Abstract
Objective
To assess patient-reported outcomes after two years of use of dual oral anti-diabetes drug (OAD) therapy in elderly people (≥65 years) with type 2 diabetes mellitus (T2DM) from Italy under real-life settings.
Methods
3-AGE was a prospective, non-interventional study in elderly people with T2DM inadequately controlled on metformin monotherapy (defined as glycated hemoglobin [HbA1c] 7.0–9.0%), in whom a second OAD was prescribed. Primary endpoint was to assess the physical and psychological symptoms associated with T2DM from baseline to 24 months using the Diabetes Symptom Check List revised (DSC-R) questionnaire. Patient’s quality of life and health status, treatment satisfaction, consumption of healthcare resources, and physician satisfaction with treatment were also assessed (secondary endpoints) using validated questionnaires. Additionally, safety and clinical characteristics were also evaluated.
Results
The mean age of the study population (N = 860) was 71.5 ± 5.2 years. Addition of a second OAD significantly (p < .0001) reduced the DSC-R score from baseline (0.73 ± 0.68) to both Months 12 and 24 (0.63 ± 0.59 and 0.61 ± 0.56), and HbA1c from baseline (7.72% ± 0.54%) to Month 12 (6.95% ± 0.82%). Adding a second OAD improved quality of life and health status (baseline, 71.31 ± 15.16 to Month 12, 74.49 ± 13.64; p < .0001), patient’s treatment satisfaction (p < .0001), and consumption of healthcare resources per patient. Physicians expressed good satisfaction with patients’ treatment (across efficacy, tolerability and compliance domains) at Month 12. Overall, 32 adverse reactions (in 24 patients) and four hypoglycemic episodes were reported during the 24 months.
Conclusion
Addition of a second OAD improved physical and psychological symptoms associated with T2DM and was well tolerated in elderly people under real-life settings.
Introduction
The aging population is increasing worldwide due to improved life expectancy, and so is the prevalence of type 2 diabetes mellitus (T2DM) in elderly peopleCitation1,Citation2. Reports from the Italian Association of Clinical Diabetologists (Associazione Medici Diabetologi, AMD) showed that out of more than 500,000 people with T2DM included in the database, approximately 60% were ≥65 years old (33.4% aged 65–74.9 years; 27.3% aged ≥75 years)Citation3. Similarly, the ARNO Diabetes Elderly showed a 17% prevalence of diabetes in the elderly along with a progressive increase in polypharmacy and comorbiditiesCitation4. As such, T2DM in the elderly is a serious health concern both because of the proportion of the population involved and the impact on health and quality of life (QoL) in these subjects. Anti-hyperglycemic treatment in elderly patients is associated with increased risk of hypoglycemia, cognitive decline, falls and fractures, and more common use of polypharmacyCitation5,Citation6. Furthermore, QoL and wellbeing may be dramatically diminished in these people due to diabetic complicationsCitation7,Citation8.
Guidelines for the management of diabetes in the elderly recommend individualization of treatment, taking into consideration the functional status, the presence of frailty, dependency, comorbidities, life expectancy, and benefits and risks of anti-hyperglycemic agentsCitation9–11. Despite all recommendations, glycemic control seems to remain suboptimal in these patients. Data from the AMD database showed that approximately 25% of people with T2DM aged ≥65 years had glycated hemoglobin (HbA1c) ≤6.5%, and 11% had HbA1c >9.0%, suggesting that they are either over-treated and, therefore, at greater risk of hypoglycemia, or inadequately controlledCitation12.
Most randomized controlled trials on the pharmacological treatment of T2DM include few elderly people, and there is therefore limited evidence on the efficacy and safety of new oral anti-diabetes drugs (OADs) in this population. The INTERVAL study was the first trial to explore the feasibility of individualized HbA1c targets in elderly people with T2DMCitation13. The study demonstrated that the use of more recent oral agents such as the dipeptidyl peptidase-4 inhibitor (DPP-4i) vildagliptin allowed achievement of such targets more efficiently with no tolerability issuesCitation13. Information on health-related quality of life (HRQoL) and patient-reported outcomes (PROs) in the elderly T2DM population is also limitedCitation14,Citation15. Similarly, real-life data on the elderly patients’ perceptions of diabetes treatment and related day-to-day challenges, as well as clinical and psychological effects associated with treatment, are largely missingCitation16–18. The aim of this study was to assess the PROs, both from the clinician and patient perspectives, with the use of dual OAD therapy in elderly (≥65 years) subjects with T2DM in a real-life setting in Italy.
METHODS
Study design and population
3-AGE was a prospective, multicenter, non-interventional, real-world study in elderly people with T2DM inadequately controlled on metformin monotherapy, in whom a second OAD was prescribed by the investigators based on current clinical practice guidelines for the management of hyperglycemia. The study was conducted in 55 diabetes clinics in Italy and was initially planned for a period of 3 years, though it was prematurely stopped when all patients reached at least 2 years of follow-up. At the end of one year, approximately 12% of the population discontinued the study (due to the loss to follow-up, add-on of insulin, or protocol violation), and this could have led to a higher dropout rate during the third year of observation. Nonetheless, an interim analysis at one year showed improvements in glycemic control, QoL, and treatment satisfaction upon the addition of a second OAD. Since this was a longitudinal, observational study with no active comparator(s), a 2-year observation was deemed sufficient to provide substantial information on treatment intensification, whereas a third-year follow-up would have risked jeopardizing the interpretation of the results due to potential further patient dropout. The reason for not including an active control group was to determine the effect of treatment intensification on the QoL in elderly type 2 diabetes subjects under conditions as close as possible to the real-life setting. All investigators were therefore requested to intervene based on current clinical guidelines for the management of hyperglycemia. Hence, no pre-specified rescue therapy was planned.
We included men and women, aged ≥65 years with HbA1c 7.0–9.0% while on metformin monotherapy, with a second OAD added within 2 weeks before the study entry (baseline). Subjects with a history of low treatment compliance, significant psychiatric disorders, or life expectancy <3 years (at investigator’s discretion) were precluded from participation in the study. The rationale for defining HbA1c levels of 7.0–9.0% in people inadequately controlled on metformin monotherapy was based on standard T2DM guidelines. If glycemic goal (HbA1c <7.0%) is not achieved after ∼3 months of monotherapy, then a second antihyperglycemic agent is recommended. On the other hand, if HbA1c levels are >9.0% then the probability of prescribing a single oral agent is very low, and instead insulin or two or more oral agents are recommended.
Data collection
Demographic information, anthropometric details, and medical history were recorded at study entry (baseline visit) in an electronic case report form (eCRF) by individual investigators. PROs data on diabetes-related symptoms and changes therein following the addition of a second OAD were collected using standard and validated questionnaires such as the Diabetes Symptom Checklist-Revised (DSC-R) questionnaireCitation19,Citation20, EuroQoL 5D (EQ-5D) questionnaireCitation21 and Treatment Satisfaction Questionnaire of Medication-9 (TSQM-9)Citation22. DSC-R questionnaire is used to measure the symptoms score by 34 questions across eight domains (Psychological fatigue, Psychological cognitive function, Neuropathic pain, Neuropathic sensory function, Cardiovascular symptoms, Ophthalmological function, Hypoglycemia, and Hyperglycemia) with the results summarized by a total score: the higher the score, the greater the symptom burden. The health status of each individual was assessed by means of the EQ-5D questionnaire, which consists of a descriptive EQ-5D system and the EuroQol Visual Analog Scale (EQ VAS). The EQ-5D system is used to capture QoL across mobility, self-care, usual activities, pain/discomfort, and anxiety/depression dimensions. EQ VAS records patient’s self-assessed state of health on a 0 − 100 visual analog scale. Patient satisfaction following the addition of a second OAD was assessed across the efficacy, convenience, and overall satisfaction domains by means of the TSQM-9. Consumption of healthcare resources associated with dual OAD therapy (i.e. hospitalizations and/or additional visits, complications, and surgical/interventional procedures) was assessed by the physician using the Resource Consumption Questionnaire. Physician satisfaction with regards to efficacy, tolerability, and compliance to treatment was determined using the Physician’s questionnaire.
Study assessments
The primary endpoint was the change in physical and psychological symptoms and complications associated with T2DM following initiation of dual OAD therapy as recorded using DSC-R questionnaire. The secondary endpoints included assessment of QoL, health status, patient’s satisfaction, consumption of healthcare resources, and investigator’s satisfaction with treatment. Other assessments, such as HbA1c, vital signs, body weight, and body mass index (BMI), were also recorded during the observation period.
Safety
Suspected adverse reactions, drug-related hypoglycemia, and deaths during the study were recorded in eCRFs. The incidence of adverse reactions and serious adverse reactions were summarized according to System Organ Class and Preferred Term of MedDRA dictionary, version 18.1, overall and by OAD class. Further, we have collected the data pertaining to adverse drug reactions by the name of the related drugs.
Statistical analysis
The 3-AGE study was an observational study with no confirmatory aims. Overall, 860 patients met all inclusion criteria and were therefore eligible for analysis. Based on sample size and 12% dropout at one year, the 95% confidence interval for the mean of total DSC-R score with an estimated standard deviation of 0.5 to 1.5 was calculated to range from 0.036 to 0.107.
Descriptive statistics were used to present the demographics and baseline characteristics of eligible patients. Categorical data were presented as absolute and relative frequencies or contingency tables. Paired t-tests were used to analyze changes from baseline for normal data distribution and the Wilcoxon signed rank test was used in case of non-normally distributed data. Medical history/current medical conditions were summarized by system organ class and by OAD class. All statistical analyses were performed using SAS® for Windows release 9.4 (64-bit) (SAS Institute Inc., Cary, NC, USA).
Ethics and good clinical practice
The study complied with the applicable principles of the International Conference on Harmonisation Good Clinical Practice (ICHGCP), the Declaration of Helsinki, and the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) guidelines for the classification and conduct of observational studies on medicinal products (determination of 31 May 2010)Citation23, and was approved by the Ethics Committees of the participating centers. Informed consent from each patient was obtained before entering the study.
Results
Patient disposition and baseline characteristics
Of the 871 patients enrolled, 860 were eligible for analysis (). Overall, 210 patients discontinued the study prematurely (22 due to the need of insulin treatment, 13 changed background therapy, 10 required add-on therapy). The baseline demographics and clinical characteristics of the study population are summarized in . The mean age of the study population was 71.5 ± 5.2 years and 54.5% of patients were men. The mean BMI was 29.8 ± 4.7 kg/m2, mean HbA1c was 7.72% ± 0.54%, and mean diabetes duration was 9.4 ± 6.5 years (). At baseline, 81.1% of patients reported at least one comorbidity; vascular disorders were reported in two-thirds of the population (66.9%; primarily hypertension, 64.9%). The majority of patients (627 patients, 72.9%) were prescribed a DPP-4 inhibitor plus metformin (DPP-4i + MET), while a much lower proportion of patients received an alpha-glucosidase inhibitor plus metformin (AGI + MET; n = 70, 8.1%), a sulfonylurea plus metformin (SU + MET; n = 72, 8.4%), or other OAD combinations (n = 81, 9.4%). Ten patients (1.2%) remained on metformin alone. Of these 10 patients, three patients received a second OAD later on, six patients dropped out of the study due to protocol violation and one patient remained in the metformin treatment group. However, due to the observational nature of the study, these patients continued on metformin for a long time, and so we have results for the metformin alone group in a very small sample size.
Figure 1. Patient disposition. *One patient was excluded for all four reasons, one patient was excluded because of age (<65 years) and HbA1c >9.0%. Abbreviations. HbA1c, glycated hemoglobin; OAD, oral anti-diabetes drug.
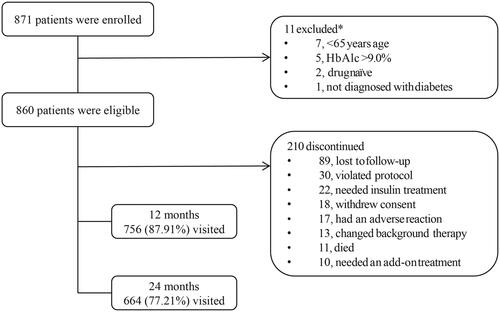
Table 1. Patient demographics and baseline characteristics (eligible patients).
Changes in HbA1c levels from baseline to Month 12
HbA1c levels were significantly reduced (−0.76% ± 0.90%; p < .0001) from baseline to Month 12 (6.95% ± 0.82%) after the addition of a second OAD. No relevant changes in vital signs, body weight, or BMI were reported at Month 24 (Table S1, Figure S1).
Disease symptom score
Addition of the second OAD significantly reduced (p < .0001) total DSC-R symptom score from 0.73 ± 0.68 at baseline to 0.63 ± 0.59 at Month 12 (−0.08 ± 0.59) and 0.61 ± 0.56 at Month 24 (−0.12 ± 0.62). Analysis of DSC-R scores by domain reported a significant improvement from baseline to Month 12 in the Psychological Fatigue (p = .0011), Hypoglycemia (p = .0010), and Hyperglycemia (p < .0001) domains. Similarly, improvements in DSC-R score from baseline were reported in all domains except Neuropathic Pain, Neuropathic Sensory Function, and Cardiovascular Symptoms at Month 24 (Table S2).
Quality of life
The EQ-5D scores indicated an overall good QoL in the study population. Across all domains of the questionnaire, the largest improvements in QoL were observed in mobility, pain/discomfort, and anxiety/depression dimensions at Month 12. A small proportion of patients (<5%) reported extreme problems in all five dimensions (, Table S3). The mean EQ-VAS scores demonstrated a significant increase (p < .0001) in overall health status from baseline (71.31 ± 15.16) to 12 months (+2.95 ± 11.92).
Table 2. EQ-5D scores: summary statistics by visit and domain (eligible patients).
Treatment satisfaction
The TSQM-9 mean scores revealed an overall greater treatment satisfaction with dual therapy at Months 12 and 24 (p < .0001) compared with baseline in all domains of Effectiveness (Month 12: +5.92 ± 18.75; Month 24: +9.48 ± 18.53), Convenience (Month 12: +3.40 ± 15.33; Month 24: +5.66 ± 16.65), and Global Satisfaction (Month 12: +8.76 ± 18.56; Month 24: +12.38 ± 19.80) (, Table S4).
Table 3. TSQM-9 scores: summary statistics by visit and domain (eligible patients).
Resource consumption
The addition of a second OAD resulted in statistically significant lower consumption of resources per patient at Months 12 and 24 across all domains with the exception of hospitalization and/or visits to the emergency room at Month 24 (). The frequency of consumption of resources by domain and study visit revealed that the most commonly utilized resources were for cardiovascular complications under “Hospitalization and/or emergency room visits”, angioplasty under “Surgical/interventional procedure”, diabetology, cardiology unit consultations under “Medical visits”, and HbA1c test under “Diagnostic procedure”. “Out-of-hours healthcare services” for hypoglycemia and hyperglycemia were utilized at the baseline visit only. Notably, physicians recommended a diagnostic procedure (81–91% of patients) and HbA1c test (94–97% of patients) in majority of the patients at all visits.
Table 4. Consumption of healthcare resources per patient: summary statistics by visit and domain.
Physician’s questionnaire
The mean scores for the Physician questionnaire on treatment satisfaction at Month 12 were higher, being 8.20 ± 1.29 (min–max: 0–10) in the Efficacy domain, 8.48 ± 1.12 (min–max: 4–10) in the Tolerability domain, and 8.40 ± 1.22 (min–max: 3–10) in the Compliance domain.
Safety
Four patients (two on DPP-4i + MET, one each on SU + MET and other combinations) presented with one hypoglycemic event (plasma glucose: 48–71 mg/dL) each during the observation period. Three of these events were likely triggered by a skipped/late meal. One of them required hospitalization.
A total of 32 adverse reactions were reported by 24 patients (on DPP-4i + MET); no serious adverse reactions were reported. The most frequent adverse reaction was gastrointestinal disorders (1.6%; Table S5). Eleven (5.2%) deaths (3 unexpected/sudden deaths; 2 myocardial infarctions; 2 cardio-circulatory arrests; and one subject each with pancreatic carcinoma and pulmonary neoplasia) occurred in 8 men and 3 women, aged between 65 and 87 years, and treated with DPP-4i + MET (n = 9) or SU + MET (n = 2).
Discussion
The 3-AGE study assessed the PROs with the use of dual OAD therapy in elderly people with T2DM from Italy under real-life settings. Addition of a second OAD resulted in improvements in PROs with significantly lower scores in the DSC-R symptom questionnaire at both Months 12 and 24 accompanied by an overall good QoL, improvement in health status, greater treatment satisfaction, and lower consumption of resources per patient at all study visits as compared with baseline. The mean score of the Physician questionnaire concerning treatment satisfaction was high (more than eight) for all domains. Overall, the addition of a second OAD was well tolerated with a low incidence of hypoglycemic events.
In the present study, addition of a second OAD was associated with a significant improvement of overall DSC-R score across all 8 domains at Month 12 that persisted through Month 24. This result suggests a lower symptom burden. The reductions in diabetes symptoms and symptom distress over 24 months correlated with improvements in DSC-R symptom score reported in earlier studies of treatment intensification with OADs and insulinCitation24,Citation25. Furthermore, consistent with glycemic control at Month 12, the reduced symptom burden in this population suggests an association between glycemic control and improvement in physical and psychological symptoms of T2DM in this population.
In elderly people with T2DM, the HRQoL is poorer than in those without diabetes, and can be affected by the number and the type of comorbiditiesCitation7,Citation8. The good QoL observed in our study suggests maintenance of high EQ-5D scores from baseline to Month 12, irrespective of the second-line OAD. This is in general agreement with the UKPDS-37 study, which demonstrated that QoL in people with T2DM was not affected by the drug used for treatment intensification, but rather by the complications of the diseaseCitation26. Furthermore, the maintenance of high EQ-5D in all five domains (Mobility, Self-care, Usual activities, Pain/discomfort, and Anxiety/depression) and improvement in overall state of health from baseline to Month 12 with the addition of a second OAD was similar to that reported in an earlier long-term study of treatment intensification with dapagliflozin in patients with metformin failureCitation27.
The overall treatment satisfaction with the addition of a second OAD is in line with the findings from a European observational studyCitation28. In that study, however, lower treatment satisfaction was apparent in subjects with hypoglycemic symptoms. Therefore, the improvement in treatment satisfaction documented in the current study might be due to the very few hypoglycemic episodes.
The consumption of healthcare resources across domains at each study visit decreased along with improvement in treatment satisfaction, suggesting that this was due to the addition of a second OAD. This is in line with the finding from a recent observational study in Italy, which demonstrated increased work productivity, reduced healthcare costs, and improved QoL in people with T2DM (age group, 28–88 years) treated with a fixed-dose combination of vildagliptin/metforminCitation29.
It is tempting to hypothesize that the improvement in PROs observed in the current study could be due to the improvement in glycemic control associated with the low rate of adverse events (in particular, hypoglycemia), which might have resulted in fewer diabetes-related symptoms and a perception of better QoL and health status that, in turn, could have led to greater treatment satisfactionCitation30. The same factors might also account for the high scores reported by physicians with respect to treatment satisfaction. Furthermore, the glycemic control achieved with the addition of a second OAD to failing metformin monotherapy suggests treatment intensification by the physicians, in line with local treatment guidelines. Thus, in agreement with the Italian guidelines, the vast majority of elderly patients included in the present survey received a DPP-4i as an add-on therapy, a pharmacologic treatment with a well-established favorable risk-to-benefit ratioCitation31. Interestingly, the reduction in HbA1c observed in this predominantly DPP-4i-treated population is in line with the HbA1c reduction reported in DPP-4i studies involving elderly people with T2DM (−0.90% to −0.62%)Citation13,Citation32,Citation33.
Overall, only a few adverse drug reactions and hypoglycemic events were reported in the study population. This might be attributed to the good tolerability profile of the second-line OADs prescribed and in particular to the predominant use of a DPP-4i. Such a low rate of adverse events, in particular of hypoglycemia, may reflect a conservative approach by physicians concerned about polypharmacy and frailty of their patients. This would be in agreement with the prevailing concept of treatment individualization, and it should be appreciated that a significant improvement in glycemic control was indeed achieved. These findings are reassuring because they show that a safe risk-to-benefit ratio can be ensured in elderly patients with T2DM in whom treatment intensification is deemed necessary. Even more importantly, such an improvement can be achieved without imposing further burden to the patient who has already shown improvement in a number of patient-related outcomes.
Strengths and limitations
The current study is the first to report a comprehensive analysis of PROs as well as physical and psychological symptoms associated with T2DM in elderly people. While this information adds to that already available in the literature, our study’s main advantage is that it was conducted in a real-life setting, thus enabling better transferability of the observation to a broader population of elderly individuals. In addition, failure to treatment was apparent in no more than 45 patients out of 210 patients discontinued (22 required insulin, 13 changed background therapy, 10 required add-on therapy), along with the reported average HbA1c reduction, suggests an overall efficacy of dual versus monotherapy. The main limitation of the study may be its early termination at 2 years, rather than the originally planned 3 years. Nonetheless, the 2-year observation period still provided information on a follow-up that is longer than the duration of available studies on PROs in elderly patients with diabetesCitation24,Citation29. No specific educational program was implemented in the present study to strengthen diet and physical activity. These, of course, remain key in the treatment of diabetes and their role in the elderly vulnerable population is of great interest. However, it was our scope of this study to assess changes in QoL parameters in a condition as close as possible to the real-world. Future and ad hoc studies will be necessary in the future to appreciate the effect of diet and physical activity on glycemic control and its correlation with respect to improved physical and psychological symptoms in the elderly type 2 diabetes population. Similarly, more studies will be required to determine to which extent improvement in glycemic control could also result in the improvement of physical and psychological symptoms.
Conclusions
Addition of a second oral anti-diabetes drug in elderly people who were inadequately controlled on metformin monotherapy was well-tolerated and resulted in improvements in the severity of symptoms, treatment satisfaction, and quality of life as shown by improvements in the scores of patient-reported outcome questionnaires, as well as better glycemic control. Results from this study will help in understanding patient perspectives and thus improve treatment decisions and patient care, especially in a vulnerable population such as elderly patients with type 2 diabetes mellitus.
Transparency
Declaration of funding
The study was funded by Novartis Pharma AG, Basel, Switzerland. The study sponsor participated in the study design, data collection, data review, data analysis, writing of the report, and decision to submit the article for publication.
Declaration of financial/other relationships
AL and GS declare no competing interests. SG received research funding from Novartis and has been a consultant for, or received honoraria from, Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, Bruno Farmaceutici, Eli Lilly, Hikma Pharmaceuticals, Janssen, Johnson & Johnson, Menarini, Merck Sharp & Dohme, Mundipharma, Novartis, Novo Nordisk, Sanofi, and Takeda. FG served on advisory panels for AstraZeneca, Novo Nordisk, and Sanofi; served as a consultant for AstraZeneca, Boehringer Ingelheim, Lifescan, Roche Diabetes Care, Sanofi, and Takeda; and received research support from Eli Lilly and Takeda. OD received fees for her role as an advisory board member for Eli Lilly, Boehringer, Astra Zeneca, Sanofi, Novo Nordisk, and Takeda; lecture fees from Novo Nordisk; travel support from Astra Zeneca, Novo Nordisk, Sanofi, Eli Lilly, Boehringer, and Menarini Diagnostics; consulting fees from Novo Nordisk; and clinical trial support for her institution from Novo Nordisk, Sanofi, and Eli Lilly. MB is an employee and shareholder of Novartis. SDP received research funding from Novartis Pharmaceuticals Co., MSD (Merck & Co.), and Novo Nordisk and has been a consultant for, or received honoraria from, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Laboratories Servier, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi-Aventis, and Takeda Pharmaceuticals. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author Contributions
All authors were responsible for conception and design of the study, had full access to the final data and interpreted them, critically revised the manuscript for important intellectual content, and approved the final version for submission.
3-AGE_study_Supplementary_-_5_Aug_2019.docx
Download MS Word (64.3 KB)Acknowledgements
The authors thank all the physicians who participated in this study. The authors also thank Sashi Kiran Goteti, PhD, and G. Lakshmi Deepa, PhD, of Novartis Healthcare Pvt. Ltd, Hyderabad, India, for providing medical writing support for the manuscript, which was funded by Novartis Pharma AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
References
- World Health Organization. Global health and aging [Internet]; October 2011 [cited 2018 July 10]. Available from: http://www.who.int/ageing/publications/global_health.pdf.
- International Diabetes Federation. IDF diabetes atlas [internet], 8 ed. Brussels, Belgium: International Diabetes Federation; 2017 [cited 2018 July 10]. Available from: http://www.diabetesatlas.org/resources/2017-atlas.html.
- Rossi MC, Candido R, Ceriello A, et al. Trends over 8 years in quality of diabetes care: results of the AMD Annals continuous quality improvement initiative. Acta Diabetol. 2015;52:557–571.
- Osservatorio ARNO Diabete Anziani Il profilo assistenziale della popolazione anziana con diabete [Internet]; 2015 [cited 2018 July 10]. Available from: http://www.siditalia.it/pdf/2017%20%20SID%20%20Osservatorio%20ARNO%20Diabete%20Anziani.pdf.
- Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLOS One. 2009;4:e4144.
- Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107–115.
- Chin YR, Lee IS, Lee HY. Effects of hypertension, diabetes, and/or cardiovascular disease on health-related quality of life in elderly Korean individuals: a population-based cross-sectional survey. Asian Nurs Res (Korean Soc Nurs Sci). 2014;8:267–273.
- Hajian-Tilaki K, Heidari B, Hajian-Tilaki A. Solitary and combined negative influences of diabetes, obesity and hypertension on health-related quality of life of elderly individuals: A population-based cross-sectional study. Diabetes Metab Syndr. 2016;10(2 Suppl 1):S37–S42.
- International Diabetes Federation. IDF Global guidelines for managing older people with type 2 diabetes [Internet] [cited 2017 Jun 23]. Available from: http://www.idf.org/sites/default/files/IDF-Guideline-for-older-people-T2D.pdf
- Sinclair A, Morley JE, Rodriguez-Mañas L, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13:497–502.
- Associazione Medici Diabetologi, Società Italiana di Diabetologia. Standard italiani per la cura del diabete mellito. Rome, Italy: AMD-SID [internet]; 2014 [cited 2018 Jul 10]. Available from: http://www.siditalia.it/pdf/Standard%20Cura%20Diabete%20-%20Capitolo%205%20-%20Terapia.pdf
- Tondini S, Boemi M, Marnini P, et al. Quality of healthcare provided to elderly with type 2 diabetes: data from the AMD annals initiative. J Diabetes Metab. 2015;6:542.
- Strain WD, Lukashevich V, Kothny W, et al. Individualised Treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382:409–416.
- Marrett E, Stargardt T, Mavros P, et al. Patient-reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. Diabetes Obes Metab. 2009;11:1138–1144.
- Nicolucci A, Pintaudi B, Rossi MC, et al. The social burden of hypoglycemia in the elderly. Acta Diabetol. 2015;52:677–685.
- Bradley C, de Pablos-Velasco P, Parhofer KG, et al. PANORAMA: a European study to evaluate quality of life and treatment satisfaction in patients with type-2 diabetes mellitus—study design. Prim Care Diabetes. 2011;5:231–239.
- Chew BH. Medication adherence on quality of life among adults with type 2 diabetes mellitus: an exploratory analysis on the EDDMQoL study. Qual Life Res. 2015;24:2723–2731.
- Manan MM, Husin AR, Alkhoshaiban AS, et al. Interplay between oral hypoglycemic medication adherence and quality of life among elderly type 2 diabetes mellitus patients. J Clin Diagn Res. 2014;8:JC05–JC09.
- Grootenhuis PA, Snoek FJ, Heine RJ, et al. Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabet Med. 1994;11:253–261.
- Naegeli AN, Stump TE, Hayes RP. A psychometric evaluation of the diabetes symptom checklist-revised (DSC-R) cognitive distress, fatigue, hyperglycemia, and hypoglycemia subscales in patients with type 1 and type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:27–30.
- EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
- Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 200927;7:36.
- Agenzia Italiana del Farmaco. Guideline for the classification and conduction of the observational studies on medicines [Internet]; [cited 2018 Jul 10]. Available from: https://www.agenziafarmaco.gov.it/ricclin/sites/default/files/files_wysiwyg/files/CIRCULARS/Circular%2031st%20May%202010.pdf
- Abbatecola AM, Spazzafumo L, Fabbietti P, et al. Diabetes-related quality of life is enhanced by glycaemic improvement in older people. Diabet Med. 2015;32:243–249.
- Rubin RR, Peyrot M, Chen X, et al. Patient-reported outcomes from a 16-week open-label, multicenter study of insulin pump therapy in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2010;12:901–906.
- Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). U.K. Prospective Diabetes Study Group. Diabetes Care. 1999;22:1125–1136.
- Grandy S, Langkilde AM, Sugg JE, et al. Health-related quality of life (EQ-5D) among type 2 diabetes mellitus patients treated with dapagliflozin over 2 years. Int J Clin Pract. 2014;68:486–494.
- Alvarez Guisasola F, Tofé Povedano S, Krishnarajah G, et al. Hypoglycaemic symptoms, treatment satisfaction, adherence and their associations with glycaemic goal in patients with type 2 diabetes mellitus: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) Study. Diabetes Obes Metab. 2008;10(Suppl 1):25–32.
- Genovese S, Tedeschi D. Effects of vildagliptin/metformin therapy on patient-reported outcomes: work productivity, patient satisfaction, and resource utilization. Adv Ther. 2013;30:152–164.
- Ali MK, Feeney P, Hire D, et al. Glycaemia and correlates of patient-reported outcomes in ACCORD trial participants. Diabet Med. 2012;29:e67–74.
- Incalzi RA, Ferrara N, Maggi S, et al. Personalizzazione del trattamento dell'iperglicemia nell'anziano con diabete tipo 2 [internet]; [cited 2018 Jul 10]. 2017. Available from: http://www.sigg.it/docs/Documento-ufficiale.pdf
- Schweizer A, Dejager S, Bosi E. Comparison of vildagliptin and metformin monotherapy in elderly patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Obes Metab. 2009;11:804–812.
- Schernthaner G, Barnett AH, Patel S, et al. Safety and efficacy of the dipeptidyl peptidase-4 inhibitor linagliptin in elderly patients with type 2 diabetes: a comprehensive analysis of data from 1331 individuals aged ≥ 65 years. Diabetes Obes Metab. 2014;16:1078–1086.
