Abstract
Objective
To compare real-world outcomes with newer (insulin glargine 300 U/mL; Gla-300) versus standard of care (SoC) basal insulins (BIs) in the REACH (insulin-naïve; NCT02967224) and REGAIN (basal insulin-treated; NCT02967211) studies in participants with uncontrolled type 2 diabetes (T2DM) in Europe and Brazil.
Methods
In these open-label, parallel-group, pragmatic studies, patients (HbA1c > 7.0%) were randomized to Gla-300 or SoC BI for a 6-month treatment period (to demonstrate non-inferiority of Gla-300 vs SoC BIs for HbA1c change [non-inferiority margin 0.3%]) and a 6-month extension period (continuing with their assigned treatment). Insulin titration/other medication changes were at investigator/patient discretion post-randomization.
Results
Overall, 703 patients were randomized to treatment in REACH (Gla-300, n = 352; SoC, n = 351) and 609 (Gla-300, n = 305, SoC, n = 304) in REGAIN. The primary outcome, non-inferiority of Gla-300 versus SoC for HbA1c change from baseline to month 6, was met in REACH (least squares [LS] mean difference 0.12% [95% CI –0.046 to 0.281]) but not REGAIN (LS mean difference 0.17% [0.015–0.329]); no between-treatment difference in HbA1c change was shown after 12 months in either study. BI dose increased minimally from baseline to 12 months in REACH (Gla-300, +0.17 U/kg; SoC, +0.15 U/kg) and REGAIN (Gla-300, +0.11 U/kg; SoC, +0.07 U/kg). Hypoglycemia incidence was low and similar between treatment arms in both studies.
Conclusions
In both REACH and REGAIN, no differences in glycemic control or hypoglycemia outcomes with Gla-300 versus SoC BIs were seen over 12 months. However, the suboptimal insulin titration in REACH and REGAIN limits comparisons of outcomes between treatment arms and suggests that more titration instruction/support may be required for patients to fully derive the benefits from newer basal insulin formulations.
Introduction
Many people with type 2 diabetes (T2DM) who initially achieve glycemic control with oral antihyperglycemic drugs (OADs) eventually require basal insulin, either alone or in combination with other agentsCitation1. Although a recent ADA/EASD consensus report recommends that glucagon-like peptide-1 receptor agonists (GLP-1 RAs) be used as the first injectable therapy optionCitation2, basal insulins still play a key role in the management of people with T2DM who are uncontrolled on OADs with or without GLP-1 RAsCitation3. In clinical practice, basal insulin initiation is frequently delayed until glycated hemoglobin (HbA1c) is >9% (>75 mmol/mol)Citation4.
Newer second-generation basal insulin analogs such as insulin glargine 300 U/mL (Gla-300) were developed to optimize glycemic control while minimizing the risk of hypoglycemia. Gla-300 provides a more constant and prolonged pharmacokinetic and pharmacodynamic profile than insulin glargine 100 U/mL (Gla-100, a standard of care [SoC] in basal insulin treatmentCitation5), and equivalent glycemic control with reduced risk of anytime (24 h) or nocturnal hypoglycemia, in randomized controlled trials (RCTs) of people with T2DMCitation6–9. Furthermore, a lower risk of hypoglycemia with Gla-300 during the initial weeks of treatment (the titration period) has been consistently shown in regulatory RCTs versus Gla-100, and has also been demonstrated versus insulin degludecCitation6–10. Consistent with RCTs, Gla-300 has shown comparable HbA1c reduction with fewer hypoglycemic episodes versus first-generation basal insulin analogs in retrospective, non-interventional real-world evidence (RWE) studiesCitation11,Citation12.
Even after initiation, those using basal insulin frequently experience poor glycemic control, which may be related to insulin dose up-titrationCitation13, that is often suboptimal in real-world practiceCitation4,Citation14. Such therapeutic inertia (a phenomenon that includes delayed therapy initiation, lack of dose adjustment and delayed therapy intensificationCitation13), which probably contributes to the low level of HbA1c target achievement in routine clinical practiceCitation4,Citation15, may be related to perceived barriers such as hypoglycemia and weight gainCitation13,Citation14,Citation16–18. We hypothesized that the use of newer basal insulin (such as Gla-300) in clinical practice may allow people with T2DM to titrate appropriately to achieve glycemic control with minimal hypoglycemia, thereby overcoming therapeutic inertia. However, although results from non-interventional real-world studies are interesting and may better reflect clinical practice than regulatory RCTs, comparisons between treatments in such studies may be confounded even if factors are fully controlled by appropriate statistical methods such as propensity score matching.
Here we present the results of two studies that combine real-world relevance with the advantages of randomization, a methodology which, uniquely, is a perfect instrument to address confounding. These studies aim to evaluate the real-world effectiveness of newer basal insulin, Gla-300, compared with other SoC basal insulins, in insulin-naïve (the REACH CONTROL trial) and basal insulin-treated (the REGAIN CONTROL trial) participants with uncontrolled T2DM. REACH and REGAIN are the first randomized real-life studies (often known as pragmatic studies as they aim to assess the outcomes of treatment in routine clinical practice, rather than in the controlled explanatory setting of a regulatory RCTCitation19) using basal insulin treatments in Europe and Brazil, and the first such studies in the field of basal insulin therapy. The studies were intended to allow for direct treatment comparisons in a real-life setting, and also provide an opportunity to determine whether this pragmatic study design is a valid approach to investigate the impact of novel therapies on therapeutic inertia.
Materials and methods
Study design and participants
REACH CONTROL (NCT02967224) and REGAIN CONTROL (NCT02967211) were multicenter, open-label, randomized, active-controlled, 2-arm, parallel-group, comparative, pragmatic real-world studies in patients with uncontrolled T2DM who were either insulin-naïve but considered eligible for basal insulin therapy (REACH; conducted in Brazil, France, Germany, Italy, Romania, Spain, United Kingdom) or already on basal insulin therapy (REGAIN; conducted in Brazil, Finland, France, Italy, Romania, Spain, Switzerland, United Kingdom). Participants underwent a 1-week screening period, followed by a 6-month treatment period (for assessment of the primary outcome) and a 6-month extension period (during which patients continued within their assigned treatment arm) (Supplementary Figure 1), to evaluate durability of clinical benefit, as well as patient-reported outcomes (PROs) and healthcare resource utilization. Patients attended four study visits during the 12-month randomization period. Both trials were approved by local/national institutional review boards/independent ethics committees, as appropriate, and were performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. All participants provided written informed consent.
Participants were aged ≥18 years with uncontrolled T2DM (HbA1c >7.0%) after ≥6 months of treatment with or without GLP-1RA therapy and either (i) current OADs (REACH) or (ii) basal insulin therapy with/without OADs (REGAIN). Permitted OADs were metformin, sulfonylureas (SU), thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium-glucose co-transporter 2 (SGLT-2) inhibitors, glinides, α-glucosidase inhibitors. SoC basal insulin therapy included Gla-100, insulin detemir, neutral protamine Hagedorn (NPH) insulin or insulin degludec. Participants in REGAIN were also required to have a fasting plasma glucose (FPG) concentration of >130 mg/dL (>7.2 mmol/L) to justify the need to intensify the current basal insulin treatment.
Exclusion criteria included the presence of any clinically significant abnormality that would restrict successful participation in the study, use of OADs not specified in the inclusion criteria, use of GLP-1RA not approved for use with insulin, or use of any drug in the context of a clinical trial within 3 months prior to screening. Participants in REGAIN were also excluded if they had received short-/rapid-acting insulin (e.g. human recombinant insulin, insulin lispro, insulin aspart, insulin glulisine, inhaled human insulin, pre-mix insulin, and biosimilars) during the 3 months prior to screening, other than for temporary use during hospitalization. Additional exclusion criteria are listed in the Supplementary Material.
Randomization and treatment
Randomization (1:1) either to Gla-300 or to any SoC basal insulin (all commercially available long- or intermediate-acting basal insulins, chosen at the investigator’s discretion based on their clinical practice) was performed centrally by an interactive response technology (IRT) system; the investigator called the IRT system after the screening visit for allocation of the patient number, and again on Day 1 when the system randomized the patients to one of the treatment arms. Randomization was stratified according to HbA1c category (<9% or ≥9%) at screening, SU use at the time of randomization, and GLP-1RA use within 6 months prior to randomization. Gla-300 was to be self-administered once-daily at approximately the same time of day. SoC basal insulins were to be administered once- or twice-daily according to approved labeling and clinical practice. If needed, participants randomized to SoC basal insulin could switch to any other SoC basal insulin, except Gla-300, during the treatment period. Where available, participants were offered either a specific, designated patient support program (PSP) (Gla-300 arm only) or a PSP as per the investigator’s usual practice (any patient assigned to SoC), to help them achieve successful insulin initiation and overall self-management, but this was not compulsory. The PSP for Gla-300 (COACH) has been described previouslyCitation20,Citation21, and includes tailored disease education, product support, and encouragement for lifestyle changes, but does not include specific titration support.
The approved insulin delivery devices for study drugs were distinguishable; therefore, this was an open-label study. However, HbA1c and FPG were analyzed in central laboratories that were blinded to study drug received by the patient, and the sponsor and investigators were blinded to the results prior to database lock.
Dose and titration
The administered dose of Gla-300 or SoC basal insulin was at the investigator’s discretion according to their standard practice and the patient’s characteristics and requirements, and dependent on self-monitored plasma glucose data and occurrence of hypoglycemia. The recommended starting dose of Gla-300 was in line with the country-specific label, taking into account glucose control and patterns of hypoglycemia in each patient. In brief, the starting dose of Gla-300 was to be 0.2 U/kg in REACH and the same dose as the previously used basal insulin (adapted as per the Gla-300 label in those previously on a twice-daily regimen) in REGAIN. The recommended target range for FPG was 80–130 mg/dL (4.4–7.2 mmol/L), as per American Diabetes Association (ADA) guidelinesCitation22. Insulin dose could be adjusted in order to achieve this target or other local practice guidelines, and glycemic targets could be adapted if it was deemed necessary by the investigator according to individual participant considerations. Importantly and in keeping with the real-world nature of the study, no protocol-recommended titration algorithm or oversight of titration outside of local clinical practice was performed. It was recommended that Gla-300 dose changes did not occur more frequently than every 3–4 days, in line with the EU labelCitation23. Modification of concomitant antihyperglycemic medication use (i.e. discontinuation or initiation) was performed according to local clinical practice and based on the investigator’s discretion.
Outcomes
The primary outcome was change in HbA1c from baseline to month 6, to demonstrate the non-inferiority of Gla-300 versus SoC basal insulin therapy. Secondary outcomes included the proportion of participants reaching HbA1c targets (<6.5%, <7.0%, <7.5% and <8.0%) at months 6 and 12, the percentage of participants whose HbA1c decreased by ≥1% (REACH) or ≥0.5% (REGAIN) at months 6 and 12, change in FPG from baseline to months 6 and 12 and the proportion of participants remaining on assigned basal insulin therapy during the 6- and 12-month randomized periods, with or without intensification (defined as addition of any OAD, GLP1-RA and/or rapid-acting insulin medication, or an increase in dose of antihyperglycemic medication already in use).
Safety outcomes included hypoglycemia episodes, including incidence of nocturnal (00.00am–05.59am) and anytime (24 h) hypoglycemic events, and documented symptomatic or severe events. Hypoglycemia was classified according to ADA criteriaCitation24, similar to the definition used in the pivotal studies of Gla-300Citation6,Citation7. Documented symptomatic hypoglycemia was defined as an event with typical symptoms of hypoglycemia accompanied by a PG concentration of ≤70 mg/dL, (≤3.9 mmol/L) (<54 mg/dL [<3.0 mmol/L] was also analyzed). Severe hypoglycemia was defined as an event requiring assistance from a third party to administer carbohydrate, glucagon or other resuscitative actions. Other safety outcomes included adverse events (AEs) and serious adverse events (SAEs), injection-site and hypersensitivity reactions, vital signs and change in body weight from baseline to months 6 and 12. Although not classified as a safety outcome, basal insulin dose (U and U/kg) was also assessed.
Statistical methods
For REACH and REGAIN, a sample size of 340 and 290 patients, respectively, per treatment group was determined in order to provide 90% power to ensure that the upper confidence limit of the 2-sided 95% confidence interval (CI) for the mean difference between Gla-300 and an SoC basal insulin did not exceed 0.3% (a non-inferiority margin recommended by the EMACitation25), assuming a particular standard deviation (SD) (1.4% in REACH and 1.3% in REGAIN) and that the true difference in HbA1c between Gla-300 and a SoC basal insulin was −0.05%. The power calculation was based on a 2-sample t-test for mean difference and was performed using the PROC POWER procedure in SAS software version 9.3, SAS Institute Inc., Cary, NC, USA. The primary analysis was conducted on all randomized patients following the intent-to-treat (ITT) principle. Change in HbA1c from baseline to month 6 was analyzed in the ITT population using a mixed-effect model with repeated measures (MMRM), with fixed categorical effects of treatment arm, visit, treatment-by-visit interaction, multi-country organisation, randomization strata of SU use (yes/no), randomization strata of GLP-1 receptor agonist use (yes/no), as well as continuous fixed covariates of baseline HbA1c and baseline HbA1c value-by-visit interaction. The same model was used to analyze HbA1c change to month 12, with the exception that one additional level was added to the “visit” factor. For the primary endpoint, a stepwise closed testing approach was used to assess non-inferiority and superiority sequentially. Non-inferiority (and superiority, if non-inferiority was shown) of Gla-300 versus SoC basal insulin was demonstrated if the upper bound of the 2-sided 95% CI for the between-treatment difference in mean change of HbA1c from baseline to month 6 was less than the predefined margin of 0.3% (or <0% for superiority). The test for the primary outcome was performed one-sided at level α = 0.025.
Secondary outcomes were analyzed descriptively in the ITT population, where appropriate using either a mixed-effect model with repeated measures or a logistic regression approach, and no multiplicity adjustments were made. Safety outcomes were assessed descriptively, with no statistical testing, in the safety population, comprising all randomized participants who received ≥ 1 dose of study insulin. Participants were analyzed according to treatment actually received, irrespective of the randomized treatment arm. Odds ratios (ORs) and rate ratios (RRs) for Gla-300 versus SoC basal insulins and their corresponding 95% CIs were provided, where applicable and estimated using logistic and log binomial regression approaches, respectively. The difference in the annualized rate of hypoglycemic events was evaluated using an over-dispersed Poisson regression model. All analyses were undertaken using SAS software version 9.3, SAS Institute Inc., Cary, NC, USA.
Results
Study participants
REACH was conducted between November 2015 and October 2017 and REGAIN between December 2015 and October 2017. Baseline characteristics for the overall study populations of REACH (N = 703) and REGAIN (N = 609) are presented in . The REGAIN population had longer mean duration of diabetes, a lower proportion who were receiving SUs or DPP-4 inhibitors at baseline and a higher proportion receiving SGLT2 inhibitors at baseline compared with the REACH population. Patient disposition throughout both studies is shown in Supplementary Figure 2. Among patients who did not complete the 12-month randomized period in REACH (n = 30) and REGAIN (n = 28), there were 4 (Gla-300, n = 2; SoC basal insulin, n = 2) and 5 (Gla-300, n = 3; SoC basal insulin, n = 2) deaths, respectively. The number of patients taking concomitant short-acting insulin, mainly for intensification, during the 12-month randomized periods were as follows: REACH, 21 patients (6.0%) in the Gla-300 group and 31 patients (8.8%) in the SoC group; REGAIN, 16 patients (5.2%) in the Gla-300 group and 14 patients (4.6%) in the SoC group. The most commonly used SoC basal insulin over 12 months was Gla-100 in both REACH (74%) and REGAIN (67%).
Table 1. Demographics and baseline characteristics of study participants in REACH and REGAIN (randomized population).
Outcomes
Change in HbA1c and insulin dose
In REACH, mean baseline HbA1c values were 8.99 (SD 1.40) % (74.7 [15.3] mmol/mol) with Gla-300 and 9.08 (1.49) % (75.7 [16.3] mmol/mol) with SoC BIs. There was a similar change in HbA1c in both groups, with values decreasing to 7.65 (1.19) % (60.1 [13.0] mmol/mol) with Gla-300 and 7.54 (1.17) % (58.9 [12.8] mmol/mol) with SoC basal insulins at month 6. HbA1c remained stable through to month 12, when values were 7.64 (1.09) % (60.0 [11.9] mmol/mol) with Gla-300 and 7.59 (1.16) % (59.4 [12.7] mmol/mol) with SoC basal insulins. REACH met its primary outcome, demonstrating non-inferiority of Gla-300 versus SoC basal insulins in terms of HbA1c change from baseline to month 6 (LS mean difference between groups 0.12; [95% CI −0.046 to 0.281]% [1.3 (−0.5 to 3.1) mmol/mol]) (). There was also no difference between the groups in HbA1c change from baseline to month 12 (LS mean difference 0.07 [95% CI −0.087 to 0.227]% [0.8 (−1.0 to 2.5) mmol/mol]) ().
Figure 1. LS mean (±SE) change from baseline in HbA1c by visit during the 12-month randomized period in (a) REACH and (b) REGAIN and change in insulin dose in (c) REACH and (d) REGAIN (ITT populations). Abbreviations. BI, basal insulin; CI, confidence interval; HbA1c, glycated hemoglobin; ITT, intent-to-treat; LS, least squares; M, month; SE, standard error; SoC, standard of care.
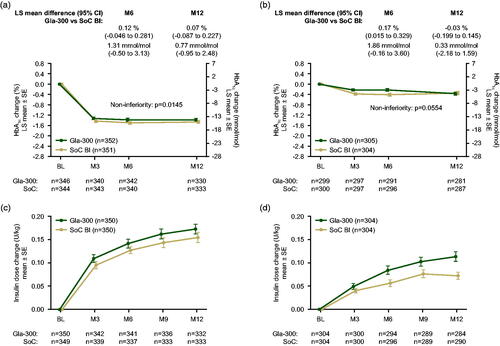
In REGAIN, mean (SD) baseline HbA1c values were 8.58 (1.10) % (70.3 [12.0] mmol/mol) with Gla-300 and 8.51 (1.18) % (69.5 [12.9] mmol/mol) with SoC basal insulins. There was only a modest decrease in HbA1c from baseline in both treatment groups, to 6-month values of 8.32 (1.24) % (67.4 [13.6] mmol/mol) with Gla-300 and 8.11 (1.18) % (65.1 [12.9] mmol/mol) with SoC BIs. From month 6 onwards there was further HbA1c reduction in the Gla-300 group (to 8.17 [1.19] % [65.8 (13.0) mmol/mol] at month 12), but little change in the SoC basal insulins group (month 12 value of 8.17 [1.28] % [65.8 (14.0) mmol/mol]). In REGAIN, the primary 6-month outcome did not achieve statistical non-inferiority of Gla-300 versus SoC basal insulins in terms of HbA1c change from baseline (LS mean difference 0.17 [95% CI 0.015–0.329]% [1.9 (0.2–3.6) mmol/mol]); however, by 12 months the difference in HbA1c between treatment groups was observed to be nominally within the non-inferiority margins (LS mean difference −0.03 [95% CI −0.199 to 0.145]% [−0.3 (−2.2 to 1.6) mmol/mol]) ().
In both studies, the degree of insulin titration reflected by dose changes was low in the Gla-300 and SoC basal insulin treatment arms (). In REACH, mean (SD) daily dose increased from baseline values of 0.18 (0.07) U/kg for Gla-300 and 0.17 (0.07) U/kg for SoC basal insulin, to month 6 values of 0.32 (0.18) U/kg for Gla-300 and 0.30 (0.18) U/kg for SoC basal insulins (mean increases of 0.14 and 0.13 U/kg, respectively). In REGAIN, mean (SD) daily dose increased from baseline values of 0.41 (0.21) U/kg for Gla-300 and 0.41 (0.23) U/kg for SoC basal insulins, to month 6 values of 0.49 (0.27) U/kg for Gla-300 and 0.46 (0.26) U/kg for SoC basal insulins (mean increases of 0.08 and 0.06 U/kg, respectively). By month 12, the daily dose had increased to 0.35 (0.19) U/kg for Gla-300 and 0.33 (0.20) U/kg for SoC basal insulins in REACH (mean increases of 0.17 and 0.15 U/kg, respectively), and to 0.52 (0.28) U/kg for Gla-300 and 0.48 (0.26) U/kg for SoC basal insulins in REGAIN (mean increases of 0.11 and 0.07 U/kg, respectively).
HbA1c targets
The proportion of patients who achieved the HbA1c target <7.0% was low and similar in the Gla-300 and SoC basal insulin arms during the 12-month period in both REACH and REGAIN (). In REACH, the percentage of patients with a ≥1% decrease in HbA1c at month 6 that was maintained at month 12 was similar in both treatment groups (Gla-300, 44.0% (155/352); SoC basal insulin, 47.9% (168/351)). In REGAIN, the percentage of patients with a ≥0.5% decrease in HbA1c at month 6 that was maintained at month 12 was similar in both treatment groups (Gla-300, 26.9% (82/305); SoC basal insulin, 27.3% (83/304)).
Table 2. Proportion of patients at HbA1c target during the 12-month randomized period by visit (ITT population).
FPG changes
In REACH, mean (SD) baseline FPG was 11.62 (3.43) mmol/L (209.3 [61.9] mg/dL) with Gla-300 and 11.67 (3.80) mmol/L (210.2 [68.5] mg/dL) with SoC basal insulins. FPG decreased similarly in both groups to month 6 values of 8.70 (2.49) mmol/L (156.7 [44.9] mg/dL) with Gla-300 and 8.42 (2.55) mmol/L (151.7 [45.9] mg/dL) with SoC basal insulins, and to month 12 values of 8.25 (2.40) mmol/L (148.7 [43.2] mg/dL) with Gla-300 and 8.35 (2.62) mmol/L (150.5 [47.2] mg/dL) with SoC basal insulins.
In REGAIN, mean baseline FPG was 9.85 (3.16) mmol/L (177.5 [57.0] mg/dL) with Gla-300 and 10.03 (3.12) mmol/L (180.7 [56.2] mg/dL) with SoC basal insulins, decreasing to 9.21 (2.75) mmol/L (165.9 [49.5] mg/dL) and 8.74 (2.91) mmol/L (157.5 [52.5] mg/dL) at month 6, and to 8.62 (3.04) mmol/L (155.4 [54.9] mg/dL) and 8.67 (3.14) mmol/L (156.2 [56.7] mg/dL) at month 12, respectively.
In both REACH and REGAIN, LS mean FPG change from baseline to month 12 was similar across treatment arms (REACH, −3.32 mmol/L [−59.9 mg/dL] with Gla-300 and −3.27 mmol/L [−58.8 mg/dL] with SoC basal insulins; REGAIN, −1.30 mmol/L [−23.5 mg/dL] with Gla-300 and −1.26 mmol/L [−22.8 mg/dL] with SoC basal insulins). In both studies, the month 12 FPG values in either treatment arm were above the ADA FPG target range specified in the protocol.
Hypoglycemia
Incidence and rates of anytime (24 h) hypoglycemia were low and similar in the Gla-300 and SoC basal insulin arms in REACH and REGAIN (). Benefits were observed for Gla-300 versus SoC basal insulins in REGAIN, for nocturnal (00.00am–05.59am) symptomatic documented (≤3.9 mmol/L) hypoglycemia (OR 0.48 [95% CI 0.26–0.87]; RR 0.45 [0.20–0.97]) and severe and/or symptomatic documented (≤3.9 mmol/L) hypoglycemia (OR 0.47 [0.27–0.84]; RR 0.42 [0.20–0.87])().
Table 3. Hypoglycemia events occurring at any time of day (24 h) during the 12-month on-treatment period in REACH and REGAIN (safety population).
Table 4. Nocturnal (00.00am–05.59am) hypoglycemia events occurring during the 12-month on-treatment period in REACH and REGAIN (safety population).
Bodyweight changes
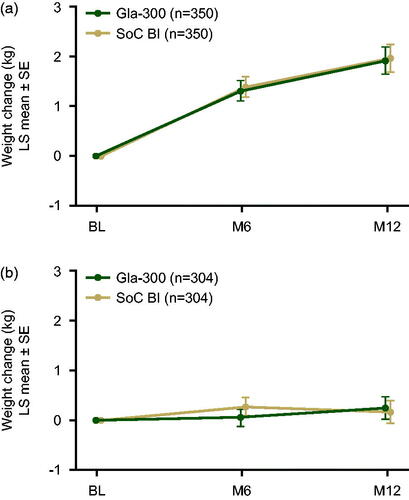
In both REACH and REGAIN, the LS mean change in patients’ body weight from baseline to month 12 was modest and similar in both treatment groups ().
Figure 2. LS mean (±SE) change in body weight from baseline to month 12 during the 12-month on-treatment period in REACH (a) and REGAIN (b) (safety populations). The 12-month on-treatment period was defined as the time from the first study drug intake until one day after last injection of the study drug. Abbreviations. BI, basal insulin; LS, least squares; M, month; SE, standard error; SoC, standard of care.
Incidence of adverse events
In both REACH and REGAIN, the AE profile was similar between treatment arms. In REACH, 208 (59.4%) and 194 (55.4%) patients in the Gla-300 and SoC basal insulin arms, respectively, had a treatment-emergent AE (TEAE), of whom 14 (4.0%) in each treatment arm had a TEAE considered as related to study drug. Two (0.6%) patients in each treatment arm had a TEAE leading to death. Two (0.6%) (Gla-300) and 3 (0.9%) patients (SoC basal insulins) discontinued treatment due to TEAEs. In REGAIN, 184 (60.5%) and 170 (55.9%) patients in the Gla-300 and SoC basal insulin arms, respectively, had a TEAE, of whom 6 (2.0%) (Gla-300) and 2 (0.7%) (SoC basal insulins) had a TEAE considered as related to study drug. Two (0.7%) patients in each treatment arm had a TEAE leading to death. Eight (2.6%) (Gla-300) and 2 (0.7%) patients (SoC basal insulins) discontinued treatment due to TEAEs.
Patient support program (PSP)
In REACH, 44.3% (155/350) of patients in the Gla-300 group at least partially participated in the core PSP, and 8.6% (30/350) of patients in the SoC basal insulin group at least partially participated in any PSP. In REGAIN, 51.0% (155/304) of patients in the Gla-300 group and 2.6% (8/304) of patients in the SoC basal insulin group participated in a PSP.
Discussion
REACH and REGAIN are the first randomized pragmatic studies in T2DM to allow for direct treatment comparisons of patient outcomes in a real-life setting, and are part of a larger real-world evidence program (including another pragmatic study, ACHIEVE-CONTROLCitation26, in ∼3300 insulin-naïve T2DM patients in North America). In both studies, the primary outcome was non-inferiority of Gla-300 versus SoC basal insulin in terms of HbA1c change from baseline to month 6. The primary outcome was met in REACH but not in REGAIN, although only a modest HbA1c reduction was seen in both treatment arms over the full study period in REGAIN, and no difference in HbA1c change between the groups was shown after 12 months in either study. It may be notable that overall basal insulin dose increases were minimal in both studies, particularly in REGAIN.
The failure to achieve non-inferiority for the difference in HbA1c change in REGAIN compared to SoC was unexpected. It is possible that this simply reflects no benefit, or even a lack of parity, with Gla-300 versus other SoC BIs in a real-world scenario. However, given the results from previous RCTsCitation6,Citation7, we feel that this is unlikely. The EDITION program of RCTs, with a treat-to-target approach, consistently demonstrated non-inferiority of Gla-300 versus Gla-100 (the most commonly used SoC basal insulin in both REACH and REGAIN) in terms of HbA1c reduction, regardless of previous insulin exposureCitation27. Compared with these RCTs, REACH and REGAIN involved nearly no restriction to participation, did not include predefined titration regimens or mandated oversight of titration during the studies, and involved less frequent contact between the patients and HCPs to ensure close titration support, so direct comparisons with REACH and REGAIN are difficult. Moreover, in REACH and REGAIN, average baseline HbA1c (8.99 and 8.58%, respectively, in the Gla-300 arms) and FPG (11.62 and 9.85 mmol/L, respectively, in the Gla-300 arms) was higher than seen in the EDITION program (HbA1c, 8.49% in EDITION 3 [insulin-naïve participants] and 8.28% in EDITION 2 [basal insulin plus OADs]; FPG, 9.93 mmol/L in EDITION 3 and 8.24 mmol/L in EDITION 2)Citation6,Citation28. The high baseline HbA1c levels are an indication of the difficulties associated with glycemic management in real-life clinical practice.
Limited insulin titration was observed in REACH and REGAIN (despite an apparent difference in dose increase between treatment arms in REGAIN) compared with the EDITION RCTs that employed a treat-to-target approach; Gla-300 dose increase over 12 months was 0.17 U/kg in REACH versus 0.48 U/kg in EDITION 3Citation28 and 0.11 U/kg in REGAIN versus 0.33 U/kg in EDITION 2Citation6. Crucially, dose titration in REACH and REGAIN was performed at the discretion of the investigators, without a predefined titration algorithm or a titration committee. In comparison, patients in the EDITION program were titrated to a fasting SMPG target of 4.4–5.6 mmol/L (80–100 mg/dL; below the ADA target range), which translated to greater increases in insulin dose and greater proportions of patients achieving glycemic targets, than was observed in REACH or REGAIN. The minimal basal insulin dose increase in REACH and REGAIN limited the ability to compare other outcomes across treatment arms and may also have driven the low incidence of hypoglycemia compared with the EDITION programCitation27 and the BRIGHT study (Gla-300 versus insulin degludec)Citation10.
Basal insulin titration in REGAIN was even more limited than in REACH, in both treatment arms, and this overall therapeutic inertia may be related to the failure to achieve non-inferiority for the primary outcome at month 6. The EDITION studies demonstrated that 12% more Gla-300 than Gla-100 was required for equivalent glycemic control owing to differences in bioavailabilityCitation27, so a lack of titration could have a greater impact on the outcomes of Gla-300. However, it has not been determined if the dose requirements seen in the EDITION RCTs are reflected in clinical practice. In addition to titration inertia, the lack of therapy intensification with short-acting insulins is notable in REGAIN, even though investigators were allowed to intensify therapy with oral antihyperglycemic agents and/or injectable therapy as they do in their clinical practice.
There are many reasons why patients may not have up-titrated their basal insulin dose to achieve glycemic control, including perceived barriers such as hypoglycemia and weight gainCitation13,Citation14,Citation16–18. However, it is likely that the lack of standardized titration-to-target algorithms for basal insulins is key to the suboptimal basal insulin titration seen in both REACH and REGAIN and may highlight the need for tools to facilitate such titration. The recent ADA/EASD consensus report recognizes the necessity of titrating basal insulins to a fasting blood glucose target using an evidence-based algorithmCitation2. Empowering patients to increase their own basal insulin dose is also importantCitation2, and self-titration has been shown to be superior to physician-led titration in the recent TAKE CONTROL studyCitation29. In TAKE CONTROL, which included both insulin-naïve and insulin pre-treated participants with T2DM, 72% of participants achieved the ADA-defined PG target of 4.4–7.2 mmol/L (80–130 mg/dL) in the self-titration group.
Substantially more people in the Gla-300 group than in the SoC basal insulin group, in both REACH and REGAIN, utilized a PSP. The minimal basal insulin dose increases and HbA1c reduction with Gla-300, despite the availability of a PSP, maybe a further indication of substantial titration inertia. However, although the Gla-300 PSP (COACH) has been shown to improve persistence and adherence to basal insulin therapyCitation20,Citation21, it does not include any titration-to-target guidance. There is no evidence that the COACH PSP would influence the outcomes of REACH and REGAIN, and so it should not necessarily be expected to improve insulin dose titration. The impact of PSPs on outcomes in REACH and REGAIN was not a predefined objective of either study and could not be analyzed because the use of a program was determined after randomization (patients could decide to use a PSP at any point throughout the study), so this remains a potential confounder.
In addition to real-life therapeutic inertia, the results presented here may be partly influenced by the study design, keeping in mind that REACH and REGAIN were the first randomized pragmatic studies in the field of basal insulin treatment and so novel in this field. Power calculations for REACH and REGAIN were based on data from regulatory RCTs and therefore assumed a slight benefit in favor of Gla-300, which may have unduly constrained patient numbers. HbA1c targets may require tailoring to the individual patient and given the less-stringent inclusion criteria for REACH and REGAIN compared with regulatory RCTs, the predefined targets (<7%) may have been too ambitious for some patients. Such a shift towards patient-centered care is advocated in the recent ADA and EASD consensus report on hyperglycemia managementCitation2, and future real-world studies of basal insulins should consider individualized targets. It is notable that both REACH and REGAIN were initiated close to the launch of Gla-300, therefore real-world experience of its use was lacking during the studies. Investigators may have exercised caution in using new basal insulin but would likely have had long-term experience with the basal insulin used in the SoC group. A potentially cautious approach to Gla-300 use could have been reinforced by the lack of an enforced titration algorithm. In addition, investigators may have been more familiar with participating in regulatory RCTs, in which visit frequency is higher and monitoring of study protocol execution and oversight of dose titration and other procedures is much stricter than in pragmatic studies. The wide range of concomitant antihyperglycemic therapies used by the heterogeneous populations included in REACH and REGAIN, that may not reflect regulatory RCTs (for example, a relatively high proportion of people using DPP-4 inhibitors in both studies), change in dose of these non-insulin antihyperglycemic therapies during the studies and the previous basal insulin use in REGAIN (in particular whether a once- or twice-daily regimen was used), may also have impacted on outcomes.
In contrast with the results presented here, other RWE studies employing retrospective analysis of electronic health records have demonstrated benefits of Gla-300 versus first-generation basal insulins in clinical practice, with comparable glycemic improvement and lower hypoglycemia risk in propensity score-matched cohortsCitation11,Citation12. In these RWE studies, the investigators chose basal insulins based on patient characteristics, and there was no intervention that may have led to a “study effect”; however, there was no randomization to control for unknown confounders and no way to determine if more basal insulin titration occurred compared with REACH and REGAIN.
Conclusions
Therapeutic inertia is a global unmet medical need, one that the ADA and EASD are seeking to address in their recent consensus report. In both REACH and REGAIN, no differences in glycemic control or hypoglycemia outcomes were seen between treatment arms over 12 months. However, the suboptimal basal insulin titration seen in both trials reflects considerable therapeutic inertia, limiting the ability to compare outcomes between the newer basal insulin (Gla-300) and SoC basal insulin arms. Results from REACH and REGAIN show that, in addition to appropriate insulin therapy, more dedicated basal insulin titration support using evidence-based algorithms may be required for patients and healthcare providers in order to help patients with uncontrolled T2DM to achieve glycemic targets. These studies also highlight the importance of appropriate patient selection and treatment. Appropriate titration of Gla-300 and other second-generation basal insulin analogues may be required to realize their potential clinical benefits of decreasing the risk of hypoglycemia versus older basal insulins.
Transparency
Declaration of funding
The REACH and REGAIN trials were sponsored by Sanofi. Sanofi was involved in designing the trials and conducted the trials and analyzed the data. The authors received editorial/writing support in the preparation of this manuscript provided by Simon Rees, PhD, of Fishawack Communications Ltd, who was funded by Sanofi.
Declaration of financial/other relationships
NF has received research support from Sanofi-Aventis, AstraZeneca, Ipsen, Akcea, Takeda, Accelovance, Allergan, Biopharma, and Tesareo. He has served as a speaker for Sanofi-Aventis. DM has served on advisory panels for Ferrer, Sanofi, AstraZeneca, Janssen, and Merck Sharp & Dohme (MSD) and received speakers’ bureau fees from Almirall, Boehringer Ingelheim, Menarini, GlaxoSmithKline, Eli Lilly, Sanofi, Novartis, Novo Nordisk, and MSD. Institutional research support was obtained from AstraZeneca, GlaxoSmithKline, Eli Lilly, Sanofi, Novo Nordisk, and MSD. AG has received honoraria or consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, and Sanofi and research funding from AstraZeneca. TB has received research support from Abbott, Ascensia, BD, Boehringer Ingelheim, Calibra Medical, Capillary Biomedical, Companion Medical, Dance Biopharm, Dexcom, Diasome, Eli Lilly, Glysens, Kowa, Lexicon, Medtronic, Novo Nordisk, POPS! Diabetes Care, Sanofi, Senseonics, Taidoc, vTv Therapeutics, Xeris, and Zealand; consulting honoraria from Abbott, Capillary Biomedical, Eli Lilly, Medtronic, and Sanofi; and speaking Honoraria from Abbott, MannKind, Medtronic, Sanofi, and Senseonics. RR is an advisory panel member for AstraZeneca, AbbVie, Sanofi, MSD, Eli Lilly, Janssen, Novo Nordisk, Diabnext, Vaiomer, and Physiogenex; is a speaker for Bayer and Servier; and has received research funding and provided research support to Danone Research, Amgen, Sanofi, and Novo Nordisk. DF has received honoraria from advisory boards for Sanofi, Eli Lilly, Abbott, and Pfizer and speaking engagements for Sanofi, Novo Nordisk, Eli Lilly, Merk Sharp & Dohme, and Medtronic and has received research grants from Novo Nordisk, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Sanofi, Novartis, MSD, Boehringer Ingelheim, Amgen, and OPKO. BB is a consultant for Sanofi. VP, JW, ZB, MB, AC, M-LN-P are employees and shareholders of Sanofi. AP is an advisory panel member for AstraZeneca, MSD, and Novo Nordisk, and is a speaker for Abbott, Amgen, Bayer MSD, Sanofi, Novo Nordisk, Eli Lilly, and Medtronic. MP-M has acted as consultant and speaker for Novo Nordisk, Sanofi-Aventis, Lilly, Novartis, MSD, Boehringer Ingelheim, and AstraZeneca and as an advisory board member for Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, and AstraZeneca. JS has acted as an advisory board member for Abbott, AstraZeneca, Boehringer Ingelheim, GI-Dynamics, Janssen, LifeScan, Mundipharma, Novartis, Novo Nordisk, and Sanofi-Aventis and as a speaker for Abbott, AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Lilly, MSD, MedScape, Novartis, Novo Nordisk, Omniamed, and Sanofi-Aventis. He has received grants in support of investigator and investigator-initiated trials from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, GI-Dynamics, Intarcia, Ipsen, Janssen, Novartis, Novo Nordisk, Sanofi Aventis, and Ypsomed. SD has received research support from Sanofi. JW has acted as a consultant and advisory board member for Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, Napp, Novo Nordisk, MSD, Mundipharma, Sanofi, and Wilmington Healthcare (paid into University funds). He has received grants for investigator-initiated trials from AstraZeneca and Novo Nordisk and has acted as a speaker for AstraZeneca, Boehringer Ingelheim, Napp, Novo Nordisk, Mundipharma, Sanofi, and Takeda. CW has acted as an advisor, consultant, and/or speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen, Novo Nordisk, and Sanofi. MD has acted as a consultant, advisory board member, and speaker for Novo Nordisk, Sanofi-Aventis, Lilly, MSD, Boehringer Ingelheim, AstraZeneca, and Janssen; an advisory board member for Servier; and a speaker for Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc. She has received grants in support of investigator and investigator-initiated trials from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, and Janssen. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
NF, AMGC, and SDS were involved in the concept and design of both studies. BB performed statistical analyses. All authors were involved in the interpretation of the data, writing and reviewing drafts of the manuscript, and approved the final version for submission.
Supplemental Material
Download Zip (2.2 MB)Acknowledgements
None reported.
References
- Horton ES. Defining the role of basal and prandial insulin for optimal glycemic control. J Am Coll Cardiol. 2009;53(5):S21–S27.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2701.
- American Diabetes Association. 9. pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(1):S90–S102.
- Khunti K, Caputo S, Damci T, et al. The safety and efficacy of adding once-daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012;14(12):1129–1136.
- Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 Units mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units mL−1. Diabetes Care. 2015;38(4):637–643.
- Yki-Järvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus insulin glargine 100 U/mL in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17(12):1142–1149.
- Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37(10):2755–2762.
- Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37(12):3235–3243.
- Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/mL compared with glargine 100 U/mL in insulin-naive people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386–394.
- Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 Units/mL versus insulin degludec 100 Units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care 2018;41(10):2147–2154.
- Sullivan SD, Bailey TS, Roussel R, et al. Clinical outcomes in real-world patients with type 2 diabetes switching from first- to second-generation basal insulin analogues: comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D + cohort study. Diabetes Obes Metab. 2018;20(9):2148–2158.
- Zhou FL, Ye F, Berhanu P, et al. Real-world evidence concerning clinical and economic outcomes of switching to insulin glargine 300 units/mL vs other basal insulins in patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20(5):1293–1297.
- Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20(3):488–496.
- Garber AJ. The importance of titrating starting insulin regimens in patients with type 2 diabetes. Diabetes Obes Metab. 2009;11(5):10–13.
- Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19(8):1155–1164.
- Ahren B. Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag. 2013;9:155–163.
- Brod M, Rana A, Barnett AH. Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin. 2012;28(12):1933–1946.
- Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682–689.
- Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20(8):637–648.
- Goldman JD, Gill J, Horn T, et al. Improved treatment engagement among patients with diabetes treated with insulin glargine 300 U/mL who participated in the COACH support program. Diabetes Ther. 2018;9(5):2143–2153.
- Zhou FL, Yeaw J, Karkare SU, et al. Impact of a structured patient support program on adherence and persistence in basal insulin therapy for type 2 diabetes. BMJ Open Diabetes Res Care. 2018;6(1):e000593.
- American Diabetes Association. Standards of medical care in diabetes–2016. Diabetes Care 2016;39(1):S1–S112.
- Sanofi. Toujeo: Summary of product characteristics [Internet]. Paris (France): Sanofi; 2019 [cited 2019 Jan 23]. Available from: https://www.ema.europa.eu/documents/product-information/toujeo-epar-product-information_en.pdf.
- Standards of medical care in diabetes-2017: summary of revisions. Diabetes Care 2017;40(1):S4–S5.
- EMA. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus [Internet]. Amsterdam (The Netherlands): European medicines Agency; 2012. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf
- Oster G, Sullivan SD, Dalal MR, et al. Achieve control: a pragmatic clinical trial of insulin glargine 300 U/mL versus other basal insulins in insulin-naive patients with type 2 diabetes. Postgrad Med. 2016;128(8):731–739.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes. Diabetes Obes Metab. 2015;17(9):859–867.
- Bolli GB, Riddle MC, Bergenstal RM, et al. Glycaemic control and hypoglycaemia with insulin glargine 300U/mL versus insulin glargine 100U/mL in insulin-naive people with type 2 diabetes: 12-month results from the EDITION 3 trial. Diabetes Metab. 2017;43(4):351–358.
- Russell-Jones D, Dauchy A, Delgado E, et al. Take control: a randomized trial evaluating the efficacy and safety of self- versus physician-managed titration of insulin glargine 300 U/mL in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21(7):1615–1624.
