Abstract
Objective
Oral supplementation with iron is a standard intervention for treating or preventing iron deficiency with or without anemia. Over the last few decades, various forms of oral iron have been developed to improve treatment tolerability and iron bioavailability. In this review, we gathered research data regarding the use of iron protein succinylate since it was first marketed in the 1980s.
Methods
Electronic databases – PubMed and the Cochrane Library – were searched for studies published up to March 2019. Clinical or observational studies reporting data on the tolerability of oral iron protein succinylate were included. Results were statistically described to evaluate and compare the efficacy and safety of iron protein succinylate with the comparators under study.
Results
Iron protein succinylate was investigated in 54 studies: 38 randomized clinical trials and 16 observational studies, with a total of 8454 subjects. Of them, 8142 were included in the efficacy analysis: patients were divided into three population subtypes: general (n = 1899), gynecological/obstetric (n = 5283), and pediatric (n = 960). In total, 6450 patients received iron protein succinylate, experiencing a significant change in hemoglobin and ferritin in all populations. The change in all parameters was similar or higher with iron protein succinylate compared to other iron treatments evaluated. Overall, study groups receiving iron protein succinylate reported the lowest rate of adverse events.
Conclusions
Although all iron treatments analyzed are effective and safe, our results suggest that iron protein succinylate may be an excellent choice to treat iron deficiency and anemia due to its superior effectiveness and tolerability.
Introduction
Anemia caused by iron deficiency is a widespread nutritional disorder, with important consequences for human healthCitation1. Although the overall prevalence of anemia drops in middle- and high-income countries due to better access to adequate food, specific populations in these countries remain at high risk of iron deficiency. This is the case of pregnantCitation2,Citation3 and menstruating womenCitation4,Citation5, particularly adolescents, in whom the onset of menstruation overlaps with a stage of rapid growth and increased iron requirementsCitation6. Iron-deficiency anemia causes lethargy, fatigue, irritability, and breathlessness. However, various authors have suggested that iron deficiency in the absence of anemia may impair exercise performance and affect muscle fatigue and workCitation4,Citation7,Citation8.
The traditional approach to the treatment of iron deficiency was based on supplementation with ferric or ferrous salts, being ferrous sulfate the most popular form. Although ferrous sulfate has proven effective to restore iron stores in patients with iron-deficiency anemiaCitation9, gastrointestinal adverse effects (typically epigastric discomfort and nausea) may compromise treatment adherence in real-life practiceCitation10. These adverse effects can be attenuated by administering the iron supplement with meals, but absorption may be reduced by approximately 40% under these conditionsCitation11.
In the last decades, various forms of oral iron have been developed to overcome the limitations of traditional iron salts. Among these alternatives, iron protein succinylate is a highly soluble complex of iron bound to succinylated milk proteins (in addition to intolerance/hypersensitivity to the excipients, the origin of the protein, particularly casein, makes the product unsuitable for people with hypersensitivity to milk proteins). One remarkable characteristic of iron protein succinylate is that the whole complex precipitates at pH < 4, allowing iron to pass through the stomach inside a protein shell and, therefore, avoiding direct contact with the gastric mucosa. Once the complex reaches the duodenum, it resolubilizes and is hydrolyzed by pancreatic enzymes, thus releasing iron molecules in the gut lumenCitation12–14. This uptake pathway reduces the gastrointestinal adverse effects typically associated with iron supplementation. Since it was first marketed in 1988, various studies have investigated the tolerability and effectiveness of iron protein succinylate compared with other iron formulations.
In this systematic review, we analyze all therapeutic outcomes reported over three decades of use of iron protein succinylate in both the research and routine practice settings.
Methods
Data sources and search strategy
Potentially relevant publications were retrieved from the PubMed and Cochrane Library databases from January to March 2019. Searches targeted studies investigating the outcome of treatments with iron protein succinylate, irrespective of the comparator and the patient profile. Therefore, the term “iron protein succinylate” was used, and no filters or limits regarding the time of publication, study design or language were established. Articles without full text available were excluded from the record. In addition to manuscripts retrieved from the databases, the medical department of the companies that marketed oral supplements with iron protein succinylate were contacted and asked for additional reports (gray literature) regarding this formulation.
Study selection and eligibility
All records resulting from the electronic search were screened for adequacy. After removing duplicates, all manuscripts with full text available were assessed for eligibility. Reviews were not included in the record; however, relevant publications cited in the review and not retrieved in the electronic search were considered for eligibility.
Eligible articles had to be written in English, Spanish or Italian (or any other language with a translation available). All participants of any age or gender with oral iron treatment indicated for iron deficiency, anemia or prophylaxis of anemia (in case of pregnancy) were considered, as well as all comparators, including ferrous sulfate, ferritin extractive, ferrimannitol ovalbumin, iron polystyrene sulfonate, ferric gluconate complex, iron polysaccharide complex, or placebo. Studies evaluating the efficacy of iron protein succinylate with concomitant treatments were also included, but the latter group of comparators was excluded from the efficacy results analysis to minimize bias in this regard.
Data extraction and analysis
All data were extracted and recorded in a database specifically designed for this review. Descriptive data included the number of patients (total and in each study arm), presentation of the iron protein succinylate and dose, the comparator and dose, target population, indication and treatment duration of the treatment, and study design. All measurements of variables that lacked information about the testing method and/or units were not considered for the analysis.
Treatment outcomes included change (baseline and final means) in hemoglobin (Hb), ferritin, adverse events (AEs), and if mentioned, gastrointestinal adverse events (GAEs) and whether AEs/GAEs were related with the treatment or not. To minimize the risk of bias, all analyses were weighted by the number of patients in each study and treatment.
Results were presented as the weighted average for baseline and final values of each outcome with their corresponding weighted variance. This assessment was performed for iron protein succinylate and each type of comparator: ferric and ferrous complexes. All efficacy measures were categorized into four populations. All-patient (i.e. all patients included in the efficacy analysis), general (i.e. a set of patients including, postoperative, patients with gastrointestinal pathologies, and older patients, among others), gynecologic/obstetric, and pediatric populations (each included patients from studies with their respective target population, and ). All analyses were descriptive, and no hypothesis tests were performed.
Table 1. Studies investigating iron protein succinylate treatment efficacy vs. other iron complexes. Data included in the pooled efficacy analysis.
Table 2. Other studies investigating IPS effectiveness and/or safety. Only IPS treatment data from these studies are included in the pooled efficacy analysis.
Results
Selection and characteristics of selected studies
The search of the electronic databases yielded 140 records, of which 50 were duplicates and four were considered invalid for not reporting quantitative data on the use of iron protein succinylate. Of the 86 full-text articles considered for eligibility, 64 corresponded to clinical studies investigating the use of iron protein succinylate in a total of 6946 subjects. Of these, 54 reported efficacy and tolerability results and were, therefore, included in the analysis ().
Of the 54 studies included in the analysis, 38 were randomized controlled trials, 32 of which compared the efficacy results of iron protein succinylate with other iron complexesCitation15–46 (). Six randomized controlled trialsCitation47–52 and one observational studyCitation53 compared iron protein succinylate of various presentations with or without other medications, and 15 studiesCitation54–68 investigated the effectiveness of iron protein succinylate without a comparator (). Overall, the studies selected for the analysis included a total of 8454 subjects: 8142 were considered for the efficacy analysis and 8005 for the tolerability analysis. Of all the patients considered for the efficacy analysis, 6450 (79.21%) subjects received iron protein succinylate and 1692 (20.78%) were treated with other ferrous or ferric complexes as comparator groups. Ferrous complexes included ferrous sulfate and iron polystyrene sulfonate (n = 1010) and various ferric complexes (n = 682), including: ferritin extractive, ferrimannitol ovalbumin, and ferric gluconate.
Pooled efficacy results
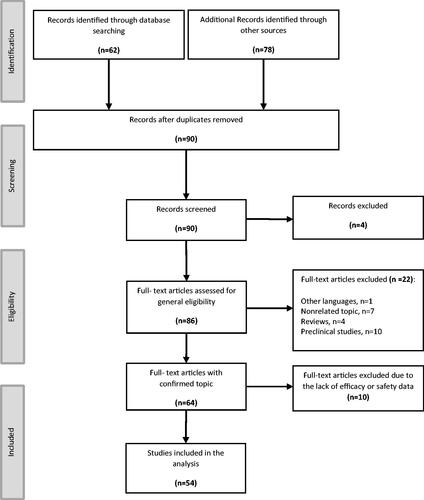
summarizes the weighted averages of baseline and final values of Hb and ferritin for all subjects treated either with iron protein succinylate or other iron complexes in the three selected populations and for all patients.
Table 3. Pooled efficacy results.
Overall, 8,142 patients were included for the efficacy analysis, with an iron treatment duration of 23–180 days. Treatment with iron protein succinylate achieved high efficacy results in the analysis including all patients. The pooled analysis of pre- and post-treatment Hb in patients receiving protein succinylate, assessed in 6450 patients with a mean treatment duration of 49 days, showed a mean increase of 16.2% (+1.74 g/dl). This value was similar or higher than that observed with ferrous salts (15.9%, +1.72 g/dl; n = 1010; mean treatment duration of 58 days) and ferric salts/complexes (8.0%, +0.85 g/dl; n = 682; mean treatment duration of 49 days). The effect of protein succinylate on ferritin change, assessed in 3071 patients, showed a mean increase of 52.8% of this laboratory parameter. This increase was also higher than that observed with ferrous salts (35.3%, n = 969) and comparable or slightly higher than the one achieved with ferric salts/complexes (48.5%, n = 494) (, ).
Figure 2. Pooled efficacy results for hemoglobin and ferritin in all patients included (A and B, respectively). Abbreviations. Hb, hemoglobin; Fer, ferritin. Labels indicate the weighted percentage of change for each endpoint in a given population.
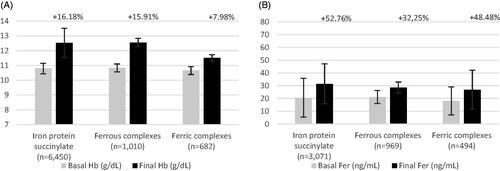
The general population analyzed included 1899 subjects treated for 23–180 days. In this population, Hb change was the most frequently reported outcome. Overall, iron protein succinylate achieved positive results regarding change in Hb and ferritin with a mean treatment duration of 2 months. Patients included in the Hb analysis of iron protein succinylate (1091) showed a mean increase of 19.9% (+2.1 g/dl). This increase was comparable or slightly higher than that observed with ferrous salts (represented only by ferrous sulfate) (16.5%, +1.83 g/dl; n = 607) and ferric salts/complexes (18.2%, +1.67 g/dl; n = 201). Ferritin change in iron protein succinylate was assessed in 871 patients, who showed a mean increase of 60.1%. This increase was higher than that observed with ferrous salts (27.7%, n = 566) and ferric salts/complexes (46.0%, n = 146) as well. (, ).
Figure 3. Pooled efficacy results for hemoglobin and ferritin in general population (A and B, respectively). Abbreviations. Hb, hemoglobin; Fer, ferritin. Labels indicate the weighted percentage of change for each endpoint in a given population.
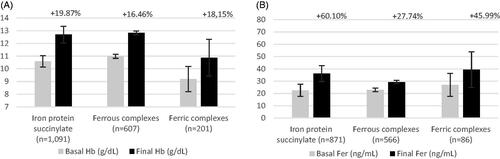
The gynecologic/obstetric population analyzed included 5283 subjects, with a treatment duration of 28–120 days. In this population, iron supplementation was initiated as preventive treatment or as active treatment for iron-deficiency anemia either for pregnant women (n = 362) or gynecological population (n = 4921). Gynecologic/obstetric subjects treated with protein succinylate experienced a mean increase of 15.8% (+1.68 g/dl) in Hb levels (n = 4838; mean treatment duration of 45 days) and 44.8% in ferritin levels (assessed in 1763 patients). Mean increase in Hb associated with ferrous salts was 19% (+1.93 g/dl, n = 138) with a longer mean treatment duration (53 days), while for ferric salts the increase was smaller: 4.4% (+0.41 g/dl, n = 307; mean treatment duration of 52 days). Consistently with the trend observed in the all-patient population, the ferritin change associated with iron protein succinylate was higher than that observed in patients treated with ferric salts/complexes (34.5%, n = 280) and ferrous salts (29.6%, n = 138) (, ). Additionally, we evaluated the outcomes of iron protein succinylate treatment in studies that only included pregnant patients with anemia. The average trend of Hb increase was similar than for the overall gynecologic/obstetric population (16.9%, n = 571) with a mean (SD) baseline Hb of 10.3 (1.2) g/dl, but reaching a final mean (SD) Hb level of 12.1 (1.2) g/dl. Ferritin levels had a lower increase in the pregnant anemic subpopulation than in the overall population or the rest of the gynecologic/obstetric population (23%, n = 571) after treatment with iron protein succinylate (mean [SD] baseline and final ferritin, 18 [22.5] and 22.1 [19.2] ng/ml, respectively).
Figure 4. Pooled efficacy results for hemoglobin and ferritin in gynecological/obstetric population (A and B, respectively). Abbreviations. Hb, hemoglobin; Fer, ferritin. Labels indicate the weighted percentage of change for each endpoint. Labels indicate the weighted percentage of change for each endpoint in a given population.
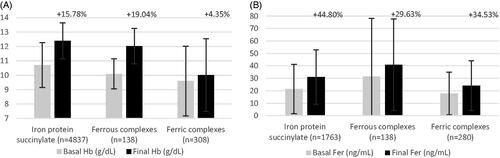
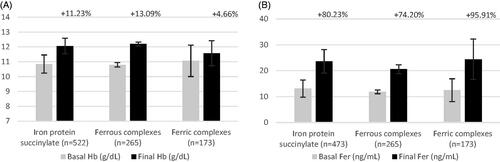
The pediatric population included 960 subjects. Age ranged from premature infants to 14-year-old children, and treatment duration was from 30 to 60 days. Most subjects in this population were treated with iron protein succinylate and iron polystyrene sulphonate ferrous salt (n = 522 and n = 265, respectively), mostly at a weight-adjusted dose. In the pediatric population, the values for every outcome increased similarly for all treatments analyzed. Hb and ferritin changed from baseline after treatment with iron protein succinylate: 11.2% (n = 522) and 80.2% (n = 437), respectively (, ).
Figure 5. Pooled efficacy results for hemoglobin and ferritin in pediatric population (A and B, respectively). Abbreviations. Hb, hemoglobin; Fer, ferritin. Labels indicate the weighted percentage of change for each endpoint in a given population.
In addition to the populations categorized in .e. all patients, general adult, gynecologic/obstetric, and pediatric), various studies assessed for efficacy included subjects with specific disorders or conditions. This was the case of 27 patients with gastroenteric pathologies that were medically or surgically managed, who received iron protein succinylate to treat iron-deficiency anemiaCitation15,Citation54. Likewise, 35 patients included in the efficacy analysis had received iron protein succinylate in the surgery settingCitation15,Citation44. All these patients experienced a mean increase of Hb of 25% or more, reaching normal values of Hb after the therapy (i.e. >12 g/dl).
Additionally, the search of the electronic databases allowed the identification of two trials involving 30 patients with subclinical hypothyroidism who received iron protein succinylate: of these, 22 patients combined iron therapy with L-thyroxineCitation48,Citation61. All of these had a mean increase in Hb of 18.25%, reaching Hb normal values after treatment (i.e. >12 g/dl).
Finally, the analysis also included one trial involving 50 patients treated with iron protein succinylate while receiving H2 antagonists as antiulcer therapyCitation53. These patients normalized their hematologic parameters as expected, without clinical interactions between therapies. Mean Hb percentage change was 19.65%Citation53.
Pooled tolerability results
In this review, tolerability of all iron treatments reported was assessed in terms of adverse event rate per patient and whether this AE was gastrointestinal and/or related to the treatment, when this information was available. Forty-seven (87%) studies reported AE frequency, although only 32 (59.3%) provided information on the causality regarding iron supplementation.
A total of 924 adverse events were reported. Of these, 823 were gastrointestinal, and 438 were considered to be treatment-related. None of the AEs reported was serious. Iron protein succinylate was the formulation with the lowest adverse event rate, either related or non-related. Overall, both ferrous and ferric complexes showed adverse event rates that were more than three times higher compared to iron protein succinylate (, ). Moreover, the relative AE rate of ferrous and ferric complexes vs. iron protein succinylate increased more than six fold when considering only treatment-related AEs (, ).
Figure 6. Pooled tolerability adverse events rate and related adverse events rate (A and B, respectively) for all patients included in the tolerability analysis. The “n” shows the sample size of each iron supplement in which AE rate and RAE rate has been calculate.
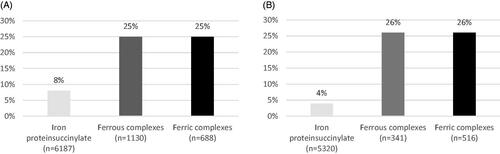
Table 4. Pooled safety data.
Studies investigating the tolerability of iron protein succinylate in pregnant women revealed a similar adverse event rate than that observed in the overall population (0.06, n = 504 vs. 0.07, n = 6187, respectively)Citation26,Citation27,Citation41,Citation49,Citation57. In these patients, almost all reported AEs were GAE (GAE rate: 0.05, n = 504). None of the studies including patients who received iron protein succinylate in the surgery setting reported any AECitation15,Citation44. Finally, the trial investigating the effect of combined treatment with iron protein succinylate and H2 antagonists suggested a more favorable tolerability in patients receiving combined treatment than those receiving iron protein succinylate aloneCitation53.
Discussion
In this systematic review, we provided pooled data on the efficacy and safety of iron protein succinylate from over 30 years of clinical experience and research with this formulation. The literature burden retrieved from this search amounted to 64 studies, in which nearly 7000 subjects received iron protein succinylate. As the purpose of the review was to provide a clinical perspective of iron supplementation, ten full-text articles reporting pharmacokinetic properties of iron protein succinylate were ruled out. Despite the heterogeneity regarding the methodological approach of these studies, they persistently reported that iron protein succinylate shows adequate bioavailability and good absorptionCitation69. Importantly, unlike other iron preparations, iron protein succinylate is well absorbed both under fasting conditions and after mealsCitation70, which can potentially improve adherence to treatment.
Most clinical guidelines for the management of iron-deficiency anemia recommend unspecific oral iron supplementation to restore iron levelsCitation71,Citation72. Even though all marketed formulations have proved to adequately do this, the low gastrointestinal tolerability of some treatments – such as those based on ferrous salts – may reduce the patient’s adherence to treatment and, therefore, compromise its effectivenessCitation13. Our review compared iron protein succinylate with other iron supplements, including widely prescribed ferrous salts, such as iron sulfate. The pooled efficacy analysis showed that iron protein succinylate was consistently associated with a significant change in Hb and ferritin, irrespective of the population. It is generally assumed that ferrous salts have a better bioavailability (due to a more efficient absorption) and as result, to be more efficacious than ferric complexesCitation73. Our results suggest the same or superior efficacy results of iron protein succinylate treatment compared with the most commonly used oral ferrous salts. Additionally, iron protein succinylate treatment achieved these improved results in a 15.5% shorter mean treatment duration (49 vs. 58 days). Regardless, both iron protein succinylate and ferrous salts had better efficacy results than the ferric complexes analyzed. Importantly, differences between results of iron protein succinylate and other ferric complexes may be due to its unique formulation: iron protein succinylate precipitates at acid pH values, thus protecting the metal from gastric polymerization. The complex solubilizes at the duodenum, where the protein matrix is easily digested, and iron is absorbedCitation12. Furthermore, it is well known that the co-administration of iron supplements and organic acids (i.e. ascorbic acid) enhance the absorption of iron, largely due to their ability to reduce ferric to ferrous ironCitation74. Iron protein succinylate contains succinic acid, an organic acid that improves iron absorption up to 20–30%Citation75.
Compared to the general adult population in which the trend of higher change was maintained across analyzed outcomes, the pooled analysis in the gynecological/obstetric population yielded less, yet positive results. This attenuated trend may be partially explained by the heterogeneity of the populations included in each trial, which encompassed both pregnant women with diagnosed iron deficiency and healthy pregnant women for whom iron supplementation was prescribed as a preventive treatment for iron depletion. Concordantly, the evaluation of the percentage of change of Hb and ferritin in pregnant women with anemia compared with the general population results showed a weaker increasing trend, attributable to the different inclusion criteria between populations: various authors have observed that the effect of iron supplementation is likely to depend on changes in intestinal iron transport induced by iron deficiency and on the baseline iron statusCitation76,Citation77. Therefore, any comparison between the outcomes of iron supplementation in healthy subjects and those with iron deficiency must be taken cautiously.
The pediatric population was the least represented and included mostly ferritin extractive and iron polystyrene sulfonate as comparators. Particularly in this case, all values increased similarly for all treatments analyzed. This population included very diverse subjects in terms of age and weight and, although doses were mainly weight-corrected, the heterogeneity of dosages included in the pooled efficacy analysis and the distinct requirements for different ages may have obscured true differences between treatments on the pooled results.
In the case of ferritin, the increase in hematologic parameters observed in all populations may seem scarce at first glance; however, it might be adequate considering the treatment duration of the included studies. Most guidelines recommend 3–6 months of iron treatment continuation once the Hb levels are restored to replete the iron stores and normalize ferritin levelCitation78. The average treatment duration with iron protein succinylate was of 49 days, clearly not enough to treat the anemia (notice that most of the patients were anemic) and recover iron stores. Nevertheless, it is noteworthy that with a shorter mean treatment duration (49 vs. 58 days), iron protein succinylate achieved better results than ferrous sulfate (average increase of 53 vs. 32%).
Overall, treatment with iron protein succinylate was associated with a much lower rate of AE than other iron formulations (assessed in 6187 patients). Ferrous and ferric complexes showed a similar AE rate, being more than threefold that of iron protein succinylate. Consistently, treatment with iron protein succinylate showed a good tolerability profile, not only in the global pooled safety analysis, but also in patients with special susceptibility such as those with gastrointestinal pathologies and pregnant women. Of all AE potentially associated with iron supplementation, gastrointestinal AEs are the most frequently reported and have been associated with poorer treatment adherence, particularly to ferrous sulfateCitation14.
Regarding related adverse events, even though most studies included in the analysis reported some tolerability results, the reduced number of related events and the poor analysis concerning their relationship with the iron treatment, as it is the case for studies including patients that received iron protein succinylate in the surgery setting, limits conclusions in this regard. Nevertheless, our results showed a better tolerability profile of iron protein succinylate-related adverse events compared with ferrous and ferric complexes, achieving a RAE rate five times lower.
The influence of non-absorbed oral iron on the balance of intestinal flora has been recently highlightedCitation79,Citation80. Although various authors have reported an association between iron supplementation and overgrowth of pathogenic species in children gutCitation81,Citation82, the specific impact of iron protein succinylate on gut flora has not been assessed. Data presented in our review show that iron protein succinylate tends to cause less adverse events – including gut disturbances – than its comparators, thus suggesting a lesser impact on gut flora. Nevertheless, specific studies shall be conducted to further confirm the effect of iron protein succinylate on the intestinal flora.
Our analysis was limited by the unbalanced number of patients in different treatments and the heterogeneity of the investigated populations. Furthermore, a comparative analysis between preparations in patients with anemia or iron deficiency was not performed. However, rather than drawing strong conclusions regarding pairwise comparisons of iron treatments, our review was aimed at providing a picture of 30-year clinical experience and research with iron protein succinylate. In this regard, the exhaustive and barely limited search was likely to capture all this experience, albeit in a descriptive manner.
Conclusion
Three decades of research with iron protein succinylate have generated a considerable amount of evidence regarding its effectiveness and safety. Our pooled analysis of 54 studies indicates that iron protein succinylate achieved similar or higher efficacy results than other oral iron forms, including the widely used ferrous sulfate. Analyzed studies place iron protein succinylate as the iron complex with the lowest rate of AEs and GAEs, confirming its tolerability profile in the overall population and particular populations, such as pregnant women and patients undergoing surgery. Additional information provided in our review may help clinicians to make decisions regarding oral iron supplementation.
Transparency
Declaration of funding
This work was supported by ITF Research Pharma S.L.U. (Italfarmaco).
Declaration of financial/other relationships
AMF has no conflicts of interest to declare. JLMB is a full-time employee at ITF Research Pharma S.L.U. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors, AMF and JLMB contributed equally to the conception and design, the data acquisition, and the analysis of the results. All authors participated in drafting and critically revising the manuscript and gave the final approval to all the versions. Therefore, all authors agree to be accountable for all aspects of the work.
Acknowledgements
The authors would like to thank i2e3 Biomedical Research Institute for providing medical writing assistance, funded by ITF Research Pharma S.L.U.
References
- World Health Organization(WHO), & United Nations Children’s Foundation [Internet]. Focusing on anaemia: towards an integrated approach for effective anaemia control. 2004 [cited 2020 Jan 20]. Available from: https://www.who.int/nutrition/publications/micronutrients/WHOandUNICEF_statement_anaemia/en/
- Preventive Services Task Force Center for Primary Care Prevention and Clinical Partnerships Agency for Healthcare Research and Quality. Screening for iron deficiency anemia—including iron supplementation for children and pregnant women. Am Fam Physician. 2009;79:897–898.
- WHO, CDC. Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level. Geneva (Switzerland): World Health Organization; 2007.
- Clénin GE. The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med Wkly. 2017;147:1–17.
- Low MSY, Speedy J, Styles CE, et al. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst Rev. 2016;4:CD009747.
- Dallman PR. Changing iron needs from birth through adolescence. Nestle Nutr Work Ser. 1992;30:29–38.
- Brutsaert TD, Haas JD, Viola T, et al. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr. 2003;77(2):441–448.
- Scholz BD, Gross R, Schultink W, et al. Anaemia is associated with reduced productivity of women workers even in less-physically-strenuous tasks. Br J Nutr. 1997;77(1):47–57.
- Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309–1316.
- Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87(2):98–104.
- World Health Organization. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva (Switzerland): World Health Organization; 2001.
- Cremonesi P, Camarazza I. Chemical and biological caracterization of iron protein succinylate (ITF 282). Int J Clin Pharmacol Ther Toxicol. 1993;31(1):40–51.
- Cremonesi P, Acerbon A, Raja K. Iron absortion: biochemical and molecular insights into de importance of iron species for intestinal uptake. Pharmacol Toxicol. 2001;91(3):97–102.
- Köpcke W, Sauerland MC. Meta-analysis of efficacy and tolerability data on iron proteinsuccinylate in patients with iron deficiency anemia of different severity. Arzneimittelforschung. 1995;45(11):1211–1216.
- Ambrosini A. Efficacy and tolerability of iron proteinsuccinylate in the treatment of iron deficiemcy due to surgery. Boll Chim Farm Suppl Clinica Terapia. 1988;127(3):40–43.
- Bianchi G. Iron proteinsuccinylate in children’s iron deficiency. Boll Chim Farm Suppl Clinica Terapia. 1988;127(3):30–34.
- Bregani E, Pogliani E, Scotti A. Pilot clinical trial Legofer vs. Ferrograd – summary of final results. Internal Report.
- Careddu P, Caramia G, Giovanelli G. Iron therapy in pediatrics: comparative assessment of efficacy and tolerance of iron proteinsuccinylate vs. sodium iron gluconate; 1989. Internal Report.
- Careddu P, Scott A. Controlled, double-blind, multicenter clinical trial of iron protein succinylate inthe treatment of iron deficiency in children. Int J Clin Pharmacol Ther Toxicol. 1993;31:157–169.
- Cogo R. Comparison of efficacy and tolerability of iron proteinsuccinlate vs. a ferritin comound in iron-deficient patients. Boll Chim Farm Suppl Clinica Terapia. 1988;127(3):25–29.
- Danisi M, Frontespezi S. Terapia dell’anemia sideropenica: studio controllato ferritina versus ferroproteinsuccinilato. Riforma Med. 1987;102:457–459.
- De Pretis G, Bertozzo A, Rizza F, et al. Studio sull’assorbimento de ferroproteinsuccinilao, un composto a ase di ferro coniugato con proteine succinilate, in confronto con gluconato di ferro. Basi Raz Ter. 1988;XVIII:145–148.
- DeRenzo A, Buffardi S, Frigeri F, et al. Studio di efficacia e tollerabilià del ferroproteinsuccinilato verso ferritina nell’anemia sideropenica. Receti Progress Med. 1987;78:562–565.
- DiGiacomo G, DiVirgilio, DS, et al. Loading serum iron curve and iron absoption after the administration of three different preparations. La Riv Medica Ital. 1987;VI:4–5.
- DiSomma C, Moscatelli P, Spiga L, et al. Valutazione comparativa del trattamento con ferroproteinsuccinilato e ferritina. Riforma Medica. 1988;103:385–388.
- Grossi E, Capetta P, Buzzetti G, et al. Iron deficiency in obstetrics: efficacy and tolerability of iron protein-succinylate versus extractive ferritin. Ter Mod. 1988;2:13–16.
- Guerresi E, Ciampini M, Mazzanti C, et al. Effect of administration of two iron preparations on some hematological parameters during pregnancy. La Riv Medica Ital. 1987;VI:29–31.
- Haliotis F, Papanastasiou D. Comparative study of tolerability and efficacy of iron protein succinylate versus iron hydroxide polymaltose complex in the treatment of iron deficiency in children. Int J Clin Pharmacol Ther. 1998;36(6):320–325.
- Kim YH, Chung HH, Kang SB, et al. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase iv, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Landucci G, Frontespezi S. Treatment of iron deficiency conditions in blood donors: controlled study of iron sulphate versus iron protein succinylate. J Int Med Res. 1987;15(6):379–382.
- Leocata A, Magnano C, Caruso V, et al. Evaluation of iron therapy in pediatrics: controlled study of iron-protein succinylate versus ferritin. Acta Pediatr Mediterr. 1988;4:137–140.
- Liguori L. Iron protein succinylate in the treatment of iron deficiency: controlled, double-blind, multicenter clinical trial on over 1,000 patients. Int J Clin Pharmacol. 1993;31:105–123.
- Marcacci B, Pierfederichi P, Signorelli R, et al. Comparative evaluation of iron proteinsuccinylate efficacy and tolerability in the treatment of iron deficiency in obstetrical and ginecological patients vs. organic and inorganic iron salts; 1989. Internal Report.
- Minganti E, Farina A, Farina C, et al. Indagine Clinica Sull’efficacia Terapeutica e sulla Tollerabilità della Ferri-mannitol-albumina in Gravidanza. Giorn It Ost Gin. 1995;10:597–600.
- Minqi J, Caiyun Z, Houjie , et al. Valuazione clinica del trattamento dell’anemia del sangueper carenza di ferro con ferro proteinsuccinilato (ITF) 282 mediante il metodo del confronto casuale. Internal Report.
- Najean Y, Acuto G, Scotti A. Multicentre double-blind clinical trial of iron protein succinylate in comparison with iron sulfate in the treatment of iron deficency anaemia. Clin Drugs Investig. 1995;10(4):198–207.
- Pedrazzoli P, Scotti A, Farina D. Comparison trial of iron succinylprotein complex or iron gluconate complex in the treatment of iron deficiency anemia. Clin Ther. 1988;10:414–420.
- Poggi V, Tedesco M, Conenna R, et al. Iron proteinsuccinylate in children’s iron deficiency: a controlled study versus iron polystyrensulphonate. Ter Mod. 1987;1:197–201.
- Pogliani E, Scotti A, Acuto G. Clinical efficacy and tolerability of iron protein succinylate in comparison to slow release iron sulphate in sideropenic anemia; 1990. Internal Report.
- Farriols PR, Oriol AM, Pérez F, et al. [Iron protein-succinylate in the treatment of adult iron-deficiency anemia]. An Med Interna. 2002;19:651–652. Spanish.
- Rayado B, Carrillo JA, Fernández-Esteban J, et al. Estudio comparativo entre 2 proteínas férricas en la prevención de la anemia ferropénica gestacional. Clin Invest Ginecol Obstet. 1996;24:46–50.
- Scremin S, Caprioglio L. Studio di efficacia e tollerabilità del ferro proteinsuccinilato nella carenza marziale. Boll Chim Farm. 1988;127(3):22–27.
- Sironi G, Masucci R, Gorga M, et al. Uso clinico del complesso ferroproteinsuccinylato nelle anemie sideropeniche in gravidanza. Recent Medica. 1987;XXVI:177–184.
- Veneroni G, Contos S, Tripodi S, et al. Studio clinico controllato in doppio cieco sull’efficacia terapeutica e sulla tollerabilità di un nuovo prodotto a base di ferro organico. Policlin Sez Med. 1996;103:21–29.
- Xing Y, Tong XM. Clinical study of iron protein succinylate oral solution for preventing and treating anemia of prematurity. Chinese J Contemp Pediatr. 2013;15:1059–1063.
- Bracchitta R, Santoni G. Iron proteinsuccinylate in the treatment of iron deficiency in obstetrics and gynecology. Boll Chim Farm Suppl Clinica Terapia. 1988;127(3):35–39.
- Abelli G. Open Clinical trial with Ferrolat 40 in the treatment of iron deficiency anemia; 1984. Internal Report.
- Duntas L, Krassas G, Mantzou E, et al. Effectiveness of combined treatment with L-thyroxine and iron proteinsuccinylate in patients with subclinical hypothyrodism and manufested sideropenic anemia. Nutr Nueroscience. 2000;3(6):407–414.
- Juarez-Vazquez J, Bonizzoni E, Scotti A. Iron plus folate is more effective than iron alone in the treatment of iron deficiency anaemia in pregnancy: a randomised, double blind clinical trial. BJOG: An Intern J Obs Gyn. 2002;109(9):1009–1014.
- Mollica G. Valuazione dell’efficacia della terapia marziale nell’anemia gravidica; 1984. Internal Report.
- Nicola P. Sperimentazione clinica aperta con il prodotto Ferrolat-20- nel trattamento della sideropenia e dell’anemia sideropenica; 1984. Internal Report.
- Piccoli G. Assessment of the biological safety and the subjective tolerability of iron proteinsuccinylate in patients with chronic renal failure and iron deficiency; 1990. Internal Report.
- Bianchi F, Cavassini G, Leo P. Iron proteinsuccinylate in the treatment of iron deficiency: potential interaction with H2-receptor antagonists. Int J Clin Pharmacol Ther Toxicol. 1993;31(5):209–217.
- Bedarida G. Clinical trial of the product Ferrolat of Italfarmaco S.p.A.-Milan; 1983. Internal Report.
- Belloni C. Clinical trial to evaluate the tolerance and the antianemic activity in infants of the compound Ferrolat drinkable ampoules; 1983. Internal Report.
- Schettini F. Open clinical trial with Ferrolat in iron-deficiency anemia; 1984. Internal Report.
- Sifakis S, Angelakis E, Papadopoulou E, et al. The efficacy and tolerability of iron protein succinylate in the treatment of iron-deficiency anemia in pregnancy. Clin Exerimental Obstet Gynecol. 2005;XXXII:117–122.
- Tolino A, Chiacchio G, DiSerio C, et al. Iron proteinsuccinylate: efficacy in the treatment of iron deficiency during pregnancy. Giorn It Ost Gin. 1989;2:146–148.
- Trojachanec Z. Sport anemia and sports results. Internal Report.
- Gruppo Italiano di Studio sulla Sideropenia nell’Anziano G La carenza mariziale nella popolazione anziana instituzionalizzata: indicatori precoci e trattamento farmacologico con ferroprotein succinilato. G Gerontol. 1992;40(7):1–8.
- Duntas L, Papanastasiou E, Mantzou E, et al. Incidence of sideropenia and effects of iron repletion treatment in women with subclinical hypothyrodism. Exp Clin Endocrinol Diabetes. 1999;107:356–360.
- Goisis M. An open clinical trial of ferrolate in patients with iron deficiency anemia; 1983. Internal Report.
- Haya-Palazuelos F, Benet J, Tarragona E. Tolerance and effectiveness of iron proteinsuccinylate among women with iron deficiency anemia or iron starvation status. Toko-Gin Pr. 2001;60:421–428.
- Larramendi C, Marco F, García-Abujeta J, et al. Acute allergic reaction to an iron compound in a milk-allergic patient. Pediatr Allergy Immunol. 2006;17(3):230–233.
- Manfredi B, Finelli F. A new therapeutic approach to iron deficiency. Clin Ter. 1987;123:1–15.
- Moggi C. Clinical trial to assess clinical efficacy and tolerability of the product siderofolin 40 in patients with iron deficiency anemia; 1984. Internal Report.
- Popovska A. Out-Patient treatment of sideropenic anemia with legofer. Internal Report.
- Sallusto A, Eandi M, DeBartolo G, et al. El proteinsuccinilato de hierro: terapéutica de la carencia marcial en obstetricia y ginecología. Minerva Ginecol. 1990;42:1–6.
- Cozzi A, Franceschinelli F, Arosio P, et al. Iron mobilization from protein-iron complexes by chelating agents: an in vitro approach to study iron bioavailability. Quad Ligand Q. 1988;7(2)S:69–77.
- Deriu L, Mastrantoni M. Assorbimiento in Rapporto con il Pasto di un Composto di Ferro Coniugato con Proteine Succinilate. Riforma Med. 1988;103:389–390.
- Pavord S, Myers B, Robinson S, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2012;156(5):588–600.
- Cantor AG, Bougatsos C, Dana T, et al. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the U.S. preventive services task force. Ann Intern Med. 2015;162(8):566.
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. Sci World J. 2012;2012:1–5.
- Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74(6):403–419.
- Goñi MA, Oyarzábal FJ. Ferroterapia. Nociones Generales. Bol Ter Andaluz. 2001;17:1–10.
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91(5):1461S–1467S.
- Thankachan P, Walczyk T, Muthayya S, et al. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr. 2008;87(4):881–886.
- De Franceschi L, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16–23.
- Stein J, Aksan A, Farrag K, et al. Management of inflammatory bowel disease-related anaemia and iron deficiency with specific reference to the role of intravenous iron in current practice. Expert Opin Pharmacother. 2017 ;18(16):1721–1737.
- Yilmaz B, Li H. Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals. 2018 ;11(4):98.
- Zimmermann MB, Chassard C, Rohner F, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr. 2010;92(6):1406–1415.
- Jaeggi T, Kortman GA, Moretti D, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64(5):731–742.