Abstract
Objective: To estimate the prevalence and economic burden of hyperkalemia in the United States (US) Medicare population.
Methods: Patients were selected from a 5% random sample of Medicare beneficiaries (01 January 2010–31 December 2014) to estimate the prevalence and economic burden of hyperkalemia. The prevalence for each calendar year was calculated as the number of patients with hyperkalemia divided by the total number of eligible patients per year. To estimate the economic burden of hyperkalemia, patients with hyperkalemia (cases) were matched 1:1 to patients without hyperkalemia (controls) on age group, chronic kidney disease [CKD] stage, dialysis treatment, and heart failure. The incremental 30-day and 1-year resource utilization and costs (2016 USD) associated with hyperkalemia were estimated.
Results: The estimated prevalence of hyperkalemia was 2.6–2.7% in the overall population and 8.9–9.3% among patients with CKD and/or heart failure. Patients with hyperkalemia had higher 1-year rates of inpatient admissions (1.28 vs. 0.44), outpatient visits (30.48 vs. 23.88), emergency department visits (2.01 vs. 1.17), and skilled nursing facility admissions (0.36 vs. 0.11) than the matched controls (all p < .001). Patients with hyperkalemia incurred on average $7208 higher 30-day costs ($8894 vs. $1685) and $19,348 higher 1-year costs ($34,362 vs. $15,013) than controls (both p < .001). Among patients with CKD and/or heart failure, the 30-day and 1-year total cost differences between cohorts were $7726 ($9906 vs. $2180) and $21,577 ($41,416 vs. $19,839), respectively (both p < .001).
Conclusions: Hyperkalemia had an estimated prevalence of 2.6–2.7% in the Medicare population and was associated with markedly high healthcare costs.
Introduction
Hyperkalemia is an electrolyte abnormality, defined as abnormally high serum potassium (≥5.0 mEq/L)Citation1. While hyperkalemia may be asymptomatic, a high serum potassium concentration can lead to muscle cramps and weakness, nausea, vomiting, diarrhea, dyspnea, and cardiac arrhythmiasCitation2–4. Left untreated, hyperkalemia can lead to life-threatening neuromuscular and cardiac complicationsCitation5–7.
Hyperkalemia often results from impaired urinary potassium excretion due to acute or chronic kidney disease (CKD)Citation1. A primary cause of hyperkalemia may be reduced renal function and, consequently, lowered potassium excretion and impairments to the renin-angiotensin-aldosterone system (RAAS; e.g. Addison’s disease or adrenal enzyme deficiencies) can also be contributing factorsCitation3,Citation8,Citation9. Along with renal impairment, heart failure and diabetes mellitus can also increase the risk of hyperkalemiaCitation2,Citation4,Citation10–12. The reduced cardiac output of patients with heart failure may compromise renal perfusion and increase the risk of hyperkalemiaCitation13, while diabetic insulin deficiency may raise serum potassium concentrations due to impairment in transcellular potassium transportCitation14. Additionally, the use of RAAS inhibitors (RAASi), such as aldosterone receptor antagonists, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and direct renin inhibitors (which are currently recommended by clinical guidelines for heart failure, CKD, and proteinuria) have been to shown to increase the frequency and severity of hyperkalemia episodesCitation10,Citation15.
In the United States (US) population, the prevalence of hyperkalemia was estimated to be 1.2–1.6% across the calendar years 2010–2014Citation11, and it was higher among patients with CKD and/or heart failure (5.0–6.6%)Citation11. A separate administrative claims database study estimated the prevalence of hyperkalemia to be 13.3% among patients with CKD in 2014 and found that its prevalence among the Medicare population was higher (2.3%) than among the commercially-insured population (0.09%)Citation10. However, comprehensive estimates of the prevalence of hyperkalemia in the Medicare population over time in patient subgroups defined by comorbidities are still sparse.
Evidence suggests that hyperkalemia is associated with significant economic consequences, which vary across patient populations. Costs are generally higher in patients with hyperkalemia, but the magnitude of the cost increases appear to vary by country, year (as healthcare costs continue to increase), comorbidities, and age. The primary drivers of cost differences may also vary. In the general US population, patients with hyperkalemia were found to incur $15,983 higher 1-year total healthcare costs compared with matched controls ($31,844 vs. $15,861) with increased inpatient admissions associated with hyperkalemia being the primary drivers of the cost differences (constituting 67% and 45% of short- and long-term costs, respectively)Citation16. These differences in incurred costs were greater for patients with hyperkalemia compared with matched controls within subgroups of patients with CKD and/or heart failure (cost difference: $24,133) and patients with CKD (cost difference: $21,857)Citation16. A recent study among Medicare beneficiaries found that patients with hyperkalemia had higher costs compared with patients without hyperkalemia ($4945 vs. $2137 per-patient-per-month [PPPM]) and the PPPM cost was five times higher in patients with both CKD and hyperkalemia than in the total Medicare population ($5645 vs. $1035, respectively)Citation10. However, it is unclear how much additional healthcare cost hyperkalemia incurs among the overall Medicare population and subgroups of patients with hyperkalemia-related comorbidities.
Because patients who are older and have a higher comorbidity burden are at greater risk of hyperkalemia, analyses using Medicare claims data are useful for estimating both the prevalence of hyperkalemia among this population as well as the associated economic burden to the Medicare system. The present study estimated the prevalence of hyperkalemia and assessed the economic burden of hyperkalemia among matched Medicare beneficiaries (with and without hyperkalemia) in the overall US Medicare population and patient subgroups defined by hyperkalemia-related comorbidities during the period of 2010–2014.
Methods
Data source
A 5% random sample of the Medicare claims database (Centers for Medicare & Medicaid Services) was used to estimate the economic burden and prevalence of hyperkalemia from 01 January 2010 to 31 December 2014. The database includes de-identified information for 4.4 million patients aged 65 years and older, patients younger than 65 years with certain disabilities, and individuals of all ages with end-stage renal disease. These data are geographically-representative of Medicare beneficiaries throughout the US and contain Medicare Part A and Part B claims with information on enrollment history, patient demographics, and detailed medical claims (e.g. procedures, paid amounts, service dates, and diagnoses). The database is compliant with the privacy rules outlined in the Health Insurance Portability and Accountability Act (HIPAA) and followed the principles of the Declaration of Helsinki; institutional board review was not required for this study.
Sample selection
Two patient samples were selected from the 5% Medicare random sample (01 January 2010–31 December 2014): one to estimate the prevalence of hyperkalemia (prevalence sample) and another to evaluate the economic burden of hyperkalemia (cost sample). For the prevalence sample, patients were selected such that the samples in each year were representative of the general Medicare population. For the cost sample, patients with hyperkalemia were selected for the case cohort and matched with otherwise similar patients without hyperkalemia to control for potential confounding.
Selection of the prevalence sample
Adult patients (aged ≥18 years) with at least one medical service were selected. Patients were required to have had continuous non–health maintenance organization (HMO) Medicare enrollment throughout each selected calendar year (2010–2014).
Selection of the cost sample
Adult patients with and without hyperkalemia (cases vs. controls) were selected among Medicare beneficiaries who had at least one medical service. Cases were identified as adults with at least one diagnosis code of hyperkalemia (International Classification of Diseases, Ninth Revision – Clinical Modification [ICD-9-CM] code 276.7). Potential index dates for cases include all claim dates with a hyperkalemia diagnosis. Controls were identified as adults with no diagnosis codes of hyperkalemia. Potential index dates for controls include all claim dates. Patients were required to have continuous enrollment in their Medicare plan for at least 6 months prior to the potential index date (baseline period) and 12 months after the potential index date (study period) and not be enrolled in an HMO healthcare plan during the baseline period or study period. If a patient had multiple potential index dates that met all of the inclusion criteria, the final index date was randomly selected from the eligible potential index dates. This method ensures the sample is composed of patients at different points of disease (as opposed to only patients with early–stage disease). Controls were exactly matched one-to-one to cases on age group (18–64 years or 65+ years), CKD stage (stage 3, stage 4, stage 5, and unspecified stage), dialysis treatment, and heart failure diagnosis.
Identification of hyperkalemia-related comorbidities
Hyperkalemia-related comorbidities, including CKD (stage 3, stage 4, stage 5, and unspecified stage), heart failure, diabetes, and hypertension, were identified during each calendar year for the prevalence analysis and during the baseline period for the cost analysis. CKD (stage 3, 4, 5, and unspecified stage), heart failure, diabetes, and hypertension were identified by ICD-9-CM diagnosis codes. Dialysis treatment was identified using procedure codes.
Statistical analyses
Estimation of hyperkalemia prevalence
The annual prevalence of hyperkalemia in the overall population was calculated as the number of patients with at least one hyperkalemia diagnosis (ICD-9-CM code 276.7) divided by the total eligible patients included for each calendar year (2010–2014). The prevalence of hyperkalemia among comorbidity subgroups, including CKD and/or heart failure, CKD, CKD stage (stage 3, 4, 5, and unspecified stage), dialysis, heart failure, diabetes, and hypertension, was also estimated for each calendar year. In addition, hyperkalemia prevalence was assessed among the subgroup without any of the aforementioned comorbidities. Prevalence of hyperkalemia stratified by 5–year age groups and sex subgroups among patients ≥65 years of age was also calculated. Hyperkalemia prevalence was standardized to the elderly US population by applying the hyperkalemia prevalence stratified by age and sex in the 5% Medicare sample to the corresponding age and sex distribution of the US Census population aged ≥65 years.
Estimation of the economic burden of hyperkalemia
Baseline characteristics
Patient characteristics, including demographics (e.g. age, sex, geographic region, and place of service on index date), hyperkalemia-related comorbidities (e.g. CKD, diabetes, heart failure, and hypertension), dialysis treatment, and Charlson Comorbidity Index (CCI), were assessed during the baseline period. Patient characteristics were compared between patients with and without hyperkalemia using generalized estimating equation (GEE) models to account for matching.
Healthcare resource utilization and cost analyses
All-cause healthcare resource utilization (HRU) was described within 30 days and within 1 year of the index date and compared between cases and controls in the overall patient population. Rates of utilization and presence of any utilization (including inpatient, outpatient, ED, skilled nursing facility [SNF], hospice care, home health agency [HHA], and other) were described. In addition, the total length of stay for inpatient facilities, SNFs, hospice care, and HHAs during the 12-month study period were evaluated. Continuous variables were compared between cohorts using paired t-tests, and categorical variables were compared using McNemar’s tests. The HRU measures were also described and compared between cohorts in patient subgroups, including CKD and/or heart failure, CKD, and heart failure subgroups.
In the primary cost analyses, all-cause medical costs (inflated to 2016 US dollars [USD]) were estimated and compared between cases and controls during the first 30-day period and the 1-year study period. All-cause medical healthcare costs included inpatient admission, outpatient, ED, SNF admission, and other costs. Costs were compared between cohorts using paired t-tests. In the secondary cost analyses, adjusted all-cause total medical costs were estimated and compared between cohorts using multivariate gamma regressions. Covariates included age, sex, geographic region, diabetes, hypertension, heart failure, dialysis, CKD stage (stage 3, stage 4, stage 5, and unspecified stage), CKD and/or heart failure, and CCI. GEEs were used to account for the correlation between cases and controls due to matching. The primary and secondary cost analyses were also conducted among patient subgroups, including CKD and/or heart failure, CKD, CKD stage (stage 3, stage 4, stage 5, and unspecified stage), dialysis, heart failure, diabetes, hypertension subgroups, and patients without the aforementioned comorbidities.
Statistical significance was defined as p < .05.
Results
Prevalence of hyperkalemia
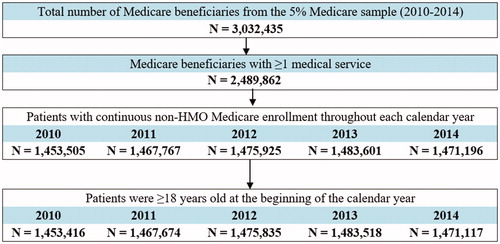
The 5% Medicare sample included 3,032,435 beneficiaries during 2010–2014, of whom 2,489,862 patients had at least one medical service (). Of this total, 1,964,905 unique patients met all inclusion criteria and were included in the analysis of hyperkalemia prevalence.
Figure 1. Sample selection (prevalence of hyperkalemia). Abbreviation. HMO, health maintenance organization.
Across calendar years, the prevalence of hyperkalemia ranged from 2.6% to 2.7% among the overall Medicare population. The prevalence of hyperkalemia was higher among patients with CKD and/or heart failure (8.9–9.3%; ) compared with the overall population. Hyperkalemia prevalence across calendar years was 10.8–12.4% for patients with CKD and was higher among stages 4 and 5 vs. stage 3 (stage 3: 9.4–11.1%; stage 4: 19.5–20.7%; stage 5: 17.8–20.2%). For patients undergoing dialysis treatment, hyperkalemia prevalence ranged from 50.2% to 52.8% across calendar years. Compared with the overall Medicare population, the prevalence of hyperkalemia across calendar years was also higher in the subgroups of patients with heart failure (8.6–9.4%), patients with diabetes (4.9–5.1%), and patients with hypertension (3.5–3.6%). Among patients without any of the aforementioned comorbidities, the prevalence was the lowest, ranging from 0.3% to 0.4% across calendar years.
Figure 2. Prevalence of hyperkalemia in the 5% Medicare sample (2010–2014). Abbreviation. CKD, chronic kidney disease.
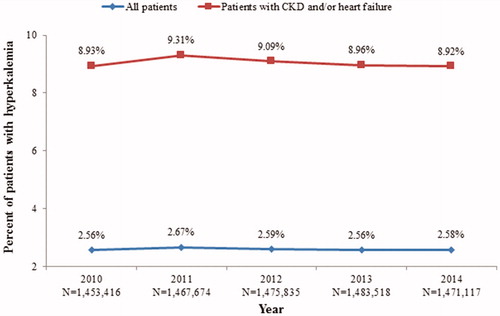
After standardizing the study population’s age and sex distribution to the US elderly population (aged ≥65 years), the annual prevalence of hyperkalemia was estimated to be 2.5–2.6%, corresponding to approximately 1.0–1.1 million patients with hyperkalemia annually in the US from 2010 to 2014.
Economic burden of hyperkalemia
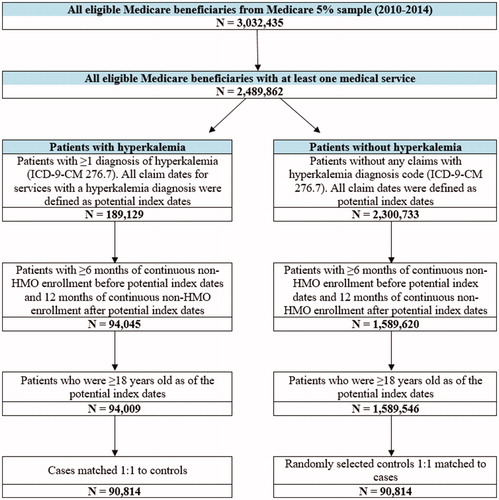
A total of 90,814 patients with hyperkalemia were matched to 90,814 patients without hyperkalemia (). Among these, 26,809 pairs had CKD, 25,603 pairs had heart failure, and 41,271 pairs had CKD and/or heart failure.
Figure 3. Sample selection (economic burden of hyperkalemia). Abbreviations. HMO, health maintenance organization; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Baseline characteristics
Among patients with and without hyperkalemia, the distribution of age was 72.8 years [standard deviation (SD): 12.3] among cases vs. 71.8 years [12.5] years among controls. Both groups had more females than males (55.4% among cases vs. 54.0% among controls) (). Both cohorts contained 29.5% with CKD (including 10.7% with stage 3, 5.0% with stage 4, 3.1% with stage 5, and 10.7% with stage unspecified), 6.9% with dialysis treatment, and 28.2% with heart failure, as a result of matching. Patients with hyperkalemia had higher CCI (3.2 vs. 2.6, respectively) and were more likely to have diabetes (48.8% vs. 35.1%) and hypertension (84.7% vs. 71.5%) compared with those without hyperkalemia (all p < .001).
Table 1. Baseline characteristics of matched patients with hyperkalemia and patients without hyperkalemia in the 5% Medicare sample (2010–2014).
All-cause HRU (overall patient cohort)
All components of all-cause HRU were higher among patients with hyperkalemia compared with patients without hyperkalemia, both within 30 days and 1 year of the index date (all p < .001) (). Specifically, cases had more inpatient admissions (30-day: 0.48 vs. 0.06; 1-year: 1.28 vs. 0.44), outpatient visits (30-day: 3.11 vs. 3.04; 1-year: 30.48 vs. 23.88), ED visits (30-day: 0.27 vs. 0.14; 1-year: 2.01 vs. 1.17), SNF admissions (30-day: 0.15 vs. 0.02; 1-year: 0.36 vs. 0.11), hospice care (30-day: 0.01 vs. 0.00; 1-year: 0.03 vs. 0.02), and HHA admissions (30-day: 0.02 vs. 0.01; 1-year: 0.11 vs. 0.04) than their matched controls. Patients with hyperkalemia also had longer inpatient, SNF, hospice care, and HHA stays for both time periods compared with patients without hyperkalemia (all p < .001). Specifically, for cases vs. controls, longer total stays were noted in inpatient (30-day: 3.79 vs. 0.34 days; 1-year: 10.54 vs. 3.04 days), SNF (30-day: 2.46 vs. 0.39 days; 1-year: 14.78 vs. 4.84 days), hospice care (30-day: 0.11 vs. 0.05 days; 1-year: 3.48 vs. 2.54 days), and HHA stays (30-day: 0.02 vs. 0.01 days; 1-year: 0.11 vs. 0.04 days) among cases. In addition, a significantly higher proportion of patients with hyperkalemia had at least one all-cause healthcare visit within 30 days and 1 year of the index date, including all categories but outpatient visits.
Table 2. Comparison of all-cause healthcare resource utilization of matched patients with hyperkalemia and patients without hyperkalemia within 30 days and 1 year of the index date in 5% Medicare sample (2010–2014).
All-cause HRU (by patient subgroups)
Similar to the results of the overall population, patients with hyperkalemia in the CKD and/or heart failure, CKD, and heart failure subgroups had higher all-cause healthcare visits (excluding outpatient visits for 30 days) and longer stays compared with patients without hyperkalemia. The results of the HRU analysis within 30 days and 1 year of the index date were generally similar across patient subgroups with CKD and/or heart failure, CKD, and heart failure. For example, in the CKD and/or heart failure subgroup, patients with hyperkalemia had significantly higher mean all-cause healthcare visits. This included inpatient (30-day: 0.55 [cases] vs. 0.09 [controls]; 1-year: 1.62 vs. 0.66), outpatient (30-day: 3.32 vs. 3.43; 1-year: 34.67 vs. 29.00), ED (30-day: 0.29 vs. 0.17; 1-year: 2.44 vs. 1.51), SNF (30-day: 0.20 vs. 0.04; 1-year: 0.49 vs. 0.18), hospice (30-day: 0.01 vs. 0.00; 1-year: 0.04 vs. 0.03), and HHA stays (30-day: 0.02 vs. 0.01; 1-year: 0.16 vs. 0.08) (all except outpatient visits for 30 days, p < .05). Patients with hyperkalemia in this subgroup also had longer inpatient admissions (30-day: 4.24 vs. 0.49; 1-year: 13.00 vs. 4.54), SNF (30-day: 3.24 vs. 0.66; 1-year: 19.47 vs. 7.81), hospice care (30-day: 0.16 vs. 0.08; 1-year: 4.90 vs. 4.27), and HHA stays (30-day: 0.02 vs. 0.01; 1-year: 0.16 vs. 0.08) than those without hyperkalemia (all p < .05).
All-cause medical costs (overall patient cohort)
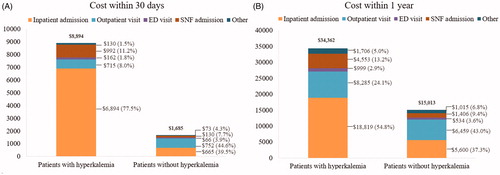
On average, patients with hyperkalemia incurred $7208 higher total medical costs over 30 days ($8894 vs. $1685) and $19,348 higher mean total medical costs over 1 year ($34,362 vs. $15,013) compared with matched patients without hyperkalemia (both p < 0.001) (). Additionally, costs were higher for inpatient admissions, ED visits, SNF admissions, hospice care, and HHA visits (excluding outpatient visits over 30 days) for patients with vs. without hyperkalemia within 30 days and 1 year of the index date (all p < .001). Inpatient costs were the primary cost driver, accounting for 68% of the 1-year total medical cost difference between cohorts. The multivariable adjusted total medical costs remained largely similar: patients with hyperkalemia incurred $7608 higher mean total medical costs over 30 days ($9282 vs. $1673) and $21,205 higher mean total medical costs over 1 year ($36,522 vs. $15,317) compared with patients without hyperkalemia (both p < .001).
Figure 4. Comparison of mean all-cause medical costs within (A) 30 days and (B) 1 year between matched patients with and without hyperkalemia in the 5% Medicare sample (2010–2014). Abbreviations. ED, emergency department; SNF, skilled nursing facility. Hospice and home health agency visits were combined with “Other claims”, including claims for durable medical equipment such as blood sugar monitors, walkers, hospital beds, etc.
All-cause medical costs (by patient subgroups)
Similar to the overall population, patients with hyperkalemia in patient subgroups defined by hyperkalemia-related comorbidities had higher mean total medical costs compared with controls over 30 days and 1 year post index date (). Multivariable adjusted total medical costs over 1 year remained largely unchanged and were significantly higher for patients with vs. without hyperkalemia in all patient subgroups (all p < .001).
Table 3. Comparison of mean all-cause total medical costs of matched patients with and without hyperkalemia in the 5% Medicare sample by subgroup (2010–2014).
Discussion
Hyperkalemia is a common electrolyte abnormality of which older patients are at increased riskCitation17. Prior studies have noted that the prevalence of hyperkalemia varied widely among patient populations, especially among those with certain comorbidities. However, to date, no studies have directly assessed the prevalence of hyperkalemia and associated incremental medical costs in the Medicare population by hyperkalemia-related comorbidity subgroups. This study provides an important piece of real-world evidence to resolve this knowledge gap.
The present study found that hyperkalemia prevalence ranged from 2.6% to 2.7% in the Medicare population during 2010–2014 and that there are 1.0–1.1 million patients aged ≥65 years diagnosed with hyperkalemia every year in the US. By comparison, a previous study shows that the prevalence of hyperkalemia among the US general population was 1.2–1.6% across the calendar years 2010–2014Citation11, confirming that elderly patients are at an increased risk of hyperkalemia. The prevalence in the overall Medicare population is in line with that in a Canadian retrospective population-based study, which reported that 2.6% of patients aged 66 years and older who presented to the ED were hyperkalemicCitation18. The present estimate is slightly higher than the 2.3% estimated by Fitch et al.Citation10 although the difference may be attributed to a more comprehensive continuous enrollment requirement in this study. (Fitch et al. required a continuous enrollment of ≥1 month eligibility, whereas the current study required a continuous enrollment period during each calendar year.)
The total medical costs were higher for patients with hyperkalemia compared with patients without hyperkalemia, both within 30 days and 1-year of their index date. Specifically, patients with hyperkalemia incurred $7208 higher 30-day costs (5.3-fold increase) and $19,348 higher 1-year costs (2.3-fold increase) than controls. Among patients with CKD and/or heart failure, the 30-day and 1-year total cost differences were higher by $7726 (4.5-fold increase) and $21,577 (2.1-fold increase), respectively (all p < .001). Inpatient admissions were the main driver of the incremental burden among patients with hyperkalemia, accounting for 68% of the 1-year total medical cost difference between cohorts. The current results also indicate that hyperkalemia was associated with an increased economic burden, including a higher number of ED visits, hospitalizations, outpatient visits, and SNF stays, which directly impact the overall cost of patient management.
Among patients with CKD and hyperkalemia, the mean 30-day cost in the present study ($8831) was higher than the monthly mean cost reported by Fitch et al.Citation10 ($5645), whereas the mean 1-year cost ($37,202) in the present study was lower than their mean annual cost ($67,740, calculated as monthly cost times 12). These differences may be due to (1) medical costs being higher closer to the hyperkalemia event; (2) the cost estimates in Fitch et al. including patient cost-sharing; and (3) Fitch et al. only adjusting for CKD stage whereas the present study also matched on a number of other factors, including age, dialysis, and heart failure. Patients with end stage renal disease (ESRD) on dialysis were included in the study population as the goal of this study was to evaluate the incremental burden of hyperkalemia on patients (including patients with and without ESRD). While we observed that patients with dialysis had a higher cost compared to the overall population (), these patients represented only ∼7% of the overall sample.
In order to control for potential confounding by underlying condition, patients with hyperkalemia were matched to controls on the key hyperkalemia-related comorbidities (dialysis treatment, CKD stage, and heart failure), as well as age group, to address potential confounding due to these key risk factors of both hyperkalemia and HRU/cost. In addition, multivariable regression analyses were used to further adjust for potential confounders, including age (continuous), sex, geographic region, diabetes, hypertension, and CCI. The consistent findings following the use of multivariable regression and the consistent incremental burden observed in all the comorbidity subgroups supported the robustness of the study’s primary conclusions. Additionally, although patients with a high comorbidity burden may receive more laboratory tests and thus be at a higher risk of being diagnosed with hyperkalemia, the matching and multivariable adjustment for these comorbidities should mitigate such potential confounding.
The study benefited from the large sample size of the Medicare population, and included approximately 1.5 million patients in the prevalence analysis and 90,000 cases and controls in the costs analysis. This study estimated the prevalence and the economic burden of hyperkalemia in the overall Medicare population as well as in patient subgroups defined by hyperkalemia-related comorbidities, comprehensively quantifying the burden of hyperkalemia from the Medicare perspective. The significant economic burden demonstrates the need for improvement in disease management and more effective treatment options for hyperkalemia. Further research is warranted to quantify hyperkalemia treatments’ impact on reducing the incremental economic burden of hyperkalemia.
This analysis is subject to several limitations, some of which are inherent to claims database studies. First, the definition of hyperkalemia was based on diagnosis codes used in the Medicare claims database. However, some patients with hyperkalemia may not have been diagnosed by their physician if their potassium was not measured. In addition, hyperkalemia may have been diagnosed by the physicians but the diagnosis code of hyperkalemia may not have been recorded in the claim. Therefore, the prevalence of hyperkalemia may be underestimated and resulted in the exclusion of some patients from the matched sample. Additionally, data indicating disease severity, such as potassium laboratory results, were not available in the database. Therefore, it was feasible only to assess the presence or absence of hyperkalemia, but not the varying degrees of hyperkalemia severity (e.g. mild, moderate, and severe). Similarly, comorbidities were also identified using diagnosis codes in the Medicare claims database. As a result, it is possible that not all comorbidities were identified.
Second, due to the observational study design there may be unobserved differences between patients with and without hyperkalemia that were not accounted for in the cost analysis. However, the results of this study were essentially unchanged in the multivariable analyses, suggesting limited impact.
Lastly, patients with and without hyperkalemia were matched on key hyperkalemia comorbidities, but not all patient characteristics were included in the match for the cost analysis. For example, due to the lack of pharmacy data in the 5% Medicare database, patients were not matched on medications associated with hyperkalemia (i.e. RAASi use). Similarly, due to the lack of data on prescription drug fills, pharmacy costs could not be estimated.
Conclusions
This study estimated that the prevalence of hyperkalemia was between 2.6% and 2.7% among the overall Medicare population and between 8.9% and 9.3% among patients with CKD and/or heart failure, with approximately 1 million patients aged ≥65 years estimated to have hyperkalemia for each calendar year (2010–2014). In addition, patients with hyperkalemia had a significantly higher economic burden compared with matched patients without hyperkalemia, both in the overall Medicare population as well as in patient subgroups. These results indicate that there is significant clinical and economic burden associated with hyperkalemia in the U.S. Medicare population, which underscores the need for effective treatment of the condition.
Transparency
Declaration of funding
This work was supported by AstraZeneca.
Declaration of financial/other relationships
JMW was an employee of AstraZeneca at the time of the study and owns stock/stock options. FM, KAB, AD, YW, JZ, and EQW are employees of Analysis Group Inc., which has received consultancy fees from AstraZeneca. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
FM: Study design, result interpretation, overseeing the conduct of analysis, manuscript drafting, review and revision; KAB: Study design, result interpretation, overseeing the conduct of analysis, manuscript drafting, review, and revision; JMW: Study design, result interpretation, and manuscript review and revision; AD: Data analysis, manuscript drafting, review and revision; YW: Data analysis, manuscript drafting, review and revision; JZ: Data analysis, result interpretation, manuscript drafting, review and revision; EQW: Study design, result interpretation, overseeing the conduct of analysis, manuscript drafting, review and revision. A peer reviewer discloses receiving honoraria from Relypsa from 2014 to 2015. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Previous presentations
Portions of this research were presented at the 2017 ASN Kidney Week, which took place October 31-November 5 in New Orleans, LA, USA.
Compliance with ethics of experimentation
This study followed the principles of the Declaration of Helsinki; institutional board review was not required for this study.
Acknowledgements
Manuscript drafts were prepared with the assistance of Shelley Batts, PhD, a professional medical writer employed by Analysis Group, Inc., Boston, MA. This study, including the manuscript preparation service, was funded by AstraZeneca.
Data availability statement
This study used a 5% random sample of the Medicare claims database provided by the Centers for Medicare & Medicaid Services to Analysis Group, Inc. and is not publically available. For information regarding access to the data used in this study please visit https://www.resdac.org.
References
- Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401.
- Luo J, Brunelli SM, Jensen DE, et al. Association between serum potassium and outcomes in patients with reduced kidney function. CJASN. 2016;11(1):90–100.
- Mushiyakh Y, Dangaria H, Qavi S, et al. Treatment and pathogenesis of acute hyperkalemia. Journal of Community Hospital Internal Medicine Perspectives. 2012;1(4):7372.
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109(10):1510–1513.
- Perazella MA, Mahnensmith RL. Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. J Gen Intern Med. 1997;12(10):646–656.
- Kovesdy CP. Epidemiology of hyperkalemia: an update. Kidney Int Suppl (2011). 2016;6(1):3–6.
- Kovesdy CP. Updates in hyperkalemia: outcomes and therapeutic strategies. Rev Endocr Metab Disord. 2017;18(1):41–47.
- Allon M. Hyperkalemia in end-stage renal disease: mechanisms and management. J Am Soc Nephrol. 1995;6(4):1134–1142.
- Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–S20.
- Fitch K, Woolley JM, Engel T, et al. The clinical and economic burden of hyperkalemia on medicare and commercial payers. Am Health Drug Benefits. 2017;10(4):202–210.
- Betts KA, Woolley JM, Mu F, et al. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018;34(6):971–978.
- Kim K, Thomsen RW, Nicolaisen SK, et al. Healthcare resource utilisation and cost associated with elevated potassium levels: a Danish population-based cohort study. BMJ Open. 2019;9(4):e026465
- Desai AS. Hyperkalemia in patients with heart failure: incidence, prevalence, and management. Curr Heart Fail Rep. 2009;6(4):272–280.
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med. 2010;25(4):326–333.
- Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. CJASN. 2010;5(3):531–548.
- Betts KA, Woolley JM, Mu F, et al. The Cost of Hyperkalemia in the United States. Kidney Int Rep. 2018;3(2):385–393.
- Dunn JD, Benton WW, Orozco-Torrentera E, et al. The burden of hyperkalemia in patients with cardiovascular and renal disease. The American journal of managed care. Am J Manag Care. 2015;21(15 Suppl):s307–s315.
- Fleet JL, Shariff SZ, Gandhi S, et al. Validity of the International Classification of Diseases 10th revision code for hyperkalaemia in elderly patients at presentation to an emergency department and at hospital admission. BMJ Open. 2012;2(6):e002011.
