalamic-pituitary-gonadal axisCitation3. The physical and emotional changes associated with early onset of puberty may result in psychological and social problemsCitation4–7 and may have a substantial negative impact on health-related quality of life (HRQoL).
Treatment of CPP with a gonadotrophin-releasing hormone analogueCitation8 suppresses the progression of puberty, increasing adult height, and decreasing long-term psychological implicationsCitation9–12. Early initiation of treatment improves outcomesCitation13,Citation14, yet there is a paucity of data on the timing of the process from the onset of symptoms to diagnosis and eventual treatment. We sought to quantify the delay in treatment initiation and describe the journey for families from initial symptoms to seeking care and treatment. Defining the timeline to treatment stresses the importance of the primary care physician’s role in evaluating children so that more optimal care can be given, and therefore better outcomes. Previous research has predominantly examined the physical outcomes of CPP, and there are few well-designed studies assessing the psychosocial impactCitation11. The aim of this study was to capture information on the patient journey from the onset of CPP symptoms to diagnosis and treatment, and to evaluate the psychosocial and HRQoL burden among children with CPP in the US.
Methods
Study design
This double-blind, cross-sectional survey conducted by One Research, LLC (private research company in Charleston, SC, USA; www.oneresearchus.com), collected data prospectively from 6 April 2018 to 14 May 2018. Potential respondents were recruited by the MAGIC Foundation (Warrenville, IL, USA), a parent-run organization that provides support for parents/caregivers of children with CPP. The MAGIC Foundation, a non-profit organization, helps families of children diagnosed with a wide variety of growth impacting medical conditions through education, networking, physician referrals and numerous other services (www.magicfoundation.org/). Parents were invited by the MAGIC Foundation to complete a screening survey. Those who had a child with a diagnosis of CPP or with symptoms of CPP were invited to complete the main survey. The non-CPP group included 300 parents/caregivers of children without CPP who were recruited from a general population sample by One Research’s partner panels; these parents/caregivers were invited by email to participate in the survey. Respondents received $30 compensation for completing the survey, which took on average 22 min to complete.
Ethical approval
This study was conducted in accordance with the ethical principles based on the Declaration of Helsinki and consistent with International Conference on Harmonisation Good Clinical Practice and Good Epidemiology Practices. Respondents provided written informed consent prior to participation in the study. Institutional Review Board (IRB) approval was provided by the central US-approved IRB, the Copernicus Group IRB (Cary, NC, USA).
Online survey
Respondents who completed the screening survey were ≥18 years of age and resided in the US. The screening survey included age, race, comorbidities, and health care providers (HCPs).
Parents/caregivers who had a child with a diagnosis of CPP were invited to participate in the burden of illness section of the survey, which included questions regarding age the child first experienced symptoms of CPP, age they received a diagnosis of CPP, and how the diagnosis of CPP was made. The survey consisted of 54 questions collecting demographic data, data about symptoms, and treatments. Respondents were asked to select which symptoms from a list of 17 CPP-related symptoms their child experienced that made them seek medical help. They also rated how their child’s symptoms changed since starting therapy using a standard, 7-point impression scale ranging from 1 (very much improved) to 7 (very much worse)Citation15–18. Parents/caregivers in both the CPP and non-CPP groups completed the previously validated instruments, PedsQL and PROMIS, to obtain information on HRQoLCitation19–24. Basic demographic information including marital status, employment status, education, income, and race/ethnicity was also requested.
The online survey was password protected and hosted by Survey Sampling International (SSI) on a secure server. SSI (now Dynata) is a market research and data collection company based in Shelton, Connecticut, and is one of the largest data solutions companies in the world.
HRQoL questionnaires
The PedsQL questionnaireCitation19 is a validated instrument used to measure HRQoL in chronically ill and healthy childrenCitation20–22. PedsQL consists of 23 items that are used to calculate 4 subscale scores (Physical Functioning, Emotional Functioning, Social Functioning, and School Functioning), an overall Psychosocial Summary score, an overall Physical Summary score, and a Total score.
To assess the quality of the relationships children with CPP have with their friends and other acquaintances, the PROMIS parent–proxy peer relationship short-form instrumentCitation23,Citation24 was included as part of the online survey.
Data analysis
A convenience sample of 150 (caregivers of a child with CPP) was targeted and there were 142 respondents with a control group recruited of double that size, 300 (caregivers of a child without CPP). Data from all eligible respondents were used in the analysis. Summary descriptive statistics included mean and SD or standard error (SE) for continuous variables, and frequency and percent distributions for discrete variables. Differences in the demographic characteristics between CPP and non-CPP groups were assessed using logistic regression to create odds ratios for categorical variables and t tests for continuous variables. Different aspects of the care pathway were assessed, such as time to referral and diagnosis.
Each item of the PedsQL was reverse scored and linearly transformed to a scale of 0–100 with higher scores reflecting better HRQoL. Raw scores from the PROMIS instrument were translated into standardized T scores with a population mean of 50 and a standard deviation (SD) of 10 using the PROMIS scoring tables (Parent Proxy Version). Lower T scores for peer relationships indicate a worse outcome. No data imputation was performed; differences between CPP and non-CPP groups were assessed with univariate analysis of variance, adjusting for differences in demographic characteristics.
Results
A total of 142 respondents with children with CPP completed the survey; 21 boys (mean age 10.7 [0.2–18] years) and 121 girls (mean age 9.2 [0–18] years). Most respondents were white (81%) ().
Table 1. Demographics.
CPP diagnostic path
The mean age (range) when a child first reported symptoms of CPP was 5.5 (0.1–10.5) years. In 11 children (7%, 4 boys ages 9–9.3 years, 7 girls 8–10.5 years) symptom onset was reported at >8 years old for girls or >9 years old for boys. In 89/142 (63%) of cases, the responder took their child to the physician because of symptoms they had noticed; in 23/142 (16%) cases, an HCP noted symptoms before the parent, and in 11/142 (8%) cases the child brought the symptoms to their attention. On average, medical help was sought 7.5 months after the child experienced symptoms. The most common symptoms were development of pubic hair (91/142, 64%) or adult body odor (68/142, 48%). In girls, the predominant symptom was development of breast buds (77%) and in boys it was development of pubic hair (81%) ().
Table 2. Major symptoms experience prior to diagnosis.
The first HCP seen by 66% of children was a pediatrician; only 9% were first seen by a pediatric endocrinologist or an endocrinologist (8%). A total of 93 (66%) children were referred to a pediatric endocrinologist, with a mean (SE) time for referral of 143 (± 32.0) days. Following this referral, on average, children waited 59 (± 7.0) days to be seen.
The mean age (range) at diagnosis was 6.6 (0.3–11.3) years. Most children received their CPP diagnosis from a pediatric endocrinologist (93 [66%]) or endocrinologist (36 [25%]) based on bone age (118 [84%]), blood tests for hormone levels (109 [77%]), breast appearance in girls (86 [71%]), or enlargement of penis or testicles in boys (23 [57%]). Pubic hair (68 [48%]), rapid growth rate based on growth charts (57 [40%]), and risk of short adult height (25 [18%]) were also reported. Other testing included gonadotrophin-releasing hormone stimulation test (73 [51%]), magnetic resonance imaging or computed tomography scan of the head (62 [44%]), pelvic or adrenal ultrasound (38 [27%]), and genetic testing (3 [2%]). Further analysis revealed that MRI was not used for diagnosis of CPP among girls under 6 years old, 53.8% (21 of 39) and among all boys, 61.9% (13 of 21).
CPP treatment
The mean age (range) when starting treatment was 6.8 (0.8–11.5) for girls and 7.6 (2.0–10.9) for boys, with mean (SE) time to treatment after a child’s visit to the pediatric endocrinologist of 220 (± 40.0) days. Overall, mean (SE) time from first symptom to treatment was 1.7 (± 0.18) years.
At the time of the survey, 86 (61%) children were receiving treatment, 32 (22%) had received treatment in the past, and 21 (17%) had never received treatment. Forty-seven (40%) patients received only depot leuprolide acetate, 45 (38%) received only histrelin acetate and 15 (13%) received both treatments. The primary reason for using leuprolide was preference by the physician (22 [36%]) or not wanting the child to undergo a surgical procedure (11 [18%]), whereas the primary reason for using histrelin was so the child did not have to think about treatment all year (21 [35%]) or because the child was afraid of injections (12 [20%]).
CPP treatment evaluation
Pubertal suppression was assessed by bone age (n = 85, 72%), growth rate (n = 78, 66%) and changes in pubertal Tanner stagingCitation25 (n = 73, 62%). Changes in luteinizing hormone levels were most commonly measured once every 6 months (n = 39, 49%) or once every 3 months (n = 23, 29%).
Of patients ever treated (n = 110), the main treatment-related concerns were side effects (short term: n = 82, 75%; long term: n = 28, 26%) and impact on emotional/mental state (n = 16, 15%). Approximately one-third (31%) of children changed or switched their initial treatment, stating the treatment was not working well enough (n = 10, 27%), child was afraid of injections (n = 6, 16%), or HCP preference (n = 6, 16%).
Most children visited the physician managing their CPP once every 3 months (n = 50, 35%) or once every 6 months (n = 44, 31%).
Of the predominant symptoms that led to the parent to seek medical help, 16% (n = 76) reported much or very much improvement in the amount of pubic hair and 17% (n = 58) reported an improvement in body odor following initiation of therapy.
Impact on HRQoL
Responses from 142 parents/caregivers in the CPP group and 300 parents/caregivers in the non-CPP group were evaluated to assess the impact of CPP on HRQoL. The average age of the parent/caregiver and the employment status were similar in the CPP versus non-CPP group (). Most respondents were women (94% and 74%, respectively). Significant differences in demographic characteristics included age group at the time the survey was conducted, sex of the parent/caregiver, martial status, and household income ().
Unadjusted mean PedsQL scores are provided in . Based on the univariate analysis of variance, adjustments were made for differences in demographic characteristics (). Adjusted mean (±SE) PedsQL total (65.3 ± 1.8 vs 75.7 ± 1.2), Psychosocial Health Summary (62.4 ± 1.8 vs 73.4 ± 1.2), and Physical Health Summary (70.7 ± 2.2 vs 79.9 ± 1.5) scores demonstrated significantly lower (p < .01) HRQoL for the CPP group compared with the non-CPP group. PedsQL subscale scores ranged from 57.6 to 70.7 in the CPP group and from 69.8 to 79.9 in the non-CPP group; in both groups, the lowest scores were reported for emotional functioning, and the highest scores for physical functioning; all values were significantly lower (p < .01) for the CPP group than for the non-CPP group (). PedsQL scores did not differ between children currently receiving treatment and those treated in the past. These scores also did not differ for children with CPP who were never treated compared with those who received treatment.
Figure 1. Parent/caregiver-reported PedsQL scores. Differences between CPP and non-CPP groups were assessed using analysis of variance, adjusting for differences in demographic characteristics (age group at time survey was conducted, sex of the parent/caregiver, marital status, and household income). The asterisk (*) denotes p < .01 for difference in adjusted mean score between the CPP and non-CPP groups. aSeven children in the CPP group and 30 children in the non-CPP group (all ≤4 years of age) were excluded because they were not enrolled in school/daycare. Abbreviations. CI, Confidence interval; SE, Standard error.
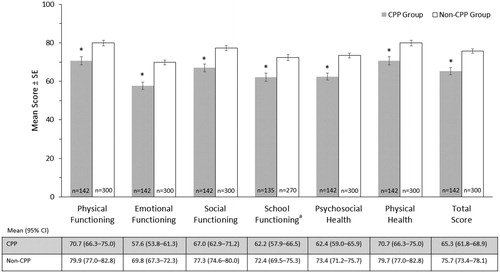
Table 3. Parent/Caregiver-reported PedsQL and PROMIS peer relationship T scores: unadjusted analysis.
The greatest impact was seen on emotional functioning where scores were significantly lower (p < .001) by at least 13 points in the CPP group versus non-CPP group for all five items. Compared with the non-CPP group, parents/caregivers in the CPP group reported that their child had more problems feeling afraid or scared, sad or blue, or angry, had more trouble sleeping and were more worried about what would happen to themselves. They also reported more teasing and trouble completing tasks or forgetting things. Children with CPP reported more hurts and aches, lower energy level, and less participation in sports.
The adjusted PROMIS peer relationship T score (±SE) was lower in the CPP group compared with the non-CPP group (45.4 ± 1.0 vs 47.4 ± 0.7, p = .11, unadjusted T scores are provided in ). Fewer parents/caregivers in the CPP group (range: 55–62%) responded ‘often’ and ‘almost always’ to statements describing positive interactions of their child with other children compared with those in the non-CPP group (range: 73–78%). For example, 76% of parents in non-CPP group reported that their child was good at making friends versus 57% in the CPP group (). More parents in the non-CPP versus the CPP group reported that other children wanted to talk to their child (76% vs 56%) and be their child’s friend (74% vs 55%).
Table 4. Parent/caregiver responses for the individual items of the PROMIS peer relationship instrument.
Significantly more parents/caregivers in the CPP group versus non-CPP group reported that their child received a diagnosis of migraines (8.5% vs 1.3%, p < .001; ). Although not statistically significant, parents/caregivers reported that 13.4% of children with CPP received a diagnosis of anxiety since the diagnosis of CPP was made, compared with 11.0% in the non-CPP group.
Table 5. Comorbid conditions.
Discussion
This study, the first of its kind to be conducted in a relatively large sample size among children with CPP in the US, sought to characterize the patient journey from the onset of CPP symptoms to diagnosis, and evaluated the psychosocial burden and HRQoL in these children and their parents/caregivers. The online survey design provides much-needed insights into the real-world experience of CPP and natural history of CPP (including symptoms, diagnosis, and treatment).
Results from this study demonstrate that on average across the US, the time from symptom onset to treatment is almost 2 years. Survey responses also demonstrated that some children, although diagnosed with CPP, never received treatment. An unexpected finding was that only 18% of caregivers were concerned about short adult height. It is also interesting that 11 children with early, but normal onset puberty, were treated for concerns that puberty was precocious. Caution is needed in interpreting this result, since it is based on caregiver recall. This highlights another need for the development of additional educational resources related to the challenge of distinguishing CPP from early normal variant pubertyCitation2. The average age for onset of puberty is decreasing for both girls and boysCitation26, with socio-economic conditions influencing age at onset as well as large variations between ethnic groups, contributing to the challenge of distinguishing between precocious and normal pubertyCitation27.
Delays introduced by parents/caregivers between symptom onset and visiting an HCP may also be markedly reduced by more widespread education highlighting that although rare, the symptoms of this disease should not be ignored. This study also demonstrates an even split between two of the most used treatment options to date, leuprolide acetate and histrelin acetate. This emphasizes the need for individualized treatment options, as physicians, parents, and patient concerns are not all similar.
The current standard of care for the evaluation of CPP includes at minimum: measurement of hormone levels, bone age x-rays, documentation of breasts in girls, or increases in testicular size in boys. Interestingly, this study based on caregiver recall, did not demonstrate that all those things were assessed. The fact that these measures were not performed may be partially a result of weakness in recall, but also reflects different provider practices. Performance of magnetic resonance imaging scan of the head is still debated in children older than 6 years of age, and is a measure that caregivers are more likely to remember; therefore, it is relevant that not quite half of the children had a magnetic resonance imaging scan done. These findings highlight a considerable variability in practice across the US and further emphasizes the need for physician and caregiver education.
Because CPP is a rare disease, the number of published studies examining CPP is limited and most studies have small sample sizesCitation7,Citation9,Citation28–35. However, a longitudinal study performed by Mensah et al.Citation35 examined psychosocial adjustment in a large sample of Australian children using PedsQL. The Mensah study found that children who experienced early puberty (by 8–9 years) had poorer Psychosocial Health Summary scores across childhood than those who did not. The lowest PedsQL sub-scores in that study were reported for emotional functioning, although lower scores were also reported for all other PedsQL sub-scoresCitation35.
Our results are consistent with those in the Mensah studyCitation35 and show that children with CPP have more unfavorable PedsQL summary scores than children without CPP. The impact compared with children without CPP was greater on psychosocial health than on physical health with the biggest difference seen in emotional functioning and included problems of feeling afraid or scared, sad or blue, and angry as well as trouble sleeping and being worried about what would happen to themselves. It is understandable that children going for frequent medical appointments would wonder more about their health. Peer relationships may also contribute to their emotional functioning. They reported more teasing, which may be related to tall stature and early physical changes. The report of increased trouble completing tasks or forgetting things is not easy to understand, and further study is needed. Children with CPP reported more hurts and aches, which is understandable since they were undergoing injections and blood tests. Lower energy level and less participation in sports is not expected as found, unless it is related to the emotional issues described.
Children treated for CPP did not have better scores than those children with CPP who did not receive treatment. However, several of the weaknesses of this study may have influenced that result. The age of onset of puberty and specifics of treatment are based on parent recall and in some cases over many years. Additionally, the study was not designed to compare results before and after treatment, which is an important area of interest for future study. Also, some children had earlier onset of normal puberty and the rate of progression of puberty is not defined. There were not enough children between 8 and 10 years of age at reported onset of pubertal symptoms to compare their results to the children with true CPP.
Peer relationships play an important role in social developmentCitation36–38. The PROMIS peer relationship scores (a measure of social development) for the CPP group were below those of the non-CPP group and were also below the population mean of 50Citation39,Citation40. Parent/caregiver responses indicated that children with CPP have more problems being accepted by other children their age and making friends. Thus, results using two separate instruments demonstrate a significant negative effect of CPP on psychosocial health in children with CPP.
Further study is needed to determine whether treatment improves psychosocial outcomes. In the meantime, these results emphasize the need for appropriate medical treatment to ease the burden for children suffering from CPP.
There are several limitations to this study which should be noted. The length of the survey might have contributed to the quality of parents’ responses to the survey questions. Information from respondents who answered ‘never received treatment’ was not captured and would have provided useful information on why treatment was not initiated. Furthermore, diagnosis was self-reported and not clinically verified. Recall bias, a limitation common to studies of this nature, and sample selection bias may have been introduced. Because responses were collected from parents who are members of the MAGIC Foundation, they may not be completely generalizable to the entire CPP population. However, one would suspect that parents involved in this group would be more attentive and thus the true delay in onset of treatment may be even greater. Bias due to unobservable covariates cannot be completely ruled out. In addition, the data rely on the opinions of the caregivers. Another limitation is that the study included both children with CPP and a few children with early puberty who were treated (8–10-year-old girls). The nature of the survey makes it impossible to know whether the children actually had the onset of puberty prior to age 8 years, whether the parents did not recall, or whether there was a reason a physician chose to treat someone in that early normal pubertal age range. Finally, this analysis was performed on a population that included patients who were currently being treated, those who had been treated in the past but were not currently being treated, as well as those who never received any treatment. Further study is needed as we would expect appropriate treatment to improve HRQoL. Awareness of these issues highlights the importance of further study regarding the relationship of treatment to changes in HRQoL.
Conclusions
Findings from this caregiver survey suggest that there exists, on average, a marked delay between CPP symptom onset, referral to an endocrinologist, and ultimately treatment for this rare disease. Furthermore, children with CPP experience a substantial disease burden with a significant negative impact on HRQoL including emotional, social, and physical functioning compared with children without CPP. Minimizing the time from onset of symptoms to treatment should have a positive impact on HRQoL in both patients and parents/caregivers, as well as treatment outcomes.
Transparency
Declaration of funding
All funding for this study was provided by AbbVie. AbbVie personnel participated in the analysis and interpretation of data, drafting, reviewing, and approving the publication. All authors contributed to the development of the publication and maintained control over the final content.
Declaration of financial/other interests
AMS and EG are AbbVie Inc. employees and may own AbbVie Inc. stocks/stock options. PN has no competing interests relevant to this manuscript. KOK has been a consultant for AbbVie Inc. but was not paid for any part of this manuscript preparation. AbbVie personnel participated in the analysis and interpretation of data, drafting, reviewing, and approving the publication. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved the submission.
Acknowledgements
The authors would like to thank the MAGIC Foundation for their participation in the study. Medical writing assistance was provided by Fiona Woodward, PhD, and Joann Hettasch, PhD, of JK Associates, Inc (a member of the Fishawack Group of Companies). These services were paid for by AbbVie.
References
- National Organization for Rare Disorders (NORD) [Internet]. [cited from 2018 Aug 14] Available from: https://rarediseases.org/rare-diseases/precocious-puberty/
- Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4(3):265–274.
- Partsch CJ, Sippell WG. Treatment of central precocious puberty. Best Pract Res Clin Endocrinol Metab. 2002;16(1):165–189.
- Mendle J, Harden KP, Brooks-Gunn J, et al. Development’s tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Dev Psychol. 2010;46(5):1341–1353.
- Graber JA, Lewinsohn PM, Seeley JR, et al. Is psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry. 1997;36(12):1768–1776.
- Ge X, Conger RD, Elder GH. Jr. Coming of age too early: pubertal influences on girls’ vulnerability to psychological distress. Child Dev. 1996;67(6):3386–3400.
- Sonis WA, Comite F, Blue J, et al. Behavior problems and social competence in girls with true precocious puberty. J Pediatr. 1985;106(1):156–160.
- Carel JC, Eugster EA, Rogol A, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123(4):e752–62.
- Wojniusz S, Callens N, Sutterlin S, et al. Cognitive, emotional, and psychosocial functioning of girls treated with pharmacological puberty blockage for idiopathic central precocious puberty. Front Psychol. 2016;7:1053.
- Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Dev Rev. 2007;27(2):151–171.
- Williams VSL, Soliman AM, Barrett AM, et al. Review and evaluation of patient-centered psychosocial assessments for children with central precocious puberty or early puberty. J Pediatr Endocrinol Metab. 2018;31(5):485–495.
- O'Sullivan E, O'Sullivan M. Precocious puberty: a parent’s perspective. Arch Dis Child. 2002;86(5):320–321.
- Klein KO, Barnes KM, Jones JV, et al. Increased final height in precocious puberty after long-term treatment with LHRH agonists: the National Institutes of Health experience. J Clin Endocrinol Metab. 2001;86(10):4711–4716.
- Neely EK, Crossen SS. Precocious puberty. Curr Opin Obstet Gynecol. 2014;26(5):332–338.
- Chen M, Eugster EA. Central precocious puberty: update on diagnosis and treatment. Pediatr Drugs. 2015;17(4):273–281.
- Kletter GB, Klein KO, Wong YY. A pediatrician’s guide to central precocious puberty. Clin Pediatr (Phila). 2015;54(5):414–424.
- Mayo Clinic Staff [Internet]. Precocious puberty: symptoms and causes. [cited 2019 Sep 26]. Available from: https://www.mayoclinic.org/diseases-conditions/precocious-puberty/symptoms-causes/syc-20351811
- Carel JC, Leger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358(22):2366–2377.
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–139.
- Varni JW, Burwinkle TM, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341.
- Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5(1):43.
- Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5(1):2.
- Dewalt DA, Thissen D, Stucky BD, et al. PROMIS Pediatric Peer Relationships Scale: development of a peer relationships item bank as part of social health measurement. Health Psychol. 2013;32(10):1093–1103.
- Varni JW, Thissen D, Stucky BD, et al. PROMIS® Parent Proxy Report Scales: an item response theory analysis of the parent proxy report item banks. Qual Life Res. 2012;21(7):1223–1240.
- Tanner JM, Weiner JS. The reliability of the photogrammetric method of anthropometry, with a description of a miniature camera technique. Am J Phys Anthropol. 1949;7(2):145–186.
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–693.
- Kelly Y, Zilanawala A, Sacker A, et al. Early puberty in 11-year-old girls: Millennium Cohort Study findings. Arch Dis Child. 2017;102(3):232–237.
- Sontag-Padilla LM, Dorn LD, Tissot A, et al. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Dev Psychopathol. 2012;24(1):211–223.
- Schoelwer MJ, Donahue KL, Bryk K, et al. Psychological assessment of mothers and their daughters at the time of diagnosis of precocious puberty. Int J Pediatr Endocrinol. 2015;2015(1):5.
- Kim YJ, Lee HS, Lee YJ, et al. Multicenter clinical trial of leuprolide acetate depot (Luphere depot 3.75 mg) for efficacy and safety in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2013;18(4):173–178.
- Baumann DA, Landolt MA, Wetterwald R, et al. Psychological evaluation of young women after medical treatment for central precocious puberty. Horm Res. 2001;56(1–2):45–50.
- Xhrouet-Heinrichs D, Lagrou K, Heinrichs C, et al. Longitudinal study of behavioral and affective patterns in girls with central precocious puberty during long-acting triptorelin therapy. Acta Paediatr. 2008;86(8):808–815.
- Kim EY, Lee MI. Psychosocial aspects in girls with idiopathic precocious puberty. Psychiatry Investig. 2012;9(1):25–28.
- Pomerantz H, Parent J, Forehand R, et al. Pubertal timing and youth internalizing psychopathology: the role of relational aggression. J Child Fam Stud. 2017;26(2):416–423.
- Mensah FK, Bayer JK, Wake M, et al. Early puberty and childhood social and behavioral adjustment. J Adolesc Health. 2013;53(1):118–124.
- Dirks MA, Treat TA, Weersing VR. Integrating theoretical, measurement, and intervention models of youth social competence. Clin Psychol Rev. 2007;27(3):327–347.
- Gifford-Smith ME, Brownell CA. Childhood peer relationships: social acceptance, friendships, and peer networks. J Sch Psychol. 2003;41(4):235–284.
- Hartup WW. Social relationships and their developmental significance. Am Psychol. 1989;44:122–126.
- About HealthMeasures Scores [Internet]. [cited 2018 Oct 15]. Available from: http://www.healthmeasures.net/score-and-interpret/about-healthmeasures-scores
- PROMIS Peer Relationship Scoring Manual [Internet]. [cited 2019 Dec 3]. Available from: http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Peer_Relationships_Scoring_Manual.pdf
