Abstract
Objective
This economic evaluation aims to provide a preliminary assessment of the cost-effectiveness of radiofrequency ablation (RFA) compared with argon plasma coagulation (APC) when used to treat APC-refractory gastric antral vascular ectasia (GAVE) in symptomatic patients.
Methods
A Markov model was constructed to undertake a cost-utility analysis for adults with persistent symptoms secondary to GAVE refractory to first line endoscopic therapy. The economic evaluation was conducted from a UK NHS and personal social services (PSS) perspective, with a 20-year time horizon, comparing RFA with APC. Patients transfer between health states defined by haemoglobin level. The clinical effectiveness data were sourced from expert opinion, resource use and costs were reflective of the UK NHS and benefits were quantified using Quality Adjusted Life Years (QALYs) with utility weights taken from the literature. The primary output was the Incremental Cost-Effectiveness Ratio (ICER), expressed as cost per QALY gained.
Results
Over a lifetime time horizon, the base case ICER was £4840 per QALY gained with an 82.2% chance that RFA was cost-effective at a threshold of £20,000 per QALY gained. The model estimated that implementing RFA would result in reductions in the need for intravenous iron, endoscopic intervention and requirement for blood transfusions by 27.1%, 32.3% and 36.5% respectively. Compared to APC, RFA was associated with an estimated 36.7% fewer procedures.
Conclusions
RFA treatment is likely to be cost-effective for patients with ongoing symptoms following failure of first line therapy with APC and could lead to substantive reductions in health care resource.
Introduction
Gastric antral vascular ectasia (GAVE) is characterised by the endoscopic appearance of “watermelon stomach” with “longitudinal rugal folds traversing the antrum and converging on the pylorus, each containing a visible convoluted column of vessels, the aggregate resembling the stripes on a watermelon”Citation2. Although the exact incidence of GAVE in a population is unknown, it is a rare cause of upper gastrointestinal bleeding (UGIB) estimated to account for approximately 4% of non-variceal UGIBCitation3. The majority of patients with GAVE have underlying systemic disease including cirrhosis of the liverCitation4, autoimmune diseasesCitation5 and chronic kidney disease, amongst othersCitation6.
Patients with GAVE often develop iron deficiency anaemia, and require treatments with packed red blood cells, oral or intravenous iron supplementationCitation7. GAVE can impact on the patients’ quality of life, requiring regular visits to hospitals. In addition, despite transfusions, many patients remain anaemic, which can impact on fatigue and exercise toleranceCitation8. Furthermore, there is a significant economic impact of the requirement for blood transfusionsCitation9,Citation10.
Medical therapies, including corticosteroids and octreotide, have been trialled and described in short case series or case reports or small clinical trialsCitation11–14. However, there is no clear benefit with any of these.
Surgical treatment can be definitive for GAVE with antrectomy to remove the affected mucosaCitation15,Citation16, but in some cases the surgical mortality and morbidity can outweigh any potential benefitCitation17.
Endoscopic therapy (ET) is, therefore, a mainstay of treatment in patients with GAVE and symptomatic anaemia. Argon plasma coagulation (APC) is a thermoablative method where a high-frequency current is passed through argon gas to cause thermocoagulation. Although other therapies such as endoscopic band ligationCitation18 and Nd:YAG LaserCitation19 can be used, APC is the usual endoscopic treatment of GAVE in most centres on account of being easy to use, relatively safe, at an acceptable costCitation20. However, it can often require multiple sessions, and overall, discontinuation of transfusion dependence is currently achieved in only one-third of the patientsCitation20. APC is not without complications including sepsis, hyperplastic polyps (which can also bleed) and gastric outlet obstructionCitation21–23.
Radio-frequency ablation (RFA) has become the mainstay of endoscopic ablation treatment in such diseases as Barrett’s oesophagus, using the BarrxFootnotei flex system (Medtronic)Citation24. This technique transmits high power energy for a short period of time to ablate tissue in the gastrointestinal tract. RFA has been used in patients for the treatment of GAVE, showing improvements in haemoglobin and reductions in transfusion requirements in patients refractory to APC without complicationsCitation25,Citation26.
The aim of this study was to analyse the cost effectiveness of RFA compared to APC in the treatment of GAVE in those patients who were refractory to first line endoscopic therapy. This was modelled on data from previously published studies and expert opinion.
Methods
Model overview
The cost-effectiveness model was developed using Microsoft Excel and was constructed from the perspective of the United Kingdom (UK) National Health Service (NHS) and Personal Social Services (PSS). The model included a hypothetical cohort of patients with persistent symptoms secondary to gastric antral vascular ectasia (severe anaemia of <8 g dl−1), refractory to first line endoscopic therapy, defined as having undergone at least one session of primary treatment (APC, Endoscopic Band Ligation or YAG-Laser). A lifetime (20-year) time horizon and annual discount rate of 3.5% were applied to the costs and benefits, as recommended by the NICE reference caseCitation27. The model used three-month cycles, with a cohort of patients with an average age of 65 yearsCitation28 entering the model. Patient level benefits were quantified in the model using quality adjusted life years (QALYs) and the main outcome measure was an incremental cost effectiveness ratio (ICER).
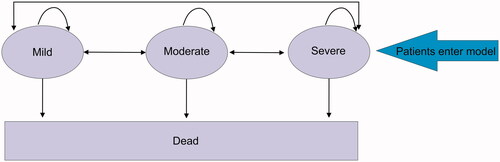
The Markov model developed included three health states based on haemoglobin (Hb) level: mild (10–12 g dl−1), moderate (8 to <10 g dl−1) and severe (<8 g dl−1)Citation1 (see ). All patients began the model in the severe health state and, thereafter, every cycle either remained in their current health state, moved between health states or died based on a series of transition probabilities. In the treatment arm patients were managed with RFA whereas in the comparator arm patients were treated with APC. The effectiveness of the interventions, RFA and APC, were determined by the movement of patients to less severe health states following treatment.
Figure 1. Model structure. The Markov model consists of three health states: mild, moderate and severe anemia, defined by hemoglobin levels. Patients can move between the health states, stay in the same health state or move to the dead state at each cycle. The patients entering the model are APC refractory chronic GAVE patients symptomatic of iron-deficiency anemia; diagnosed with chronic severe iron-deficient anemia for a duration of less than one year but over three months.
Model inputs
A summary of the key model inputs and the sources of the inputs are displayed in and . Due to a lack of available published data, expert opinion was sought to inform certain model inputs. Expert opinion was elicited via structured telephone interviews by the members constructing the model (one standard questionnaire used for all experts). Four NHS consultant gastroenterologists were interviewed, chosen due to their specialism in GAVE. Opinion was sought regarding the disease pathway and specific resource use in relation to it (for example, the proportion of people who receive supplementary iron or a blood transfusion in each health state and the adverse events relating to APC and RFA).
Table 1. Key model inputs (clinical efficacy).
Table 2. Key model inputs (others).
All patients began the model in a severe health state and received treatment. In subsequent cycles, as suggested by experts, almost all patients in the severe health state and a very small proportion in the moderate and mild health states received additional treatment. The movement of patients between health states following treatment was based on transition probabilities informed by expert input. Furthermore, general population mortality was sourced from the UK life tablesCitation29 and was applied to the cohort each cycle.
Patients who received RFA or APC were assumed to receive one treatment session per cycle. The proportion of patients requiring further resource use such as outpatient blood transfusions, endoscopies, hospitalisation, consultant visits, IV iron and oral iron was based on health state and was informed by expert opinion (). No further resource use was included in the model.
Where possible, costs were identified from publicly available sources. The NHS 2017–18 National Schedule of Reference CostsCitation30 was used for hospital-based resource use, including the cost of APC. The cost of RFA was calculated based on a number of sources, including Medtronic UK and a costing audit conducted at one of the centres supplying data for this modelling task (Royal Marsden). The Personal Social Services Research Unit (PSSRU) costsCitation31 and Prescription Cost Analysis dataCitation32 were applied to resource use in primary care. Furthermore, the British National Formulary (BNF)Citation33 was used for pharmaceutical costs. See supplementary material for the breakdown of costs for RFA treatment.
Health state preference weights (utilities) for each health state were sourced from a published systematic review and economic evaluation of treatments for anaemia associated with cancerCitation34. The utility decrements associated with being treated with RFA were extracted from a UK-based cost-utility analysis of RFA for high-grade dysplasiaCitation35. No utility decrements were identified for APC, therefore, it was assumed that patients undergoing APC would experience the same utility decrement as patients undergoing RFA.
Furthermore, utility decrements were associated with stricture and perforations (the adverse events within the model) and were identified from a published NICE clinical guideline for ablative therapy for Barrett’s oesophagus (CG106)Citation36. The adverse event rates for patients within the model were assumed to be the same following RFA and APC and were 0.2% and 0.1% for stricture and perforation, respectively.
Uncertainty analysis
Scenario analyses
Scenarios were run through the model to account for any uncertainty around the parameters estimated by clinical opinion. These included amending the clinical effectiveness of RFA, resource use and utility values. The inputs varied in each scenario are presented in .
Table 3. Scenario analysis inputs.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis (PSA) was carried out to account for the uncertainty around the parameter values by drawing at random for a total of 10,000 iterations. A gamma distribution was applied for all cost parameters and the number of consultant visits per cycle. A beta distribution was used for utility values, adverse event rates, and the proportion of patients requiring treatment, endoscopies, blood transfusions, oral iron and IV iron. Lastly, a dirichlet distribution (a multivariate beta distribution) was applied for transition probabilities.
Results
Base case
The results of the cost-effectiveness model are presented in . Using a threshold value commonly used in UK NHS reimbursement decision making (£20,000), RFA may be a cost-effective treatment for GAVE patients with persistent symptoms as a secondary treatment option for those refractory to first line endoscopic therapy, namely APC (ICER £4672 per QALY gained).
Table 4. Base case results.
Over the lifetime time horizon, on a per-patient basis, RFA is associated with higher overall costs of approximately £2400. However, RFA is also associated with improved patient outcomes of approximately 0.50 years of perfect health.
Furthermore, treatment with RFA rather than APC, over the lifetime time horizon, is associated with a reduced number of treatments: IV Iron, oral iron, blood transfusions, endoscopies and consultant visits. Per patient event counts over the time horizon are presented in . Compared to APC, RFA was also associated with an estimated 36.7% fewer procedures as well as less time in severe health states (1.77 years) and more time in mild health states (1.20 years) when treated with RFA over the 20-year time horizon.
Table 5. Base case results – event counts.
Uncertainty analysis
Scenarios
Altering the time horizon to one year shows that the ICER falls below £20,000 within the first year.
The probability of a patient remaining in a severe health state per cycle when treated with RFA would have to increase by 11% and 15% for the ICER to rise over £20,000 and £30,000 per QALY, respectively. These scenarios, in turn, decrease the probability of patients moving into the moderate health state. These scenarios generate ICERs of £21,563 and £32,248 per QALY.
Utility values for patients with CKD with anaemia were run through the modelCitation37 as CKD is associated with GAVECitation28 and, therefore, may provide more accurate health-related quality of life values for the indicated population that using values of anaemic cancer patients. Using utility values for Stage 3a and Stage 4 CKD patients resulted in an ICER of £12,793 per QALY and £10,672 per QALY, respectively, remaining below the cost-effectiveness threshold.
If almost all patients require IV iron regardless of Hb level (99%), the ICER increases from the base case value but remains below the threshold at £8893. Similarly, if almost all patients require an endoscopy (95%) regardless of Hb level, the ICER increases to £10,105 remaining below the cost-effectiveness threshold.
Further to this, if the proportion of patients needing a blood transfusion in each health state is increased (to 80% in severe, 50% in moderate and 15% in mild), the ICER increases by a minimal amount to £5080.
Finally, when increasing the stricture and perforation rates to 5% in the RFA arm, and when increasing the proportion of patients in the moderate health state that receive treatment to 10%, the ICER increases to £6942 and £7706, respectively, but remains below the cost-effectiveness threshold.
Probability sensitivity analysis
The results of the PSA show that RFA remains below the £20,000 cost-effectiveness threshold in 82.5% of the 10,000 iterations with an average ICER of £4395 (CrI: −£8989 to £44,035). Furthermore, the average net monetary benefit (NMB – representing the monetary value of intervention when a willingness-to-pay threshold for a unit of benefit is knownCitation38) is £8508 (CrI: −£6258 to £23,890). The results of the PSA are plotted in Figure S1 in the supplementary material.
Discussion
The aim of this study was to evaluate the cost-effectiveness of RFA, compared with APC, for patients with persistent symptoms secondary to gastric antral vascular ectasia and refractory to first line endoscopic therapy, namely APC. As APC has been found to be effective in only about 30% of casesCitation20, there is a need for an alternative therapy to reduce blood and iron transfusion requirementsCitation8 and thereby improve quality of life in patients with GAVE. RFA has already been shown to be effective in the treatment of GAVECitation25,Citation26. The model estimates that use of RFA for the aforementioned patient population is likely to be cost-effective in the UK when compared with APC. The per patient results show that with a lifetime time horizon, an ICER of £4672 per QALY gained is generated.
There are a number of limitations to the current study. Due to the scarcity of evidence-based literature surrounding GAVE a large proportion of the model inputs relied on expert opinion, acquired through structured telephone conversations involving four consultant gastroenterologists specialising in GAVE. Expert opinion focused on ensuring the model represented the treatment pathway, as well as resource use and model parameters.
Despite the expert opinion, outcomes from the scenario analyses demonstrate that the resource use parameters with a high level of expert opinion will likely not have a large impact on the decision as to whether or not RFA is cost effective. As shown in the results section, increasing resource use in the mild and moderate Hb states are not likely to alter the decision on whether or not the treatment would be reimbursed.
Further deterministic analysis around key inputs support that the conclusions of the model are not sensitive to variation. For example, when increasing the stricture and perforation rates to 5% in the RFA arm, and when increasing the proportion of patients in the moderate health state that receive treatment to 10%, the ICER remains below the cost-effectiveness threshold.
Altering the clinical effectiveness, and thereby reducing the relative benefit of RFA when compared with APC, does have a larger effect on the ICER and, therefore, may have a bigger effect on the reimbursement decision. However, the probability of an RFA-treated patient remaining in a severe health state would have to increase by 15% above the base case value (whereby 65% stay in the severe health state at each cycle, and only 15% of patients transition to a moderate health state) for the ICER to rise over £30,000. This situation, based on the data available on the benefit of RFA would seem to be highly improbable. This reduces the relative benefit of RFA when compared to APC by over half, in patients who are refractory to APC. The NICE Guide to the Methods of Technology Appraisal state that an ICER of intervention can rise up to £30,000 and can also rise higher than this and still achieve a positive decision if a strong enough case to support the technology can be madeCitation39. As a rare disease with a lack of a current effective treatment, causing only 4% of non-variceal upper GI bleeding in a consecutive case seriesCitation3, a strong case could be made. However, the probability of patients staying in a severe health state would still need to increase by 11% (to 61%) before the ICER rises above £20,000 per QALY.
Due to the lack of published economic modelling or relevant clinical studies in this area it was not possible to externally validate the model by comparing it to published work or to source model inputs using evidence-based literature. Instead, the model was developed through an iterative process with regular input and validation from clinical experts. Although GAVE is a rare disease, it is in the authors’ experience a disease which most gastroenterologists encounter and which can present a management quandary, particularly in those patients with disease refractory to initial therapy. The experts work in referral centres and have extensive experience in treating patients with refractory GAVE, allowing inferences to be drawn from their clinical practice.
Conclusions
Overall, this study demonstrates that, although APC is the most commonly used treatment within the UK NHS for GAVE, RFA is likely to be cost-effective for patients with ongoing symptoms of APC-refractory GAVE. Furthermore, the results indicate that the use of RFA in this patient population could lead to substantive reductions in health care resource as well as a notable impact on a patient’s state of health. As a rare disease, clinical data in this area are limited and data from ongoing registry studies will support more sophisticated assumption(s) beyond expert input.
Transparency
Declaration of funding
Funding for this study was provided by Medtronic, who commissioned SM, JM, and HD to provide consultancy, develop the early economic model, and prepare the manuscript. Medtronic also employs CL.
Declaration of financial/other relationships
SM, JM and HD are employees of York Health Economics Consortium, who were commissioned by Medtronic to provide consultancy and develop the early economic model and to prepare the manuscript. LL reports grants from Medtronic and Pentax Medical, outside the submitted work. CL is a full time employee of Medtronic. There is no further support from any organisation for the submitted work and no further financial relationships with any organisations that might have an interest in the submitted work in the previous three years. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
JM, HD, MS and SM have been involved in model design and construction. All authors have been involved in drafting and editing or reviewing the manuscript. DG, LL, CMurray, CMagee, HS and RH have been involved in providing clinical advice for inputs in the model.
Supplemental Material
Download MS Word (52.1 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Notes
i Barrx is a trademark of Medtronic, Sunnyvale, CA, USA.
References
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017.
- Jabbari M, Cherry R, Lough JO, et al. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87(5):1165–1170.
- Dulai GS, Jensen DM, Kovacs TO, et al. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004;36(1):68–72.
- Ward EM, Raimondo M, Rosser BG, et al. Prevalence and natural history of gastric antral vascular ectasia in patients undergoing orthotopic liver transplantation. J Clin Gastroenterol. 2004;38(10):898–900.
- Marie I, Ducrotte P, Antonietti M, et al. Watermelon stomach in systemic sclerosis: its incidence and management. Aliment Pharmacol Ther. 2008;28(4):412–421.
- Gretz JE, Achem SR. The watermelon stomach: clinical presentation, diagnosis, and treatment. Am J Gastroenterology. 1998;93(6):890–895.
- Novitsky YW, Kercher KW, Czerniach DR, et al. Watermelon stomach: pathophysiology, diagnosis, and management. J Gastrointest Surg. 2003;7(5):652–661.
- Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion. 2008;77(2):131–137.
- Oge T, Kilic CH, Kilic GS. Economic impact of blood transfusions: balancing cost and benefits. EAJM. 2014;46(1):47–49.
- Stokes EA, Wordsworth S, Staves J, et al. Accurate costs of blood. Transfusion. 2018;58(4):846–853.
- Fuccio L, Mussetto A, Laterza L, et al. Diagnosis and management of gastric antral vascular ectasia. WJGE. 2013;5(1):6–13.
- Barbara G, De Giorgio R, Salvioli B, et al. Unsuccessful octreotide treatment of the watermelon stomach. J Clin Gastroenterol. 1998;26(4):345–346.
- McFarlane M, O’Flynn L, Ventre R, et al. Emerging role of thalidomide in the treatment of gastrointestinal bleeding. Front Gastroenterol. 2018;9(2):98–104.
- Bhowmick BK. Watermelon stomach treated with oral corticosteroid. J R Soc Med. 1993;86(1):52–52.
- Sherman V, Klassen DR, Feldman LS, et al. Laparoscopic antrectomy: a novel approach to treating watermelon stomach. J Am Coll Surg. 2003;197(5):864–867.
- Kruger R, Ryan ME, Dickson KB, et al. Diffuse vascular ectasia of the gastric antrum. Am J Gastroenterol. 1987;82(5):421–426.
- Spahr L, Villeneuve JP, Dufresne MP, et al. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44(5):739–742.
- Elhendawy M, Mosaad S, Alkhalawany W, et al. Randomized controlled study of endoscopic band ligation and argon plasma coagulation in the treatment of gastric antral and fundal vascular ectasia. United European Gastroenterol J. 2016;4(3):423–428.
- Mathou NG, Lovat LB, Thorpe SM, et al. Nd:YAG laser induces long-term remission in transfusion-dependent patients with watermelon stomach. Lasers Med Sci. 2004;18(4):213–218.
- Nakamura S, Mitsunaga A, Oi I, et al. Long-term follow-up of gastric antral vascular ectasia treated by argon plasma coagulation. Gastrointest Endosc. 2005;61(5):AB177.
- Roman S, Saurin JC, Dumortier J, et al. Tolerance and efficacy of argon plasma coagulation for controlling bleeding in patients with typical and atypical manifestations of watermelon stomach. Endoscopy. 2003;35(12):1024–1028.
- Farooq FT, Wong RC, Yang P, et al. Gastric outlet obstruction as a complication of argon plasma coagulation for watermelon stomach. Gastrointest Endosc. 2007;65(7):1090–1092.
- Baudet JS, Salata H, Soler M, et al. Hyperplastic gastric polyps after argon plasma coagulation treatment of gastric antral vascular ectasia (GAVE). Endoscopy. 2007;39(S 1):E320–E320.
- Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145(1):87–95.
- Magee C, Lipman G, Alzoubaidi D, et al. Radiofrequency ablation for patients with refractory symptomatic anaemia secondary to gastric antral vascular ectasia. United European Gastroenterol J. 2019;7(2):217–224.
- McCarty TR, Rustagi T. Comparative Effectiveness and safety of radiofrequency ablation versus argon plasma coagulation for treatment of gastric antral vascular ectasia: a systematic review and meta-analysis. J Clin Gastroenterol. 2019;53(8):599–606.
- National Institute for Health and Care Excellence (NICE). Developing NICE guidelines: the manual. London: NICE; 2014.
- Jalil A, Ponichtera J, Hameed H, et al. Gastric antral vascular ectasia: clinical features, associations and prognosis. Poster Gut. 2013;62(Suppl 2):A46.2–A47. A1–A50.
- Office for National Statistics. National Life Tables, United Kingdom, 1980–82 to 2014–16. 2018.
- NHS improvement. National schedule of reference costs 2017–18. 2017.
- Personal Social Services Research Unit. Unit Costs of Health and Social Care 2018. London 2018.
- NHS Digital. Prescription Cost Analysis – England, 2018. [PAS]. 2018.
- National Institute for Health and Care Excellence (NICE). British National Formulary. 2019.
- Wilson J, Yao GL, Raftery J, et al. A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Asses. 2007;11(13):1.
- Boger PC, Turner D, Roderick P, et al. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s oesophagus. Aliment Pharm Ther. 2010;32(11-12):1332–1342.
- National Institute for Health and Care Excellence (NICE). Barrett’s oesophagus: ablative therapy: Cost Effectiveness Analysis for Barrett’s Oesophagus. Clinical guideline [CG106]. London: NICE; 2010.
- van Haalen H, Jackson J, Salehi H, et al. SP333 health state utility of patients with chronic kidney disease and anemia: analysis of EQ-5D in a real world population. Nephrol Dial Transpl. 2018;33(suppl_1):i456–i468.
- York Health Economics Consortium. Net Monetary Benefit [online]: York Health Economics Consortium. 2016. [cited 2019 May 9]. Available from: https://www.yhec.co.uk/glossary/net-monetary-benefit/
- National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. 2013.
