Abstract
Objective
Atrial fibrillation (AF) is present in up to 17% of patients in skilled nursing facilities (SNFs). This study compared healthcare resource utilization (HRU) and costs between AF patients initiating rivaroxaban or warfarin in SNFs.
Methods
Using de-identified claims from Optum Clinformatics Extended Data Mart (1 January 2013 to 31 December 2017), this retrospective cohort study indexed AF patients with first SNF admission during which rivaroxaban or warfarin was initiated within 3 days of admission. To adjust for selection bias, inverse probability of treatment weighting (IPTW) was applied for baseline characteristics. Logistic regression and generalized linear models were used to compare HRU and costs.
Results
519 rivaroxaban and 1129 warfarin patients met inclusion criteria. After IPTW, the cohorts were well balanced for baseline characteristics. The average length of index SNF stay was 32.07 and 37.44 days for rivaroxaban and warfarin patients, respectively. During SNF stay, rivaroxaban patients had 27% lower odds of hospitalization (p < .0001), 2.7 fewer international normalized ratio (INR) tests per-patient-per-month (PPPM; p < .001), and 2.3 fewer pathology/laboratory encounters PPPM (p < .0001) than warfarin patients. All-cause healthcare costs were $2638 lower with rivaroxaban versus warfarin (p < .0001) during the index SNF stay, with lower medical costs (p < .0001) but higher pharmacy costs (p < .0001). Total all-cause healthcare costs 100 days post-index SNF were $8746 lower with rivaroxaban versus warfarin (p < .0001).
Conclusions
In the SNF setting, AF patients treated with rivaroxaban had 5-day shorter length of stay, lower HRU, and lower all-cause total and medical costs compared to warfarin, despite higher treatment costs. These findings may help inform clinical decision-making to reduce economic burden.
Introduction
Atrial fibrillation (AF) affects approximately 5 million adults in the United States (US), most of whom are elderlyCitation1–3, and the prevalence of AF is expected to increase nearly 3-fold over the next 30 yearsCitation2. AF is associated with substantial healthcare resource utilization and costs, with more than 750,000 hospitalizations related to AF each year and an annual cost of $6 billion in the USCitation2,Citation4,Citation5. Nonvalvular atrial fibrillation (NVAF) affects more than 70% of people with AF and is associated with a 5-fold increased risk of strokeCitation6. Approximately 15% of all strokes occur in patients with existing AFCitation7. Elderly patients with NVAF are often hospitalized and may subsequently be discharged to a skilled nursing facility (SNF)Citation1,Citation8. Given the association between AF and advanced age, it is a common condition among patients in SNFs, with AF prevalence ranging from 7.5% to 17% in this populationCitation1.
Oral anticoagulants are standard of care for stroke prevention in patients with NVAF and a CHA2DS2-VASc score ≥2 in men and ≥3 in women, and direct-acting oral anticoagulants (DOACs) are now recommended over warfarin in the latest American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) guideline updateCitation9. Rivaroxaban, a DOAC, inhibits factor Xa and is an alternative to warfarin for patients with NVAF. The ROCKET-AF trial demonstrated a reduced number of strokes and strokes leading to poor outcomes in NVAF patients treated with rivaroxaban compared with warfarinCitation10. Stroke or systemic embolism occurred in 1.7% and 2.2% per year in the rivaroxaban and warfarin cohorts, respectively (p < .001), and rates of major bleeding were similar between treatment groups, but rivaroxaban was associated with a significantly lower rate of intracranial hemorrhage compared with warfarin (0.5% vs 0.7%, p = .02). In addition, analyses of real-world evidence support the effectiveness and safety of rivaroxaban for stroke prevention in NVAF patientsCitation11. These observational studies included patients from primary care settings, community hospitals, and insurance claims databases along with patient registries. However, limited information exists on anticoagulation treatment and associated healthcare resource utilization and the cost of managing NVAF patients in acute care facilities, such as SNFs. The objective of this observational study was to compare healthcare resource utilization and costs for NVAF patients initiating rivaroxaban or warfarin in SNF settings.
Methods
Study design
This retrospective cohort study used de-identified claims from Optum Clinformatics Extended Data Mart Date of Death Database (Eden Prairie, MN, USA) between 1 January 2013 and 31 December 2017 (Supplementary Figure 1). This is an adjudicated administrative health claims database in the US for members of private health insurance, including commercial plans, administrative services only, Legacy Medicare Choice Lives (before January 2006), and Medicare Advantage (starting January 2006). The population is primarily representative of commercial claims patients ranging in age from 0 to 65 years, with some Medicare patients aged 65 years and older; however, the ages are capped at 90 years. The database includes administrative claims from inpatient and other medical services (e.g. outpatient services), prescriptions as dispensed, and outpatient laboratory tests processed by large national laboratory vendors who participate in data exchange with Optum.
Study population
Adult patients were indexed on the first SNF admission during which treatment-naïve AF patients were prescribed rivaroxaban or warfarin within 3 days of the SNF admission (Supplementary Figure 1). The 3-day requirement was intended to capture anticoagulant treatment initiation associated with a new admission to SNF in AF patients and to avoid additional confounders by extending the time from SNF admission. AF was identified based on diagnostic codes (International Classification of Diseases, Ninth Revision [ICD-9]: 427.31; International Classification of Diseases, Tenth Revision [ICD-10]: I48.0, I48.2, I48.91). Patients had continuous health plan enrollment from 6 months prior to the index SNF admission through discharge, death, or end of the study period, which has been shown in prior studies to be a sufficient time period to collect baseline demographic information prior to the index date (i.e. date of SNF admission in this analysis). Individual SNF claims with a maximum 8-day gap were consolidated into 1 stay. Patients were excluded if they had evidence of valvular heart disease, mitral stenosis, or organ or tissue transplant at any time prior to the index SNF admission. Patients who received any oral anticoagulant during the 6 months prior to SNF admission were also excluded.
Variables
Healthcare resource utilization was measured as the frequency and proportion of patients with ≥1 event and the mean (standard deviation [SD]) number of events for all-cause inpatient hospitalization, all-cause emergency room (ER) visit, international normalized ratio (INR) test (identified by Current Procedural Terminology [CPT] code: 85610), and overall pathology and laboratory encounter utilization (CPT codes: 80047-89398). All-cause healthcare costs (mean [SD]) for the index SNF stay were estimated for inpatient, ER, physician visit, other medical services (i.e. laboratory tests, outpatient visits), index SNF stay, and pharmacy costs and were stratified by medical costs and pharmacy costs. All costs are inflation-adjusted to 2017 US dollars based on the Optum Cost Inflation Multiplier. The number of events and all-cause healthcare costs were also reported on a per-patient-per-month (PPPM) basis to adjust for varying lengths of the index stay and follow-up.
Statistical methods
Descriptive analyses were used to report patient characteristics. Comparisons between cohorts were made using a Student t-test or Wilcoxon rank test for continuous variables and chi-square or McNemar’s test for categorical variables. Inverse probability of treatment weighting (IPTW) was used to account for differences in baseline demographic and clinical characteristics among patients initiating rivaroxaban or warfarinCitation12. IPTW is defined as the inverse of the estimated propensity socre for treated patients and the inverse of one minus the estimated propensity score for control patientsCitation13. Those receiving rivaroxaban were weighted up to account for the many patients like them who received treatment, while those receiving warfarin were weighted down because they are essentially overrepresented in the data. A pseudopopulation is created where the weighted treatment and control groups are representative of the patient characteristics in the overall population, resulting in estimates that are generalizable to the entire population. Baseline variables that remained unbalanced by treatment cohort after IPTW were included as covariates in the multivariable logistic regression and generalized linear models that were used to compare healthcare resource utilization and costs during the index SNF stay. These models also adjusted for varying lengths of follow-up time.
Sensitivity analyses examined the 30-day and 100-day post-index SNF admission period. During each post-index SNF admission period, the frequency and proportion of patients with ≥1 hospitalization and ER visit were reported. Per-patient-per-30-day (PPP30D) and per-patient-per-100-day (PPP100D) mean (SD) number of events, including inpatient hospitalization, ER visit, physician visit, other medical services, and pharmacy fills were determined. Mean (SD) PPP30D and PPP100D total all-cause healthcare costs were estimated as total healthcare costs (total medical + total pharmacy), total medical costs and medical costs for each type of event, and total pharmacy costs. All analyses used SAS Enterprise Guide, version 7.1 (SAS Institute, Cary, NC, USA).
Results
A total of 519 rivaroxaban and 1129 warfarin patients initiating treatment met the inclusion criteria (Supplementary Figure 2). The unadjusted warfarin cohort was sicker than the rivaroxaban cohort based on higher mean comorbidity and risk scores (), highlighting the need to adjust the populations. After IPTW adjustment, the 2 cohorts were well balanced for demographics, baseline clinical characteristics, and comorbid conditions, with a standardized difference <10% (). There was a slight imbalance in mean age in the IPTW adjusted rivaroxaban versus warfarin cohorts (78.0 vs 77.1 years; standardized difference, 10.4%).
Table 1. Demographic and baseline clinical characteristics.
Healthcare resource utilization and costs
The average length of stay in SNF was 5.36 days (95% confidence interval [CI]: 4.98, 5.73) shorter for rivaroxaban-treated relative to warfarin-treated patients (). Patients initiating rivaroxaban during their SNF stay had significantly fewer hospitalizations, INR tests (as expected), and pathology/laboratory encounters than those initiating warfarin (). Interestingly, 7.3% of patients treated with rivaroxaban were tested for INR, which is not meaningful for these patients, and pathology/laboratory encounters were approximately 20% lower with rivaroxaban versus warfarin treatment. ER visits were similar between treatment cohorts. The mean number of events PPPM showed a reduction of more than 2 INR tests or other pathology/lab tests per month with rivaroxaban versus warfarin ().
Table 2. Healthcare resource utilization during the index skilled nursing facility stay.
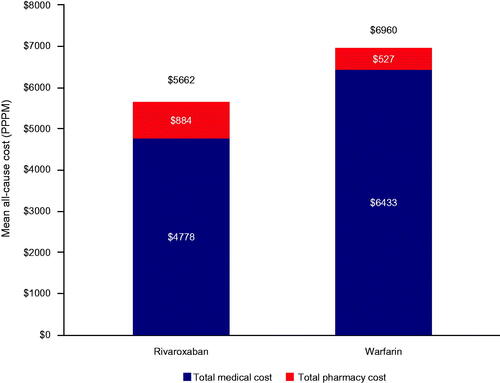
All-cause total costs during the index SNF stay were significantly reduced with rivaroxaban ($6054 [$9027]) versus warfarin ($8685 [$16,581]), with a mean difference of –$2638 (95% CI: –$3277, –$1924). This difference was a result of shorter length of stay and attributed to lower all-cause medical costs despite higher all-cause pharmacy costs. PPPM all-cause total costs were also significantly reduced with rivaroxaban versus warfarin, with a difference of –$1303 (95% CI: –$1752, –$814; ).
Figure 1. Total all-cause PPPM costs during the index skilled nursing facility stay. All differences between rivaroxaban and warfarin costs were significant with p < .0001.
Results for all-cause healthcare resource utilization over 30 days and 100 days post-index SNF admission period support the primary analysis findings. Significant reductions in hospitalizations, physician visits, and other medical services PPP30D and PPP100D were observed for rivaroxaban versus warfarin (). Total all-cause healthcare costs over 30 days and 100 days post-index period were significantly reduced with rivaroxaban compared with warfarin, with mean differences that were –$2396 lower (95% CI: –$3179, –$1529) at 30 days and $8746 lower (95% CI: –$10,591, –$6706) at 100 days (). Reductions in total medical costs PPP30D with rivaroxaban were primarily driven by the costs of other medical services; while reductions in PPP100D costs were driven by costs of hospitalizations and other medical services.
Table 3. Healthcare resource utilization and costs during the 30-day and 100-day post-index periods.
Discussion
Results of this study indicate that NVAF patients initiating rivaroxaban in the SNF setting have lower healthcare resource utilization and costs than those initiating warfarin. Significant reductions in hospitalizations, INR testing, and pathology/laboratory encounters were responsible for the reductions in medical costs. Pharmacy costs were higher with rivaroxaban compared with warfarin, but these costs only accounted for approximately 20% of the difference in medical costs PPPM, resulting in lower total costs for rivaroxaban.
The reduction in INR testing and other pathology/laboratory encounters observed with rivaroxaban is consistent with the lack of required routine blood monitoring to determine its anticoagulant effect. Despite this, we identified that 7.3% of rivaroxaban-treated patients were tested for INR during the SNF stay. INR testing is not needed for patients receiving rivaroxaban, so this testing translates to an extra nursing time to collect patient samples and a waste of resources that increase the cost of SNF care. In contrast, warfarin therapy requires close INR monitoring to maintain optimal coagulation levelsCitation14–16.
Many factors associated with an aging population can affect the achievement of target INR, including interactions with other commonly used drugs, such as selective-serotonin reuptake inhibitors, lipid-lowering agents, gastrointestinal drugs, and analgesics, as well as food and alcoholCitation14–16. In a survey administered in the long-term care setting, nurses reported an average of 5.9 h per patient initiating warfarin therapy, including initiation procedures, monitoring, and management. Approximately 41% of this time was spent on initiation procedures, including INR and other lab procedures, dietary consultation for food–drug interactions, drug-drug interactions review, and patient education. Among stable warfarin patients, 2.4 h per patient was reported to monitor and manage their warfarin therapyCitation15. Nursing shortages pose a threat to optimal anticoagulant management. In addition, several reports in long-term care populations indicate that the percentage of patients in the therapeutic range for warfarin is lowCitation14,Citation17. A chart review of 105 residents in long-term care facilities with over 3000 INR values found that time in the therapeutic range was 54%Citation17. In a cohort study of 25 nursing homes with 490 residents receiving warfarin, only 50% of patients were in therapeutic INR rangeCitation14.
Additionally, studies have assessed the use of warfarin in long-term care facilitiesCitation8,Citation18–20. Despite a high risk of stroke in AF patients in long-term care databases, only half of warfarin candidates received anticoagulant prophylaxisCitation18. In a systematic literature review of 22 studies, AF was the most common indication for warfarin in long-term care, but use was low in 5 studies, ranging from 17% to 57%Citation19. Using the 2004 National Nursing Home Survey, 54% of residents with AF and no contraindications to warfarin use were not receiving anticoagulation with warfarin and/or antiplatelet agentsCitation20. An analysis of hospitalized frail elderly patients identified AF prevalence to be 47%Citation8. Among these AF patients, 63% were prescribed an anticoagulant, but of those not prescribed an anticoagulant, only 56% had a documented contraindication to its use. Outcomes during 12 months of follow-up found significantly more events of ischemic stroke and/or bleeding among AF patients without anticoagulation.
Taken together, these results suggest that the challenges associated with warfarin use may prevent optimal anticoagulation and outweigh the benefits of stroke risk reduction in the long-term care setting. Although randomized, controlled trials of anticoagulation for AF patients have not been conducted in the SNF population, other types of data have emerged and offer evidence to inform decisionsCitation21. A recent systematic review indicated that DOACs reduce hemorrhagic strokes and intracranial bleeding more effectively than warfarin in older patients with NVAF (≥65 years of age) and should be considered particularly for those who have labile INRs or spend less than 55% of time in the therapeutic range on warfarinCitation22. The availability of data, particularly in elderly individuals with AF, that demonstrate the efficacy, safety, and cost-effectiveness of DOACs, such as rivaroxaban, may lead to increased adoption of these agents for stroke prevention. Results of the ORBIT-AF II study indicate that physicians are initiating treatment with DOACs more often than warfarin for treatment of outpatients with AFCitation23. Based on comparative trials of DOACs versus warfarin that consistently demonstrate at least noninferiority for the combined endpoint of stroke or systemic embolism and superior safety, the 2019 focused update of the AHA/ACC/HRS guidelines recommends first-line use of DOACs in eligible AF patientsCitation9.
Results of this observational analysis using real-world AF patients provides important information about healthcare resource utilization and costs related to thromboprophylaxis in the SNF setting. Approximately 80% of the total cost of care for the first 30 days in these AF patients occurred in the SNF. The potential for reducing resources and costs should be evaluated in the SNF setting, particularly with regard to inefficiencies observed in this study related to INR testing and pathology/laboratory encounters. The Centers for Medicare and Medicaid Services (CMS) implemented the SNF value-based purchasing program that links Medicare Part A payments to the hospital readmission rate of a SNFCitation24. Of nearly 15,000 SNFs subject to the CMS program, 73% received a penalty for poor 30-day readmission rates, and the average risk-standardized readmission rate increased following implementation of the program, which has been attributed to pressure from providers to shorten the length of stayCitation25. In the current analysis, healthcare resource utilization and costs remained significantly lower with rivaroxaban versus warfarin in the 100-day period after index SNF admission and reductions were related to differences in hospitalizations and other medical costs.
The design of this study included geographically diverse US claims data such that results are generalizable to both commercially insured and Medicare Advantage beneficiaries. The retrospective nature of this study provides the ability to look backward and forward from a given point in time without having to actively follow patients over time as in a prospective study. Continuous health plan enrollment enables researchers to better understand the population characteristics and longitudinally evaluate the outcomes during follow-up without interruption. The use of IPTW increased the precision of estimated treatment effects by creating a pseudopopulation that limits an association between confounders and treatmentCitation13. Stabilized weights do not increase or decrease bias but increase precision in the estimates by reducing variance of the weights. However, it is possible that potential confounders were not measured, thereby leading to residual bias. In addition, claims data are limited by potential coding errors and incomplete data, particularly related to costs, which are standardized and not representative of the actual paid amounts. Out-of-pocket expenses were not captured and may have underestimated the length of SNF stay in this population. Disease coding may reflect financial incentives used for reimbursement rather than clinical and systematically verified definitions. Prescriptions were based on those filled, not prescribed; thus, the number of unfilled prescriptions cannot be determined, and a filled prescription does not indicate that the medication was taken as prescribed. Outpatient laboratory results were only available for a small proportion of the populations whose tests were processed by the large national laboratory vendors who participate in data exchange with Optum and were not included in this study.
Conclusions
In the SNF setting, NVAF patients who initiated treatment with rivaroxaban had lower healthcare resource utilization and costs compared to those initiating warfarin during both the SNF stay and follow-up. These findings may help inform clinical decision-making regarding the choice of anticoagulation in acute care facilities to reduce healthcare resource utilization and corresponding cost of patient care.
Transparency
Declaration of funding
This study was funded by Janssen Scientific Affairs, LLC (Titusville, NJ, USA). The sponsor was involved in the study design, the collection and analysis of data, and the drafting of the manuscript.
Declaration of financial/other relationships
VSM and D. Mahajan have nothing to disclose. BW, JS, D. Milentijevic, and VA are full-time employees of Janssen Scientific Affairs, LLC. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors participated in the design and analytical approach of the study and contributed to the manuscript development. Results were summarized and interpreted in collaboration with all authors. Authors vouch for the accuracy and completeness of the data reported and the adherence of the study to the protocol. All authors gave final approval of the version to be published.
Mittal_appendix.docx
Download MS Word (114.9 KB)Acknowledgements
Medical writing support was provided by Michelle McDermott, PharmD, of MedErgy (Yardley, PA, USA), and was funded by Janssen Scientific Affairs, LLC.
Data availability statement
The data that support the findings of this study are available from Optum but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Optum.
References
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc. 2012;13(8):688–691.
- Morin DP, Bernard ML, Madias C, et al. The state of the art: atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin Proc. 2016;91(12):1778–1810.
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147.
- Centers for Disease Control and Prevention [Internet]. Atrial fibrillation fact sheet. 2017 [cited 2019 Jan 23]. Available from: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm.
- Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639–654.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988.
- National Stroke Association [Internet]. AFib-stroke connection. 2018 [cited 2019 Feb 25]. Available from: https://www.stroke.org/understand-stroke/preventing-a-stroke/afib-stroke-connection/.
- Ekerstad N, Karlsson T, Soderqvist S, et al. Hospitalized frail elderly patients – atrial fibrillation, anticoagulation and 12 months’ outcomes. Clin Interv Aging. 2018;13:749–756.
- January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132.
- Patel MR, Mahaffey KW, the ROCKET AF Steering Committee, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Beyer-Westendorf J, Camm AJ, Coleman CI, et al. Rivaroxaban real-world evidence: validating safety and effectiveness in clinical practice. Thromb Haemost. 2016;116(suppl 2):S13–S23.
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statist Med. 2015;34(28):3661–3679.
- Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–611.
- Gurwitz JH, Field TS, Radford MJ, et al. The safety of warfarin therapy in the nursing home setting. Am J Med. 2007;120(6):539–544.
- Patel AA, Nelson WW. Nurses’ self-reported time estimation of anticoagulation therapy: a survey of warfarin management in long-term care. BMC Nurs. 2015;14(1):8.
- Reardon G, Patel AA, Nelson WW, et al. Usage of medications with high potential to interact with warfarin among atrial fibrillation residents in long-term care facilities. Expert Opin Pharmacother. 2013;14(2):165–173.
- Verhovsek M, Motlagh B, Crowther MA, et al. Quality of anticoagulation and use of warfarin-interacting medications in long-term care: a chart review. BMC Geriatr. 2008;8(1):13.
- Reardon G, Nelson WW, Patel AA, et al. Warfarin for prevention of thrombosis among long-term care residents with atrial fibrillation: evidence of continuing low use despite consideration of stroke and bleeding risk. Drugs Aging. 2013;30(6):417–428.
- Neidecker M, Patel AA, Nelson WW, et al. Use of warfarin in long-term care: a systematic review. BMC Geriatr. 2012;12(1):14.
- Ghaswalla PK, Harpe SE, Slattum PW. Warfarin use in nursing home residents: results from the 2004 national nursing home survey. Am J Geriatr Pharmacother. 2012;10(1):25–36 e22.
- Alcusky M, Lapane KL. Treatment of atrial fibrillation in nursing homes: a place for direct acting oral anticoagulants? J Nurs Home Res Sci. 2018;4:15–19.
- Sommerauer C, Schlender L, Krause M, et al. Effectiveness and safety of vitamin K antagonists and new anticoagulants in the prevention of thromboembolism in atrial fibrillation in older adults – a systematic review of reviews and the development of recommendations to reduce inappropriate prescribing. BMC Geriatr. 2017;17(S1):223.
- Steinberg BA, Shrader P, Thomas L, et al. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II). Am Heart J. 2017;189:40–47.
- American Health Care Association [Internet]. SNF value-based purchasing (SNF VBP). 2017 [cited 2019 Apr 2]. Available from: https://www.ahcancal.org/facility_operations/Pages/SNF-Value-Based-Purchasing.aspx.
- Castellucci M [Internet]. Most skilled-nursing facilities penalized by CMS for readmission rates. 2018 [cited 2019 Apr 2]; Available from: https://www.modernhealthcare.com/article/20181128/NEWS/181129930/most-skilled-nursing-facilities-penalized-by-cms-for-readmission-rates.
