Abstract
Aims: Clinical data have shown that patients with diabetes require shorter training time to use Ateos versus FlexTouch. Using data acquired from a previous study, self-administration procedures that necessitated more time and repetition during mock injection were evaluated.
Methods: In this open-label task- and interview-based crossover study, 48 self-injection naïve participants with type 2 diabetes mellitus (T2DM) were randomized to 1 of 2 sequences to perform a mock injection of Ateos and FlexTouch into a rubber pad after receiving training. Time needed to conduct mock injection steps (preparation, pre-injection set-up, injection, clean-up), and the number and time needed for repeated steps due to procedural errors, were measured as post-hoc analyses.
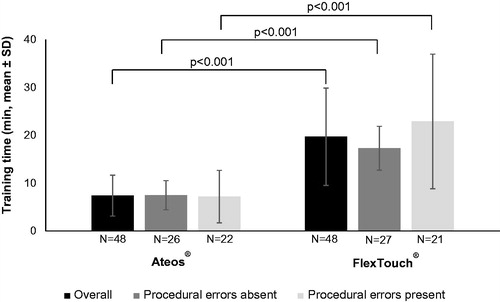
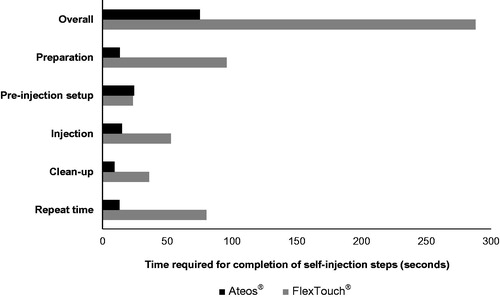
Results: Mean time for preparation, injection, and clean-up was shorter for Ateos (13, 15, 9 s) versus FlexTouch (96, 53, 36 s). Overall time for administration including repeated steps was 75 s for Ateos and 288 s for FlexTouch. Nine participants repeated procedures due to errors when using Ateos (preparation: 6; pre-injection set-up: 2; injection: 1), and 7 participants when using FlexTouch (preparation: 2; pre-injection set-up: 2; injection: 5). There was 1 repeat per person for Ateos injections versus multiple repeats for FlexTouch injections.
Conclusions: Post-hoc analysis demonstrates the time needed for overall administration was shorter for Ateos than FlexTouch, and time for each procedure was shorter or similar for Ateos versus FlexTouch. Ateos was easy for participants with T2DM to learn with fewer repeated steps due to procedural errors, and easy for healthcare professionals to introduce to their patients.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, progressive disease affecting over 400 million people worldwideCitation1. Glycemic control is essential to prevent diabetes-related health complications, with inadequate control leading to serious long-term comorbidities and increased mortalityCitation2. Japan Diabetes Society guidelines recommend insulin and glucagon-like peptide-1 receptor agonists (GLP-1 RA) to manage T2DM if diet, exercise, and prescribed oral antidiabetic medications are inadequate to achieve glycemic controlCitation3. One such therapy is Ateos, a single-use self-injection pen that delivers 0.5 mL dulaglutide (Trulicity, Eli Lilly and Company) subcutaneously via a spring-loaded mechanismCitation4. Ateos has been shown to be safe and effective for self-injection naïve patients with T2DMCitation5.
Healthcare professionals in Japan have limited time available for patient visits in their officeCitation6,Citation7. Time constraints in clinical practice can lead to missed opportunities for patient training on medication self-administration, that can compromise quality of care and contribute to procedural errors. Patient-centered care and self-management support are critical for quality diabetes care and managementCitation8.
A crossover task- and survey-based comparative study evaluated training time for device training, self-administration procedures, and ease of use by participants who were injection-naïve and trained to perform mock self-injections with label-masked pensCitation9. Participants showed a preference for Ateos over FlexTouch (Tresiba, Novo Nordisk), a reusable insulin degludec pen, and overall training time to perform a mock self-injection was shorter for Ateos than FlexTouchCitation9. Here we report results of a post-hoc analysis of data obtained in that study, that was conducted to identify steps that were difficult to learn and required more time to master due to procedural error.
Methods
Study design and participants
This was a multi-center, open-label, 2-period crossover study conducted at 2 sites in Japan, where 48 eligible participants with T2DM were randomly assigned to 1 of 2 sequences to perform mock self-injections with Ateos and FlexTouch into a rubber pad after receiving training on each device, which had masked labels. The same number of participants was recruited at each site. Participants were self-injection naïve adults (≥20 years of age) with T2DM, taking ≥1 oral antidiabetic medication, and able to read and understand Japanese. Exclusion criteria included cognitive impairment, hearing difficulty, visual impairment, severe psychopathology, gestational diabetes, type 1 diabetes mellitus, T2DM treated only with diet and exercise, prior treatment with regular self-injectable medication (e.g. insulin, epinephrine) or insulin pump for any medical condition including T2DM, and employment in a position having a direct role in treating patients with diabetes. All participants gave written informed consent, and the study followed applicable ethical guidelinesCitation9. Details of the primary study were previously reportedCitation9.
Training steps and procedures
Participants received self-injection training by a healthcare professionalCitation9. The training steps were:
the trainer instructed participants on each step of the injection,
participants performed mock injection with support from a trainer, and
participants performed mock injection into a rubber pad without support of a trainer.
Ateos administration procedural steps
(1) Preparation started when the trainer asked the participant to begin the mock injection without the trainer’s support, and ended when the cap was removed from the device by the participant. (2) Pre-injection set-up started when the participant removed the cap and ended when the device was unlocked. (3) Injection started when the device was unlocked and ended when the dose was injected. Dose injection was defined as the time when the participant removed the device from the rubber pad following injection. (4) Clean-up was defined as the time from when the participant injected the dose until they disposed of the device.
FlexTouch administration procedural steps
(1) Preparation started when the participant correctly attached a needle to the device and the inner cap on the device was removed and ended when the participant pushed the injection button to check the flow of medication from the needle. (2) Pre-injection set-up started with the flow check and ended when dose setting occurred. Dose setting was defined as when the participant finished selecting the dose of 10 international units (IU) on the device. (3) Injection started at dose setting and ended upon injection of the dose. Dose injection was defined as the time when the participant removed the insulin device from the rubber pad following injection. (4) Clean-up was defined as the time from when the participant injected the dose until the pen cap was correctly replaced back onto the device.
Statistical analysis
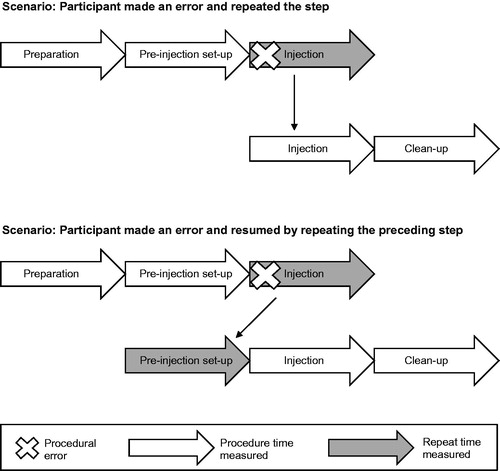
Descriptive statistics were used to report results, with training time analysis stratified by presence or absence of procedural errors. Time required for each procedure was summarized, including number of repeated steps due to procedural error and time associated with repetitions. Participants were video-recorded while performing procedures. Two investigators independently measured the time for each procedural step based on the video. When a participant made an error, the trainer corrected the mistake and the participant repeated the step until no mistakes were made (). Time associated with correctly performed steps and repeated steps was measured. If investigators’ measurements differed by >30 s, both investigators performed the measurement again. Differences between investigators’ measurements were resolved at the discretion of the principal investigator. Time associated with errors was calculated as the difference between the overall time participants performed an injection into a rubber pad without the support of a trainer, and the time for correct performance of preparation + pre-injection + injection + clean-up steps. p-Values were generated from the Student’s t-test for comparisons. The authors confirm that the data supporting the findings of this post-hoc analysis are available within the article.
Figure 1. Study schematic. For this post-hoc analysis, time was measured by investigators. When participants made an error, a trainer corrected the mistake and participants repeated the steps until mastery of the procedure. Number of repeats due to procedural errors (marked with an “X”) were counted. Time was measured for correctly performed procedural steps (white arrows) and steps repeated due to procedural errors (gray arrows).
Results
Demographics
Of the 48 patients with T2DM who participated in the study, the mean (standard deviation) age was 63.5 (8.5) years and 77.1% of the participants were male (). Overall, 41.7% of participants had been diagnosed with diabetes for >10 years and 52.1% had HbA1c ≥7.1% at the time of the survey. Most participants (70.9%) were taking ≥2 antidiabetic medications.
Table 1. Demographics and clinical characteristics.
Training time
Average time for training steps (i) through (iii) was statistically significantly shorter for Ateos than for FlexTouch (p < 0.001) for participants in all populations, overall and the subgroups defined by presence or absence of procedural errors ().
Self-injection time
Mean time was shorter for Ateos versus FlexTouch for preparation (13 versus 96 s), injection (15 versus 53 s), and clean-up (9 versus 36 s) (). The greatest time difference was observed for the preparation step, for which Ateos required approximately seven times less preparation time than FlexTouch. Mean pre-injection set-up time was similar between Ateos and FlexTouch (24 versus 23 s). Mean overall time for procedures including repeated steps was shorter (75 versus 288 s) for Ateos than for FlexTouch. Nine participants repeated steps when using Ateos (preparation: 6; pre-injection set-up: 2; injection: 1), and 7 participants repeated steps when using FlexTouch (preparation: 2; pre-injection set-up: 2; injection: 5) (). The number of repeated steps during injection was 1 per person using Ateos, compared with 8 repeated steps observed in 5 participants using FlexTouch.
Table 2. Self-injection repetitions.
Discussion
Diabetes is a serious disease and growing epidemic that requires individualized therapy managed by healthcare professionalsCitation10. Diabetes management depends upon achieving and maintaining glycemic control to prevent or minimize longer-term risks of diabetes complications, with an aim to ultimately improve and extend patients’ quality of lifeCitation2. Pen devices designed for ease of use to self-administer diabetes medications represent one approach to improve patient self-management and long-term quality of care. This was the first study to identify and compare time requirements and difficulty associated with learning to successfully utilize 2 pen devices, Ateos and FlexTouch, in injection-naïve Japanese patients with T2DM. The clinical relevance of this study is the identification of specific procedures that proved challenging during self-injection training.
This post-hoc analysis showed the time requirement for participants with T2DM who were trained on self-injection administration, and then subsequently performed a mock injection without the assistance of a trainer, was shorter overall for Ateos than for FlexTouch. The time needed to successfully achieve each step of the administration process was either shorter or similar when using the Ateos device compared with FlexTouch. Among participants who had procedural errors, there was 1 repeated step per person when using Ateos, compared with multiple repeated steps per person observed for FlexTouch. When using the Ateos device, participant performance with few errors and shorter repeat time suggested no procedures were perceived as difficult to learn and, importantly, participants had no difficulty with the injection step which is critical to correct administration of the drug.
One limitation with this study was that the devices, which had product labels masked, could still potentially be identifiable by their shape if participants had prior knowledge of either device. Another potential limitation was that training received may have varied among participants since several personnel conducted different training sessions. To minimize teaching bias, we uniformly trained the healthcare professionals (i.e. “trainers”) to administer the self-injection training to study participants. After initial training of the trainers, we checked their training method before they trained any study participants. Additionally, we minimized bias using a crossover study design and assigned an equal number of participants to each trainer. Lastly, the sample size was small, so these results should be interpreted with caution with regard to demonstrating associations between device types.
Conclusions
This post-hoc analysis demonstrated Ateos was easy for patients to learn to self-administer, requiring minimal repeated procedures for preparation, pre-injection set-up, and injection steps. These results provide useful information for potential future improvement of self-injection devices, and for healthcare professionals who train people with diabetes in the use of self-injection devices.
Transparency
Declaration of funding
This study was funded by Eli Lilly Japan, K.K. (Kobe, Japan).
Declaration of financial/other relationships
T. Asakura and TY have received fees for services to Eli Lilly Japan, K.K. ZC and T. Aranashi are full-time employees of Eli Lilly Japan K. K. ZC is a shareholder of Eli Lilly and Company (Indianapolis, IN, USA). Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All of the manuscript authors were involved in aspects of the conception and/or design of the work, including acquisition and/or interpretation of the data. All of the authors made contributions to critical revision of the manuscript, approved the final manuscript version to be published, and have agreed to be accountable for all aspects of the published work.
Previous presentations
These data were published in the form of an abstract and oral presentation at the 8th Annual Meeting of Japan Pharmaceutical and Diabetes Society in Sapporo, Japan; 7–8 September 2019.
Acknowledgements
The authors thank study participants and site personnel who participated in this clinical study. Compensated medical writing and editorial support were provided by Barbara Nambu, PhD, Jennifer Bodie, PhD, and Antonia Baldo of Syneos Health Clinical (Morrisville, NC, USA).
References
- World Health Organization [Internet]. Diabetes fact sheet; 2018. [cited 2019 Jul 15]. Available from: www.who.int/news-room/fact-sheets/detail/diabetes.
- World Health Organization [Internet]. Global report on diabetes; 2016. [cited 2020 Jan 15]. Available from: www.who.int/news-room/fact-sheets/detail/diabetes.
- Japan Diabetes Society [Internet]. Treatment guide for diabetes, 2016–2017. Tokyo, Japan: Bunkodo; 2017. [cited 2019 Jun 14]. Available from: http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2016-2017.pdf.
- Eli Lilly Japan K.K., Kobe, Japan. Trulicity Ateos package insert Ver. 1. Revised May 2019. [cited 2020 March 12]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/530471_2499416G1029_1_12. (Japanese).
- Matfin G, Van Brunt K, Zimmermann AG, et al. Safe and effective use of the once weekly dulaglutide single-dose pen in injection-naïve patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9(5):1071–1079.
- Asakura T. The association between injection device and injection training time of insulin initial self-injection training. Prog Med. 2003;23:3381–3385. (Japanese).
- Yamada KC, Aoki K. Evaluation of usability of insulin self-injection devices. Jpn J Ergonom. 2006;42(2):112–118. (Japanese).
- Glasgow RE, Peeples M, Skovlund SE. Where is the patient in diabetes performance measures? The case for including patient-centered and self-management measures. Diabetes Care. 2008;31(5):1046–1050.
- Asakura T, Suzuki S, Aranishi T, et al. Comparative usability study of the dulaglutide single-use pen versus the insulin degludec FlexTouch among self-injection-naïve patients with type 2 diabetes mellitus in Japan. Curr Med Res Opin. 2018;34(6):1117–1124.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. [cited 2020 Jan 15]. Available from: https://care.diabetesjournals.org/content/diacare/37/Supplement_1/S14.full.pdf.