Abstract
Objectives: To assess long-term (2-year) biologic treatment patterns of psoriatic arthritis (PsA) patients who initiated adalimumab, certolizumab pegol, etanercept, golimumab, or ustekinumab.
Methods: Adult patients with ≥1 pharmacy or medical claim for injectable PsA biologics (index date) were identified from the Optum’s Clinformatics Data Mart (1 January 2013–31 December 2016). Adherence, persistence, post-discontinuation treatment patterns, and addition of adjunctive medications were evaluated by index biologic.
Results: Of 996 patients included (mean [SD] age: 51.5 [12.6] years; female: 49.4%), the most common index biologics initiated were adalimumab (47.9%) and etanercept (34.5%). The mean [SD] proportion of days covered was 0.48 [0.32] for the index biologics. During the 24-month follow-up period, 19.7% of patients persisted on their index biologic; ustekinumab had the highest persistence rate (27.2%), followed by adalimumab (22.0%), golimumab (18.4%), certolizumab pegol (15.6%), and etanercept (15.4%). Of the 800 patients (80.3%) who discontinued their index biologic therapy, 35.0% restarted, 40.1% switched to another biologic, and 31.8% did neither during the follow-up period. The most common biologics patients switched to were adalimumab (31.2%) and ustekinumab (18.7%). Among patients who persisted with their index biologic for ≥90 days (n = 753), ≥1 adjunctive medication was added for 50.1% of patients. The most common adjunctive medications included corticosteroids (28.0% of patients), opioids (17.0%), nonsteroidal anti-inflammatory drugs (NSAIDs) (13.8%), and conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs) (7.3%).
Conclusions: In this real-world study of use of biologic PsA therapies, 24-month persistence was low (19.7%), and treatment was frequently supplemented with adjunctive medications.
Introduction
Psoriatic arthritis (PsA) is a chronic, immune-mediated inflammatory disease characterized by peripheral and axial joint inflammation, enthesitis, dactylitis, and skin lesions associated with psoriasisCitation1. Patients with PsA can have mild to severe disease and suffer from joint and enthesial pain, stiffness, and swelling that can progress to structural tissue damage leading to decreased function and disabilityCitation1,Citation2. In the United States, PsA is estimated to affect between 0.06% and 0.25% of the general populationCitation2; among patients with psoriasis, up to 30% may develop PsACitation3.
Biologic therapies approved for the treatment of PsA in the U.S. include the tumor necrosis factor (TNF) inhibitors, adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab; the interleukin 12/23 (IL-12/23) inhibitor, ustekinumab; the interleukin IL-17 inhibitors, secukinumab and ixekizumab; and the selective T-cell costimulation modulator, abatacept. The 2018 PsA treatment guidelines from the American College of Rheumatology/National Psoriasis Foundation recommends a treat-to-target strategy and emphasizes the importance of considering an individual patient’s situation, including disease characteristics, past treatment response, comorbidity profile, and patient preferences, to choose the optimal treatment among the many available therapeutic options for PsACitation4. For patients with PsA, the treatment management goals are to minimize symptoms, delay or inhibit structural damage, and improve functional ability and quality of lifeCitation5.
Although multiple biologic therapies have demonstrated long-term response in randomized clinical trials for PsACitation6, such patient populations and settings may not reflect the general PsA population or real-life practice patterns (e.g. switching or adding therapies for patients with inadequately controlled symptoms). Treatment adherence and persistence to biologic therapies is an important determinant of patient outcomes in rheumatic diseases, including PsACitation7,Citation8. A recent administrative claims database study of patients with PsA found that less than half of patients who newly initiated a biologic were persistent with their index TNF or IL-12/23 inhibitor during a 1-year follow-up periodCitation9. This persistence rate was approximately 5–20% lower than observed in earlier conducted studies that also were based on claims database analyses with a 1-year follow-upCitation10–13. To better understand long-term patterns of injectable biologic therapies for PsA, we assessed 2-year adherence, persistence, and switching patterns of PsA patients who initiated adalimumab, certolizumab pegol, etanercept, golimumab, or ustekinumab, as well as adjunctive medication usage.
Methods
Data source
This retrospective observational cohort study used the US administrative medical and pharmacy claims data obtained from the Optum Clinformatics Extended SES Data Marti database between 1 January 2012 and 30 September 2018. The Optum claims database is a longitudinal data source primarily comprised of United Healthcare (UHC) members with commercial plans and a growing proportion of members with Medicare Advantage health plans. Claims data consist of patient demographics, enrollment start and end dates, adjudicated pharmacy claims (e.g. outpatient prescriptions), and medical claims (e.g. inpatient and outpatient medical services) submitted for payment by providers, healthcare facilities, and pharmacies. The claims data includes information on physician visits, medical procedures, hospitalizations, drugs dispensed, date of services/prescriptions, and number of days of medications supplied. The data within Optum is de-identified and fully compliant with the Health Insurance Portability and Accountability Act (HIPAA). As this was a retrospective analysis of existing claims data and no patient-identifiable information was included in the claims dataset, institutional review board (IRB) approval was not required (per Title 45 of CFR, Part 46.104(d)(4).
Patient selection
Adult patients with ≥1 pharmacy or medical claim for adalimumab, certolizumab pegol, etanercept, golimumab, ustekinumab, or secukinumab from 1 January 2013 to 31 December 2016 were identified from the Optum database. The date of the first biologic claim was designated as the index date. Data extending back to 2012 were used to look at pre-index clinical characteristics, whereas data extending forward into 2018 were used to look at post-index outcomes. To increase the specificity of patient identification, patients were required to have ≥2 medical claims with PsA diagnoses (ICD-9-CM: 696.0; ICD-10-CM: L40.50-L40.54, L40.59) that occurred ≥30 days apart during the period from 12 months pre-index to 12 months post-index, with the first diagnosis having occurred on or prior to the index date. Such criteria are similar to those used in other published studiesCitation9,Citation14–16. Patients were also required to be ≥18 years of age on the index date and have continuous health plan enrollment for ≥12 months pre-index and during the ≥24-month post-index period. Patients were excluded if they had ≥2 different biologics filled on the same day during the study period or if they had a claim for a biologic filled during the 12-month pre-index period. Patients were also excluded if they had a medical claim during the 12-month pre-index period for rheumatoid arthritis, ankylosing spondylitis, other spondyloarthropathies, or any contraindications for the index biologic. Patients were grouped into 6 study cohorts based on their index medication.
Study measures
Demographic characteristics recorded on the index date included age, gender, U.S. geographic region, and insurance type. Baseline comorbidity scores (Quan–Charlson Comorbidity Index [QCI]) based on the presence of diagnosis codes on medical claims were calculated, and general comorbid conditions (hypertension, hyperlipidemia, diabetes, obesity, anxiety, depression based on diagnosis codes on medical claims) were evaluated during the 12-month pre-index period.
Adherence and persistence with index biologic therapy were measured during a 24-month post-index period. Adherence was measured by the proportion of days covered (PDC), defined as the total number of non-overlapping days covered by prescription claims for the index biologic therapy divided by 730 days. Given that days’ supply is not available for biologics identified from medical claims, rigorous exploratory analyses were conducted to impute days’ supply of medical claims based on the most frequently observed days’ supply for each medication from pharmacy claims. For instance, of all the selected pharmacy claims for adalimumab, 96% of claims had days’ supply of 28 during the study period; therefore, 28 days were assigned for adalimumab identified from medical claims; similarly, 28 days’ supply were assigned for certolizumab pegol and etanercept; 30 days for golimumab. Given that ustekinumab is recommended to be administered every 12 weeks (84 days) after the first dose and more than half of the pharmacy claims for ustekinumab had days’ supply of 84 or 90 days, 84 days was assigned for ustekinumab days’ supply identified from medical claims.
Persistence was measured by the number of days from the index date to the discontinuation date of the index biologic therapy or the end date of the 24-month follow-up, whichever was earliest. There is no uniform definition for the appropriate length of permissible gap over multiple refill intervals; thus, definitions on discontinuation have varied among studies. As the maintenance dosing interval for ustekinumab is generally longer than that for other index biologics, this study used a variable length permissible gapCitation17. Discontinuation of index biologic therapy was defined as having a gap in therapy of ≥1.5 times days approximating the most frequently observed days’ supply from the end of days’ supply of one prescription to the date of next prescription, i.e. 45 (1.5*30) days for adalimumab, certolizumab pegol, etanercept, and golimumab; 135 (1.5*90) days for ustekinumab. The discontinuation date of the index biologic was designated as the fill date plus days’ supply of the last prescription claim filled prior to the gap in therapy. To better understand how outcomes differ using a variable permissible gap versus a fixed permissible gap, a sensitivity analysis was conducted, in which discontinuation of the index biologic was defined as a gap of ≥90 days between the end of days’ supply of one prescription and the date of the next prescription for all index biologics.
For patients who discontinued their index biologic therapy, post-discontinuation treatment patterns were evaluated during the 24-month post-index period, including discontinuing and restarting the index biologic therapy, discontinuing and switching to another biologic therapy, and discontinuing the index therapy without restarting or switching. The categories of restarted, switched, and without restart/switch were not defined as mutually exclusive groups in this study. Among those who switched to a different biologic therapy, the therapy that patients were switched to was also reported.
For patients who persisted with their index biologic therapy for ≥90 days, the usage of adjunctive medications, i.e. having at least one medical or pharmacy claim of anxiolytics, antidepressants, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), targeted synthetic DMARDs, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, corticosteroids, and sleep aids, were examined during the period from 90 days after the index date to the end of persistence or the end of the 24-month post-index period. The cutoff of 90 days was chosen because each biologic is expected to achieve maximum efficacy by 90 days after the initiation.
Statistical analyses
For descriptive analyses, mean and standard deviations (SD) were reported for continuous variables whereas frequency and percentage were reported for categorical variables. To test the statistical differences in patient characteristics and treatment patterns among the study cohorts, ANOVA was used for continuous variables and chi-square test for categorical variables. A p-value of <.05 was considered statistically significant. All data analyses were conducted using SAS Enterprise Guide 7 (SAS Institute, Cary, NC).
Results
Baseline characteristics of study patients
A total of 1005 patients who were newly initiated on biologic therapy were identified, of which 9 patients who initiated secukinumab were further excluded from analyses due to the small sample size (). Among the total patients included in the study population (n = 996; mean [SD] age: 51.5 [12.6] years; 49.4% female), the most common index biologic therapy initiated was adalimumab (47.9%), followed by etanercept (34.5%), ustekinumab (8.1%), golimumab (4.9%), and certolizumab pegol (4.5%) (). The overall mean QCI score of the study population was 0.6 [1.1] at baseline. The percentages of patients with general comorbid conditions were similar across the cohorts.
Table 1. Baseline characteristics of study cohorts.
Treatment adherence and persistence
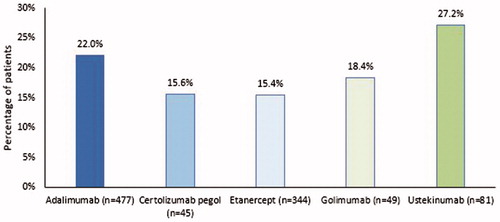
Overall, the mean [SD] PDC for index biologics was 0.48 [0.32] during the 24-month post-index period (). Mean PDC was not statistically different across the study cohorts (p = .712); numerically, similar mean [SD] PDC was observed for patients who initiated adalimumab (0.49 [0.33]), etanercept (0.48 [0.32]), golimumab (0.46 [0.34]), and ustekinumab (0.45 [0.27]). During the 24-month post-index period, 19.7% of the study patients were persistent with their index biologic therapy (mean duration [SD]: 333 [266] days), with ustekinumab having the highest persistence rate (27.2%), followed by adalimumab (22.0%), golimumab (18.4%), certolizumab pegol (15.6%), and etanercept (15.4%)) (p = .061; ; ). The mean duration of persistence was greatest among users of ustekinumab (407 [259] days) and lowest for users of certolizumab pegol (300 [268] days; p = .024 across all study cohorts). The discontinuation rate of the overall study population was high at 80.3% (mean time to discontinuation: 236 [199] days). The results of the sensitivity analysis are shown in Supplementary Figure 1.
Table 2. Adherence and persistence with index biologic therapies and post-discontinuation treatment patterns.
Post-discontinuation treatment patterns
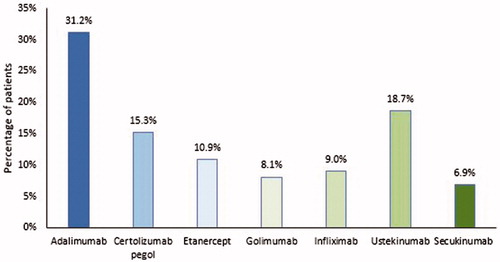
Of the 800 patients who discontinued their index biologic therapy, 35.0% of patients restarted their index biologic therapy, 40.1% switched to another biologic therapy, and 31.8% did not restart or switch their index biologic therapy during the follow-up period (). Of those who switched to another biologic therapy (n = 321), the mean time to switching was 125 [163] days and the most common biologics switched to were adalimumab (31.2%) and ustekinumab (18.7%) ().
Usage of adjunctive medications
Among patients who persisted with their index biologic therapy for ≥90 days (n = 753), 50.1% were treated with ≥1 adjunctive medication during the observation period; the most common adjunctive medications used were corticosteroids (28.0%), opioids (17.0%), and NSAIDs (13.8%) (). The usage of adjunctive medications did not statistically significantly differ across the study cohorts.
Table 3. Adjunctive medications initiated from 90 days after the index date to the end of persistence or 24 months.
Discussion
To our knowledge, this study is the first to assess long-term, two-year treatment patterns of biologics among patients with PsA in a real-world setting. Results from this study showed that the majority of PsA patients discontinued their index biologic (72.8–84.6%) in the 2-year follow up period after initiation of treatment, with an overall average persistence duration of approximately 11 months. Such findings highlight the need for improvement for better long-term management options for patients with PsA.
Previous studies have documented the poor rate of persistence with biologic therapy among PsA patientsCitation9,Citation18. Walsh et al. reported a 1-year overall persistence rate of 44.5% and a discontinuation rate of 55.5% among PsA patients treated with biologic therapies, also identified from the Optum database (1 January 2013–31 January 2015)Citation9. In another recent claims database analysis of PsA patients treated with biologic therapies identified from the IBM databases (15 January 2016–31 July 2017), Oelke et al. reported an overall 1-year rate of discontinuation of 43.6%Citation18. The findings of this novel study with a 2-year follow-up demonstrate that persistence of biologic therapy among patients with PsA substantially declines over the long term.
Of the six injectable biologic therapies evaluated in this study, ustekinumab had the most favorable persistence and lowest rate of discontinuation. However, persistence is impacted by a broad array of factors, such as efficacy, tolerability, preferences, costs, and drug availability, so greater persistence may not necessarily reflect greater efficacy. In an exploratory analysis of patients initiating secukinumab (n = 9), no patient persisted with this index biologic for 24 months; five patients did not have any restart or switch after discontinuation and 1 patient switched to adalimumab.
Additionally, this study demonstrated that results from sensitivity analyses varied across the index biologic groups; furthermore, the persistence of index biologics depends on the permissible gap definition and/or days’ supply imputed from medical claims. Consistent with our findings, Oelke et al. conducted sensitivity analyses using fixed gap cut-offs of ≥45 and ≥180 days as alternative definitions of discontinuation and found that the overall discontinuation rate across all biologics was 56.0% using a shorter gap (≥45 days) and only 29.8% using a longer gap (≥180 days)Citation18. However, Oelke et al. only used outpatient pharmacy claims to identify patients with TNF or IL-17 inhibitors and only used pharmacy claims to evaluate treatment adherence and persistence on index biologics. As biologics are covered by both medical and pharmacy benefits, outcome measures, such as adherence and persistence, could be underestimated without taking medical claims into account. Our study included medical claims of biologics and imputed days’ supply of medical claims based on results from rigorous exploratory analyses.
Previous studies have defined discontinuation as no refill for a short gap (45, 60, or 90 days) after the days’ supply of the previous prescription ran out. While this definition is appropriate for comparing persistence with biologics that are typically dispensed monthly (adalimumab, etanercept, certolizumab, golimumab), it is suboptimal for comparisons with biologics dispensed less frequently (ustekinumab) because ustekinumab has the longest maintenance dosing frequency (84 days) compared to other evaluated biologics. Given the maintenance dosing interval varies across the index biologics, the persistence rate of ustekinumab would be underestimated if a single fixed permissible gap was assigned to all of the study biologics. Instead, a permissible gap of 1.5 times the most frequently observed days’ supply was applied in this study to mitigate the potential bias due to a fixed permissible gap.
Switching to another biologic therapy after discontinuation was common among PsA patients included in this study, with 40.1% of those who discontinued their index biologic switching to a different biologic during the 2-year follow-up period. Among study patients, the most common biologics switched to were adalimumab and ustekinumab. Previous studies have shown that adverse effects and lack of efficacy are the most common reasons for PsA patients discontinuing their index TNF inhibitor and switching to another biologic. Additionally, switching to another biologic therapy with a different mechanism of action may yield a better response to treatmentCitation19,Citation20. Further in-depth study of the outcomes of PsA patients who switch biologic therapies should be pursued.
In the current study, one-half of PsA patients who persisted with their index biologic therapies also took adjunctive medications. Consistent with the findings of Walsh et al.Citation9, anti-inflammatory and pain medications were the most prevalently used, suggesting treatment with biologics alone may not adequately control all of the symptoms of PsA. Such inadequate control of PsA may contribute to compromised adherence and persistence, thereby leading to disease progression. Further study of the continued symptoms PsA patients experience while on biologic therapies is needed in the context of persistence versus interrupted therapy.
The current study is subject to limitations. The sample size of some treatment groups was relatively small and treatment patterns of biologics were limited to the 24-month observation period. As with other retrospective claims database analyses, administrative claims data are collected for the purpose of facilitating payment for healthcare services and therefore, definitive (clinician verified) diagnoses are not available. Prescription dispensing records captured in the claims data do not necessarily indicate the actual administration of the medication. Claims data may not capture generic (paid out of pocket) or over-the-counter medications and therefore usage of these medications, including over-the-counter NSAIDs and topical analgesics, was not determined. Also, it is not clear whether the adjunctive medications were used for the treatment of PsA symptoms or for conditions unrelated to PsA because such information was not available in the claims database. Similarly, causes of study withdrawal, reasons for treatment discontinuation, switching of therapies, or adding adjunctive medications could not be determined from claims. Several unmeasured factors may contribute to changes in biologic therapy, including lack of efficacy, adverse effects, and withdrawal due to minimal activity of diseaseCitation20–23. PsA is a heterogenous disease and treatment choices may be influenced by various phenotypic features, as well as preferences of patients, providers, or payers. This study was not designed to assess factors affecting treatment selection or predictors of persistence. Further study is warranted to determine potential risk factors for altering treatment patterns, so as to better develop strategies that can improve the long-term management of biologic therapy of patients with PsA. In addition, events such as discontinuation that occurred after the 24-month observed follow-up period were not captured; it is possible that discontinuations at the end of the follow-up period were captured more frequently for biologics with shorter vs. longer treatment gap definitions (45 days for adalimumab, certolizumab pegol, etanercept, golimumab vs. 135 days for ustekinumab). However, a fixed follow-up period allowed for optimal comparison of outcomes across the study groups. This study only included PsA patients covered by commercial or Medicare Advantage insurance plans (79.3% vs 20.7%, respectively); therefore, the results may not be generalizable to patients with other insurance types, such as Medicaid, the overall Medicare covered population, those without health insurance coverage, and those in other countries with different types of available health services. Lastly, due to the small sample size of certain treatment groups, matching or multivariable regression were not performed and the causal relationship between treatment patterns and patient characteristics or outcomes could not be established. However, a strength of this study was its use of large administrative claims data of patients from geographically dispersed U.S. health plans to assess outcome measures within a 2-year follow up period. This adds to existing evidence to help better understand the long-term treatment patterns of biologics and the use of adjunctive medication among patients with PsA who initiated biologics.
Conclusions
In this real-world study of use of biologic PsA therapies, 24-month persistence was low, ranging from 15.4% to 27.2%. Of the six injectable biologic therapies evaluated in this study, ustekinumab had the most favorable persistence and lowest rate of discontinuation. However, persistence can be impacted by a broad array of factors, such as efficacy, tolerability, preferences, costs, and drug availability, and thus, greater persistence may not necessarily reflect greater efficacy. Half of patients used an adjunctive therapy, many of which were anti-inflammatory or pain medications. The widespread interruptive biologic treatment patterns and high use of pain and anti-inflammatory medications observed in this real-world study highlight the significant need for improved long-term management of patients with PsA.
Transparency
Declaration of funding
This research and preparation of this manuscript was sponsored by Janssen Scientific Affairs, LLC.
Declaration of financial/other relationships
JAW has received grants and/or served as a consultant for Pfizer, AbbVie, Novartis, Lilly, and UCB. QC, IL, TF, CDP, and SDC are employees of Janssen Scientific Affairs, LLC, and stockholders of Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Supplemental_Figure_1.docx
Download MS Word (23.7 KB)Acknowledgements
The authors would like to thank Jay Lin and Melissa Lingohr-Smith of Novosys Health for their writing contribution to this manuscript and acknowledge that this contribution was supported by Janssen Scientific Affairs, LLC.
Note
Notes
i Optum, Eden Prairie, MN.
References
- Cantini F, Niccoli L, Nannini C, et al. Psoriatic arthritis: a systematic review. Int J Rheum Dis. 2010;13(4):300–317.
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545–568.
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735.
- Singh J, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32.
- Ceponis A, Kavanaugh A. Treatment of psoriatic arthritis with biological agents. Semin Cutan Med Surg. 2010;29(1):56–62.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21–37.
- Gottlieb A, Gratacos J, Dikranian A, et al. Treatment patterns, unmet need, and impact on patient-reported outcomes of psoriatic arthritis in the United States and Europe. Rheumatol Int. 2019;39(1):121–130.
- Vangeli E, Bakhshi S, Baker A, et al. A systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseases. Adv Ther. 2015;32(11):983–1028.
- Walsh JA, Adejoro O, Chastek B, et al. Treatment patterns among patients with psoriatic arthritis treated with a biologic in the United States: descriptive analyses from an administrative claims database. J Manag Care Spec Pharm. 2018;24(7):623–631.
- Chastek B, White J, Van Voorhis D, et al. A retrospective cohort study comparing utilization and costs of biologic therapies and JAK inhibitor therapy across four common inflammatory indications in adult US managed care patients. Adv Ther. 2016;33(4):626–642.
- Zhang HF, Gauthier G, Hiscock R, et al. Treatment patterns in psoriatic arthritis patients newly initiated on oral nonbiologic or biologic disease-modifying antirheumatic drugs. Arthritis Res Ther. 2014;16(4):420.
- Bonafede M, Fox KM, Watson C, et al. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real world settings. Adv Therapy. 2012;29(8):664–674.
- Zhu B, Edson-Heredia E, Gatz JL, et al. Treatment patterns and health care costs for patients with psoriatic arthritis on biologic therapy: a retrospective cohort study. Clin Ther. 2013;35(9):1376–1385.
- Merola JF, Herrera V, Palmer JB. Direct healthcare costs and comorbidity burden among patients with psoriatic arthritis in the USA. Clin Rheumatol. 2018;37(10):2751–2761.
- Kern DM, Chang L, Sonawane K, et al. Treatment patterns of newly diagnosed rheumatoid arthritis patients from a commercially insured population. Rheumatol Ther. 2018;5(2):355–369.
- Crane MM, Juneja M, Allen J, et al. Epidemiology and treatment of new‐onset and established rheumatoid arthritis in an insured US population. Arthritis Care Res. 2015;67(12):1646–1655.
- Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–457.
- Oelke KR, Chambenoit O, Majjhoo AQ, et al. Persistence and adherence of biologics in US patients with psoriatic arthritis: analyses from a claims database. J Comp Eff Res. 2019;8(8):607–621.
- Reddy SM, Crean S, Martin AL, et al. Real-world effectiveness of anti-TNF switching in psoriatic arthritis: a systematic review of the literature. Clin Rheumatol. 2016;35(12):2955–2966.
- Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29–37.
- Palmer JB, Li Y, Herrera V, et al. Treatment patterns and costs for anti-TNFα biologic therapy in patients with psoriatic arthritis. BMC Musculoskelet Disord. 2016;17(1):261.
- Huynh DH, Boyd TA, Etzel CJ, et al. Persistence of low disease activity after tumour necrosis factor inhibitor (TNFi) discontinuation in patients with psoriatic arthritis. RMD Open. 2017;3(1):e000395.
- Cantini F, Niccoli L, Nannini C, et al. Frequency and duration of clinical remission in patients with peripheral psoriatic arthritis requiring second-line drugs. Rheumatology (Oxford. 2008;47(6):872–876.