Abstract
Introduction: Treatment adherence continues to be a major challenge in psoriasis. Patient preference studies, especially discrete-choice experiments, are gaining popularity to gather insights into patient reported treatment outcomes. This systematic literature review aimed to critically assess all discrete choice experiments exploring patients’ and physicians’ preferences for psoriasis treatment characteristics.
Methods: PubMed and EMBASE databases were searched using keywords “psoriasis” and “preferences” to identify relevant literature. Discrete-choice experiments conducted in French or English from the year 2000 onwards, that focused on evaluating psoriasis treatment preferences in patients and/or physicians, were included. The relative importance of treatment attributes was assessed and studies were critically appraised using validated checklists.
Results: Out of 987 articles identified, 25 articles fulfilled the inclusion criteria. Overall, patients and physicians prioritize efficacy-specific outcomes. Patients are shown to place greater importance to process attributes when compared to physicians, especially route and location of administration. Physicians focus primarily of efficacy attributes, however when the top two attributes are considered, safety outcomes increasingly become considered important. Of the studies, 60% conducted subgroup analysis, of which many reported associations between specific patient characteristics and preferences. Factors such as age, disease severity, and duration of condition significantly affected preferences for treatment attributes.
Conclusions: This review provides insight into the types of attributes that patients and physicians value most, and therefore can help improve shared decision-making. The findings of this study also encourage regulatory agencies to continue integrating patient preferences in their decision-making.
Introduction
Psoriasis is a chronic inflammatory disease that predominantly affects the skin and joints. Epidemiological data has reported that the prevalence of psoriasis varies from 0.9% in the United States to over 8% in NorwayCitation1. Psoriasis not only significantly increases the risk of comorbidities, especially psoriatic arthritis, depression, obesity, diabetes, and cardiovascular diseaseCitation2, but many affected patients report that the disease has a significant impact on their quality-of-lifeCitation3. Consequently, this has significant societal and economic implications due to elements such as increased rates of absenteeism in patients suffering from psoriasisCitation4.
Although new therapies have revolutionized psoriasis treatment, patient treatment adherence continues to be a major challengeCitation5. Despite improved efficacy, persistently low adherence rates indicate that an unmet patient need exists regarding treatment availability. Whether this unmet need motivates a drive to explore new treatment options with increased efficacy, or to evaluate the value of procedural treatment factors such as the ease of administration, attention should aim to understand patient perspectives. Studies have demonstrated that low levels of adherence are exacerbated by individual beliefs regarding psoriasis treatment and low levels of involvement from healthcare professionalsCitation6. Therefore, shared decision-making and increased patient-involvement are of utmost importance in the successful treatment of psoriasis. Insights into patients’ preferences and increasing patient involvement in prescribing decisions can positively influence adherence rates and satisfactionCitation5,Citation6. In turn, improved adherence rates can both save scarce healthcare resources and positively impact the economy by decreasing associated and indirect costs of treatment such as unnecessary hospitalizations and productivity lossesCitation7.
The need to incorporate patient preferences in prescription decision-making is nowadays widely acknowledgedCitation8. Patient preference studies are broadening our understanding of the factors that influence treatment selection and adherence beyond traditional efficacy and safety outcome measuresCitation9. Preference elicitation studies generally categorize treatment characteristics in process, outcome and cost factors. Process factors typically consist of attributes such as mode of administration, treatment frequency, or location of administration, while outcome factors focus on efficacy or treatment adverse event (AE) profilesCitation10. Patient preference studies are helping healthcare professionals and regulatory agencies broaden their understanding of patient values and thus promote placing the patient at the focal point of treatment decision-makingCitation11. The incorporation of patient preferences in the value assessments of new treatments has been advocated by regulatory agencies such as the Food and Drug Administration (FDA) in the United StatesCitation12 and Health Technology Assessment bodies such as the National Institute for Health and Care Excellence (NICE) in the United KingdomCitation13. More importantly, the fact that these studies are being increasingly considered in the health technology assessments of treatments and in policy-context is giving patients a strong voice at the decision tableCitation14.
Patient-preference studies are generally divided into stated- or revealed-preferred methods. The former utilizes surveys and questionnaires to understand the motivations of patients when making trade-off decisions for hypothetical yet real-life-like treatment choices. The latter is based on observing choices made in real-world settings. Given that observation opportunities are limited in the context of psoriasis treatment decision-making, patient preference studies have adopted stated-preferred methodsCitation10. Discrete choice experiments (DCE), commonly categorized as a type of Conjoint Analysis (CA), are a type of stated-preference method that are frequently used to investigate patient preferences regarding psoriasis treatment characteristicsCitation15. In DCEs, patients are asked to make trade-off decisions and elicit their preference between two or more hypothetical treatment options, each being characterized by a unique profile of treatment attributes. By doing so, researchers can identify the relative importance participants place on one attribute over another. According to the International Society for Pharmacoeconomics and Outcomes Research’ (ISPOR) guide on conjoint analysis, CA and DCEs are especially useful for quantifying preferences in healthcare due to the constrained nature of a consumer’s choice of goodsCitation16. Additionally, DCE results have been described as more reliable than other types of preference elicitation methods due to their ability to mimic real-world decision-making situationsCitation17. More importantly the improved quality and validity of DCE studies are receiving more attention from policy-makersCitation18,Citation19.
Due to the prolific nature of preferences studies, there is a need to consistently update our understanding of patient and physician preferences for psoriasis treatment. Furthermore, there is value in investigating whether preferences for treatment attributes are in fact as heterogeneous as previously reported. Patient preferences have been shown to vary greatly amongst subgroups, i.e. region, age, disease severity, etc.Citation9. In a German patient-preference DCE, Schaarschmidt et al.Citation20 identified that patients value certain process attributes over outcomes attributes, suggesting that patients pay more attention to attributes that affect lifestyle factors. However, this contrasts with other studies that demonstrate that patients value efficacy and safety over process attributesCitation21,Citation22. Though Florek et al.’sCitation9 recent systematic review provided a strong general overview of patient values as they differentiate between subgroups, there was minimal focus on assessing the quality of the studies included and a much broader focus on general patient preference studies using a wide variety of preference elicitation methods.
The objective of the current study was to evaluate the treatment attributes that patients and physicians consider the most important when selecting a given treatment for psoriasis and to highlight key quality gaps to improve the validity and adoption of DCE studies in wider contexts. To do so, the current systematic review identified all relevant DCE studies and conducted a critical appraisal of the studies included to determine the current standard of quality in conducting these types of studies. Importantly, this review aimed to provide recommendations to strengthen the validity of future studies being conducted based on the limitations raised by the review. This paper then proceeded to strengthen the current understanding of preferred treatment characteristics and aimed to determine whether patients and physicians have diverging priorities when selecting treatment.
Methodology
Research type and design
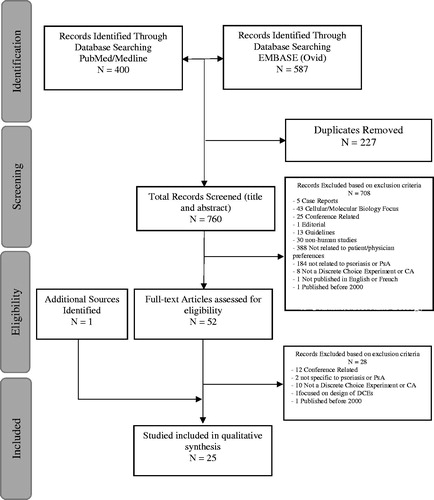
The current systematic literature review builds on two recent systematic reviews of patient preference studies in psoriatic treatment by Florek et al.Citation9 and GonzalezCitation23. This review focuses only on DCEs that evaluate patient and physician preferences. The review applied the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and incorporates recommendations by FinkCitation24 and Yu et al.Citation25 in the identification of all relevant articles and the critical appraisal of selected studies. The exact methodology of the proposed study followed the four-phase approach elaborated by LiberatiCitation26 and is illustrated in .
Data collection
Identification/search strategy
To identify all relevant literature, two databases were screened systematically; specifically, PubMed and EMBASE. The final search strategy utilized in this review derived key terms from three psoriasis-related studies and integrated the terms into a final comprehensive search strategyCitation9,Citation27,Citation28. The final search strategy combined two searches; the first being related to identifying preferences and the second in relation to the therapeutic area of interest, psoriasis. The search strategy employed Boolean Operators in order to gather all relevant material; ((“preference*”) OR (“clinic* preference*”) OR (“physician* preference*”) OR (“patient* preference*”) OR (“patient* priorities”) OR (“public preference*”) OR (“discrete choice”) OR (“DCE”) OR (“conjoint analysis”) OR (“stated preference*”)) AND (“Psoriasis”[MeSH Terms] OR “Psoriasis”[All Fields] OR “Psoria*” [Mesh Terms] OR “Psoria*” [All Fields]). This broad search strategy ensured that all studies regardless of naming convention were captured. To ensure comprehensiveness, the final selection was supplemented by a hand search. Both a backward and forward reference search strategy were employed on included studies and on related systematic literature reviews. Forward referencing identifies studies which have cited a study already included, whereas backward referencing refers to reviewing the bibliography of an article included in our reviewCitation29.
Screening and selection process
Key considerations were made in the screening process. Studies that related to both the treatment of psoriasis or psoriatic arthritis (PsA) were included given that there is significant overlap in the medications used to treat both in human beingsCitation30. Secondly, studies had to include a discrete choice exercise (a decision to be made between two or more options), be quantitative in nature, and be published (or in press) in a full-text English or French article between January 2000 and May 2019. This review excluded studies that did not use empirical measures to determine preferences such as surveys or focus groups. The current review also excluded any DCE that pooled psoriasis’ or PsA patients with other diagnoses (typically rheumatoid arthritis, ankylosing spondylitis) to avoid misinterpreting treatment selection preferences for other conditions. Lastly, case reports, commentaries, editorials, conference abstracts, and unpublished articles, as well as all grey literature were not included.
The systematic literature review employed a two-stage selection process according to PRISMA standardsCitation26. The screening process was conducted by two independent researchers to ensure the internal validity of the process. During the first phase, the primary reviewer overviewed the titles and the abstracts of the papers identified in PubMed and EMBASE and screened them for relevance based on the inclusion and exclusion criteria specified above. The second reviewer validated the selection using the same criteria. In the second stage, selected articles underwent a full text screening by two independent researchers.
Data extraction and reporting quality assessment
Data extraction and quality reporting was carried out in a four-step process. First, generic study characteristics were extracted. Extracted characteristics included title, author, year of publication, country, population, sample size, and DCE methodology characteristics (number of choice sets, number of attributes, and number of alternatives). The second segment of the data analysis comprised of a quality assessment, integrating elements from two tested quality checklists. First, it incorporated specific items from the ISPOR checklist which lays out best practices for conducting conjoint analysisCitation31. Specifically, this review included (1) Numerical items, (2) Attributes and levels, (3) Construction of tasks, (4) Experimental design, and (5) Preference elicitation from the checklist. These sections were drawn specifically to provide a more detailed assessment of the methodology of the studies included which are known to be typically lacking in qualityCitation32. Secondly the PREFS (Purpose, Respondents, Explanation, Findings, Significance) checklist was used. All five elements of the PREFs checklist were used to assess quality namely; purpose (regarding the research question), respondents (regarding the internal and external validity), explanations (regarding the methodology), findings (regarding the results and conclusions), and significance (regarding the statistical analyses conducted)Citation33. Each item was scored based on whether it was acceptable (score = 1), needed improvement (score = .5), or was unacceptable (score = 0). An “acceptable” score represented a study that both reviewers answered “yes” to the qualification questions to each section in both the PREFs and ISPOR checklist, “needs improvement” represented if at least one question was answered with a “yes,” whereas “unacceptable” was denoted by the reviewers answering “no” to all qualifying questions. An aggregate sum score (ranging from 0–9, where 9 equated to the maximal score) was then given to each study and was compared across studies.
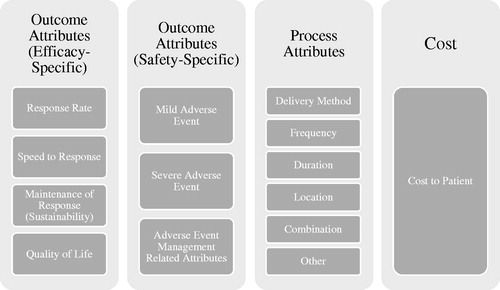
In the third step, all process, outcome, and cost treatment attributes were identified for each study. Given variability in both the types and in the nomenclature of attributes, the attributes were categorized for ease of analysis purposes. Process attributes were divided into location (of administration), frequency (of administration), duration (of administration), delivery method, and other; whereas outcome attributes were categorized as either efficacy specific, safety-specific, or quality-of-life specific. The efficacy and safety categories were further sub-categorized to simplify the interpretation of the various methods used for measuring safety and efficacy. Allocation of raw attributes into each specified category was confirmed by an independent reviewer with expertise in psoriasis.
In the final step, the top two most important attributes for both patients and physicians from each study were identified. Each study’s top two attributes were then tabulated and graphically represented. It is important to note that when reporting results, any process attribute that was identified as being within the top two most important factors in a given study was labelled as a “process attribute” instead of its specific sub-category to simplify the interpretation of the results. Also, PsA patients were agglomerated with PSO patients, given the limited number of studies and due to the similar nature of the treatments prescribed for both conditions. To structure the analysis of preferences for psoriasis treatment attributes, the number of times a given attribute category was identified as being most important in all studies was compared. Studies were categorized by either patient-specific or physician-specific and thus were compared only amongst themselves. Secondly, studies were also categorized by attributes design (outcome only vs. process and outcome vs. process, outcome, and cost). The relative importance of each attribute within the studies was then reported. When the relative importance of an attribute was available in a study reviewed, the relative importance was drawn directly from the study. However, when only the coefficients were provided, the relative importance was calculated using the range level method discussed by ISPORCitation34.
Exploratory analysis
In the final part of the review, we isolated studies that conducted subgroup analysis. Studies that included subgroup analysis were reviewed to identify qualifying characteristics that significantly impacted the preference associated with a given attribute. This review only highlighted specific associations between patient characteristics and attribute preferences if at least two studies demonstrated statistically significant results. Given that an analysis for every observable sub-group is beyond the scope of this review, this review builds on some of the characteristics reported by Florek et al.Citation9. Namely, we reported associations for age, marital status, disease severity, disease duration, and the impact of psoriasis on quality-of-life as measured by the Dermatology Life Quality Index (DLQI) and lastly comorbidity of PsA.
Results
illustrates the results of the study screening and selection process. By searching PubMed and EMBASE, 987 hits were obtained and 227 duplicate records were removed. Of the 760 records that remained, 708 records were excluded after title and abstract screening (exclusion reasons are provided in ). Fifty-two articles underwent full-text assessment, of which only 24 articles met the full inclusion criteria. Only one additional article was identified through manual search, resulting in a total of 25 articles for the analysis.
Study characteristics
The main study characteristics are provided in whereas categorical analyses of study characteristics are reported in Appendix A. Among the studies reviewed, the majority of published DCE studies were conducted since 2015 (64%)Citation17,Citation21,Citation22,Citation35–45. Most studies were conducted in Europe (64%)Citation5,Citation20,Citation40,Citation46–54, wherein 36% of all studies were conducted in GermanyCitation5,Citation20,Citation22,Citation35,Citation42,Citation49–51,Citation53. Almost a quarter of all studies were conducted in the United States (24%)Citation17,Citation21,Citation38,Citation43,Citation48. Only 12% of all studies were conducted in AsiaCitation39,Citation41,Citation45; one of which was conducted in a lower-middle income country (LMIC) (Philippines)Citation39,Citation55. Sample sizes ranged from 67Citation39 to 1,064Citation21. Finally, regarding the target population of the studies included, six studies included psoriasis patients with all degrees of severity where diagnosis was confirmed by a physicianCitation21,Citation37,Citation38,Citation45,Citation47,Citation48, 11 studies targeted solely patients with moderate–severe diagnosisCitation5,Citation20,Citation22,Citation35,Citation39,Citation49–54, two studies targeted patients with psoriatic arthritisCitation36,Citation43, two studies investigated physician preferences uniquelyCitation44,Citation46, while four studies investigated both patient and physician preferencesCitation17,Citation40–42.
Table 1. List of discrete choice experiment studies included in the systematic literature review of patient and physician preferences; including general characteristics of the studies.
Quality assessment
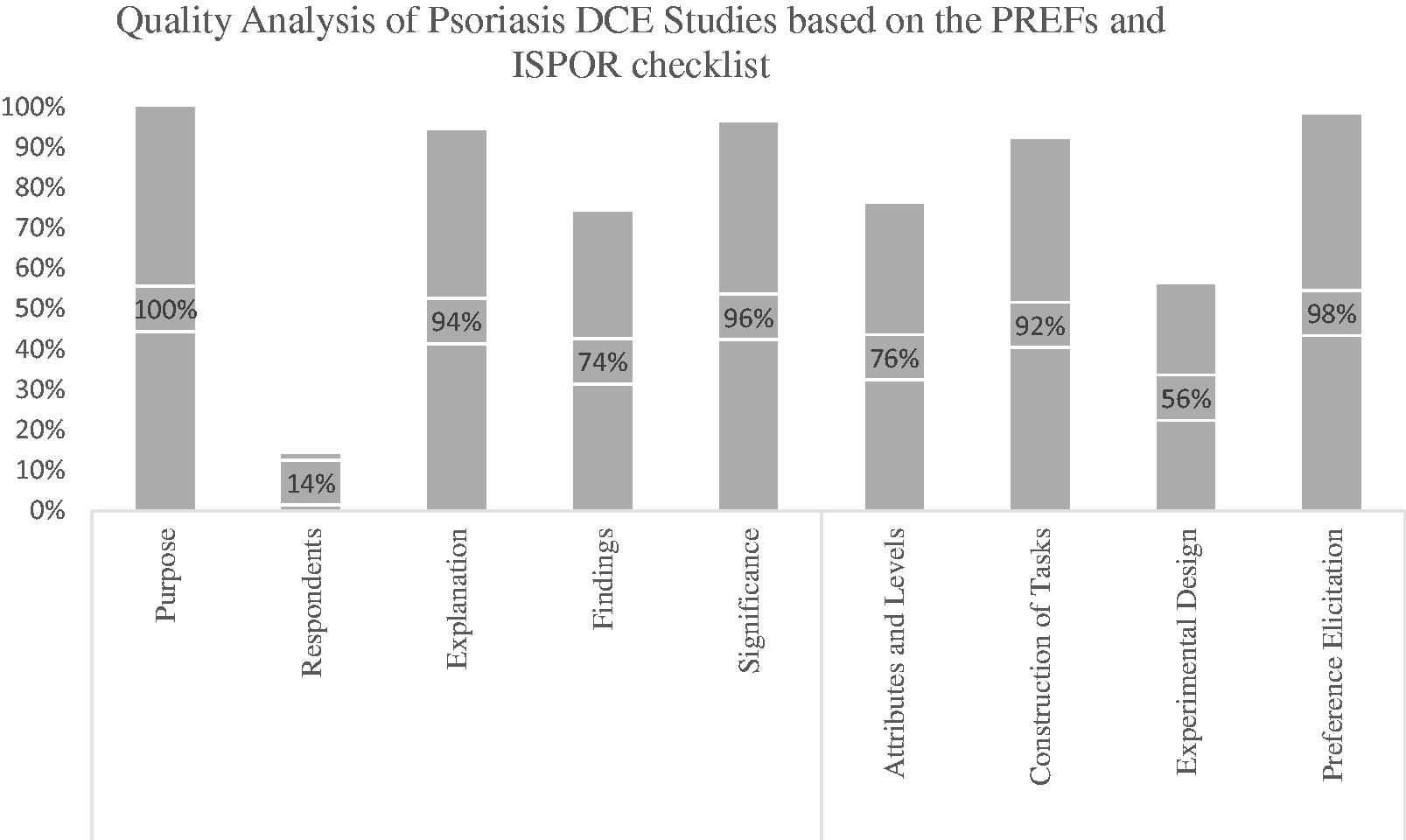
reports the quality assessment scores attributed to the DCE studies included. Using the PREFS checklist, it was determined that every study included in this review reported a clear purpose related to the identification of patient or physician preferences. Regarding the “Respondents” aspect of the checklist, only two studies addressed the differences between responders and non-respondersCitation44,Citation54. Three studies reported strategies for increasing survey response rates, however they did not explicitly address differences between responders and non-respondersCitation17,Citation41,Citation52. Most studies provided an adequate explanation of their methods (94%) and included appropriate statistical methods (96%), however only 74% of all studies reported all their findings transparently according to ISPOR recommendations. Common gaps in reporting findings were the lack of reporting all relevant coefficients, subgroup analysis scores, or relative importance scores. To allow a more in-depth evaluation of the methodologies of each study, all four methodology-specific items from the ISPOR checklist were also assessed. Overall, studies clearly stated how the discrete choice tasks were constructed (92%) and used appropriate methodologies to elicit preferences from their participants (98%). Of note, nearly a quarter of studies did not adequately justify the selection of the attributes and levels included in their study. Finally, only 56% of studies adequately justified the selection of the experimental design utilized (Appendix B). With regards to final quality scores (whereas a score of 9 is considered the maximum), eight studies received a total score of 6 or lower, eight studies received a score between 6.1 and 7.5 and nine studies received a total quality score of 7.6–9 ().
Table 2. Quality assessment of the DCE studies included in the review according to both PREFS and ISPOR quality checklists.
A more detailed analysis of key methodological considerations is represented in . When reporting on the specific methodology used to construct the DCE tasks and on deciding which attributes to include, all authors conducted a literature review or investigated relevant clinical evidence. Five studies referenced previous studies when reporting on how attributes were selectedCitation22,Citation38,Citation49,Citation51,Citation53 and 96% of studies consulted experts within the field (patients or clinicians with psoriasis knowledge). Interestingly, only 24% of studies reported to have pilot tested their DCE.
Table 3. Quality assessment continued.
Classification of attributes
The 25 DCE studies compiled a total of 191 attributes. One hundred and twenty-four (65%) attributes were classified as outcome attributes, 55 (29%) as process attributes, and 12 (6%) were cost-related. Only five studies (20%) solely included outcome attributesCitation21,Citation36,Citation40,Citation46,Citation47. It should be noted that two of the outcome-only studiesCitation21,Citation40 considered plaque location an attribute, however in this analysis plaque location was not considered as an attribute, as the location was an independent variable in these studies. Eight studies (32%) included both outcome and process attributesCitation17,Citation22,Citation35,Citation37,Citation38,Citation41,Citation44,Citation53, whereas 12 studies (48%) included outcome, process, and cost attributesCitation5,Citation20,Citation39,Citation42,Citation43,Citation45,Citation48–52,Citation54 (Appendix C). Given the high level of variability in the attribute naming conventions used by researchers, this review bucketed the attributes into new categories (Appendix D–G). Amongst all outcome attributes, 55% were efficacy-specific whereas 45% were safety specific. Efficacy-specific outcome measures were subdivided into “Response Rate” (defined by probability of achieving an effect measured by Psoriasis Area Severity Index (PASI) or Body Surface Area (BSA) reduction – 53% of all efficacy attributes), “Speed on Response” (defined by the time it takes to first experience relief of symptoms – 13% of all efficacy attributes), “Response Maintenance or Sustainability” (defined by the longevity of the effect experienced by the patient – 25% of all efficacy attributes), and quality-of-life (defined by measures of health-related quality-of-life – 9% of all efficacy attributes). Safety-specific outcome measures were divided into mild adverse events (AE) (e.g. itching, nausea, vomiting etc. − 33% of all safety attributes), severe adverse events (e.g. risk of lymphoma, serious infections, melanoma or nonmelanoma skin cancer, etc. − 47% of all safety attributes), or adverse event management related attributes (defined as reversibility of AEs – 20% of all safety attributes). Process attributes were identified using previously defined categories, namely mode of administration (21%), frequency (27%), location of treatment (20%), duration of treatment (14%), a combination of process attributes (9%), and other (9%). Five studies opted to combine multiple process attributes together, in most cases mode and frequency of administrationCitation17,Citation37,Citation48,Citation52,Citation54. Lastly, cost attributes were defined as the specific cost to the patient (out of pocket costs) ().
Significance of attributes
Certain studies evaluated both patients and physicians or had participants identify preferences for different scenarios, and thus these iterations added to a larger study sample used in this review. In particular, the study by Alcusky et al.Citation17 asked both patients and physicians to elicit preferences for both moderate and severe hypothetical patient groups and thus provided four sets of “most important” attributes. Also noteworthy, Xu et al.’sCitation43 study separated patients into commercially-insured and Medicare-covered groups, providing two data sets. Altogether we identified 32 “most important” attributes for patients and physicians and 32 “second most important” attributes. Only 17 studies (53%) reported relative importance scores (), for the remaining 15 data sets relative importance was calculated. To prevent cross-over between population-groups, we first evaluated all patient-specific preferences independently and then compared results with physician-specific preferences. Secondly, we also compared the distribution of preferences according to the included categories of attributes (i.e. outcome-only studies vs. process and outcome studies & process, outcome and cost studies). Results of our analysis are presented in .
Figure 3. Identification of the most and second most important treatment attributes, differentiated by outcome only, outcome and process and outcome, process and cost studies.
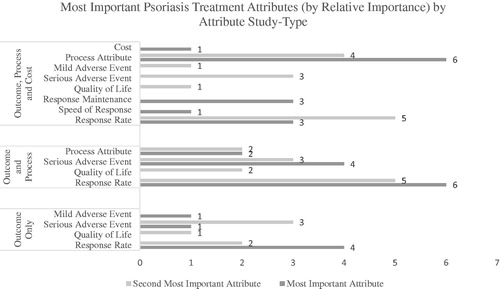
Table 4. Identification of the most and second most important attributes for each of the 25 studies included.
Patient versus physician preferences
In the patient sample (25 studies), efficacy-outcomes were identified as the most important attribute in twelve studies (48%), safety-outcomes in five studies (20%), process attributes in seven studies (28%), and cost only once (4%). Regarding the second most important attribute, efficacy was selected in 13 studies (52%), safety in seven studies (28%) and process outcomes in five studies (20%). In the physician sample (N = 7), efficacy was identified as the most important attribute 5 times (71%), whereas safety and process attributes were identified as most important in one study each (14% each). Regarding the second most important attribute, efficacy and safety were the second most important attributes in three studies (43% each) whereas process attributes were only selected by physicians once (14%). Tallying the top two preferred attributes, efficacy was identified as the most important in 50% of patient-specific studies. Safety and process attributes were ranked as the top two most important attributes 12 times each (24% each). The cost attribute was amongst the top two attributes only once. Similarly, in the physician sample, efficacy was identified within the top two most important attributes in 57% of the studies. However, safety (29%) appeared more among the top two attributes for physicians than process attributes (14%). Cost was never considered in the top two most significant attributes for physicians (). Efficacy was revealed to be the most important treatment attribute for patients and physicians ().
Preferences by study design
In the outcome-only studies, efficacy was named the most important attribute in 66% of studies, whereas safety only in 33%. Regarding the second most important attribute, there was an even split between efficacy and safety (50% each). In the outcome and process studies, we noticed an even split between the efficacy and safety attributes, both being evaluated as the most influential attribute in 50% of studies. Regarding the second most important attributes, efficacy was identified as the second most important in 58% of studies, safety in 25%, and process attributes in 17%. Lastly, when studies also included a cost element, only efficacy, process, and cost attributes were identified by patients and physicians as being the most important attribute (50%, 43%, and 7% of studies, respectively). Regarding the second most important attributes, efficacy again ranked as the top second most preferred attribute being selected (43%), whereas safety and process attributes were selected in 29% of studies (). The exact attributes identified as most important are listed in .
Subgroup preference trends
Overall, 15 out of 25 (60%) studies included subgroup analyses. Regarding age, three significant associations were identified. First, older participants have been reported to attach less importance to response rates (treatment efficacy) than younger participantsCitation20,Citation35,Citation42,Citation50,Citation52; secondly older patients are more influenced by the risk of severe AEs than younger participantsCitation35,Citation42,Citation45,Citation50,Citation52,Citation53. Lastly, older patients seem to be less concerned with the speed of response in comparison to younger participantsCitation35,Citation52,Citation53. In regards to marital status, two studies identified that patients who identified themselves as single placed more importance on response rates compared to participants in a relationshipCitation35,Citation50. In terms of disease severity, two studies identified that patients with more severe psoriasis were more tolerant to severe AEs than those participants with milder forms of psoriasisCitation41,Citation42. Also, three studies identified that patients who have more experience with their condition (years since diagnosis) were less concerned with response rates in comparison to those who have not lived as long with psoriasisCitation37,Citation42,Citation51. In terms of the impact of psoriasis on participants’ quality-of-life, as measured by the Dermatology Life Quality Index (DLQI), two studies identified that patients with greater DLQI scores (equivalent to greater impact of psoriasis on quality-of-life) placed less value on treatment efficacyCitation35,Citation37, whereas two studies demonstrated that patients with higher DLQI scores placed more value on response maintenance than those with lower DLQI scoresCitation35,Citation52. Lastly, patients who were also diagnosed with PsA were less concerned with speed to responseCitation22,Citation42 and they were more concerned with both response rates and response maintenance than patients without this comorbidityCitation22,Citation49.
Discussion
This study confirmed that DCE methodology is being used more frequently to capture preferences regarding treatment characteristicsCitation56. Despite increased adoption of DCE methodology, this study has identified that the current body of literature provides a limited understanding of patient preferences in PsA and should focus on conducting more direct comparisons of the preferences between patients and physicians. The usefulness of these studies is widely recognized, for example, earlier this year (February, 2019), NICE provided its first recommendations regarding the design of patient preference studies and have encouraged authors to seek consultation and feedbackCitation57. As patient preference insights increasingly inform regulatory and reimbursement processes of new medication applications in both North AmericaCitation58,Citation59 and EuropeCitation60,Citation61, the quality of DCE studies must improve so that the findings drawn from these studies are reliable and transferred to decision-making contextsCitation19.
To meet necessary quality standards, this review identified specific gaps intrinsic to the methodology adopted by current DCE studies, specifically in the context of capturing PSO preferences. A major gap in the current DCE literature is the lack of reporting on non-responders. Though it is admittedly difficult to gather information on participants that do not respond, careful survey construction can both attract higher response rates and can ensure that the responses truly reflect the preferences of respondents in real-world settings and are thus more generalizable. According to Bridges et al.Citation31, interviewer-led administration of surveys may improve a respondent’s comprehension of the DCE exercise. Secondly, confirming the results of an evaluation conducted by ISPOR in 2012Citation32 which determined that the experimental design of most studies was not being properly documented, a quarter of studies evaluated in this review did not adequately report the findings of their statistical analysis. Studies either omit reporting coefficient scores for all attributes or simply interpreted coefficient scores without considering the range of the levels. Applying the range method, as described by Hauber et al.Citation34, was complicated by the heterogeneity of data reporting styles. We therefore recommend that authors either report relative importance scores or are transparent in the use of coefficients for reporting the importance of attributes evaluated.
Although studies have improved in reporting the experimental design used in the last four years, there continue to be gaps. Specifically, the selection of a specific experimental design was seldom justified, and these designs were piloted in only a fifth of all studies reviewed. DCE tasks are known to be cognitively burdensome, and thus overcrowding of choices can complicate decision-makingCitation41,Citation62. This review identified that the number of attributes included has decreased in the last 4 years to comply according to ISPOR recommendations, however as the average number of attributes remains high, we echo these recommendations to try to minimize the overcrowding of attributes in DCE design. A further possibility to avoid information overload due to too many attributes is to divide the attributes into groups. The DCEs by Schaarschmidt et al.Citation20,Citation22,Citation42,Citation51 Schmieder et al.Citation49 Umar et al.Citation5 and Kromer et al.Citation35 contained 10 or 11 attributes. However, the attributes were divided into two groups with 5–6 attributes each in order to reduce the number of attributes presented in parallel, with one attribute being part of both groups to enable a later comparison; similar examples of combining treatment attributes can be found (Appendix D). Lastly, given the vast heterogeneity in attribute naming conventions we have proposed strategic categorization of attributes for psoriasis. Outcome attributes can be firstly differentiated into efficacy and safety-specific outcomes; these should then be further sub-categorized to allow one to address the full range of preferences for treatment options. We caution that although categorization may improve alignment and comparisons amongst studies, it may also take away from gaining insights into the intricacies of patient and physician preferences.
Our study has confirmed the findings of previous reviewsCitation9,Citation23, efficacy tends to be the most influential treatment attribute for both patients and physicians. We use the term efficacy broadly here to include response rate, speed of response, response maintenance, and quality-of-life measures. All these sub-categories were identified as being within the top two most influential treatment attributes for treatment selection. For psoriasis, the current standard measure for treatment efficacy is PASI90Citation63. However, it was demonstrated in some studies that patients may place more value to full clearance, especially in comparison to physiciansCitation21,Citation38,Citation48. For this reason, we recommend that future studies assess the difference in relative importance between PASI90 and PASI100. Furthermore, this finding has greater implications for future clinical studies and for pricing and reimbursement decisions that aim to make value-based decisions that closely reflect patient preferences. Another interesting finding reported by Tada et al.Citation45 was that patients placed most value on the sustainability of efficacy after treatment withdrawal, in which another study termed this attribute “bio-holiday” potentialCitation41. We acknowledge that though it may be very difficult to measure this end-point pre-marketing authorization, it currently represents an unmet patient-need that may provide opportunities for future investigation. This further supports the integration of real-world evidence and post-approval clinical evidence in value-based decision-making at a regulatory level.
Safety attributes were also considered important in treatment decision-making by both patients and physicians. As highlighted in previous reviewsCitation9,Citation23, severe AEs had a stronger influence on decision-making than mild AEs. Specifically, 10-year risk of tuberculosis, lymphoma, and of serious infections are of primary concern to both patients and physicians when selecting appropriate treatments. According to our assessment, in comparison to patients, physicians identified severe AEs as more influential in treatment decision-making. Additionally, sub-group analysis of physicians identified more experienced physicians tend to place more weight and consideration to severe AE profiles when selecting which treatment to prescribeCitation41,Citation42. Conversely to physicians, our results demonstrate that patients place great importance on process attributes. In fact, patients selected various process attributes as being the most important attribute influencing decision-making in more studies than adverse events (mild and severe) altogether, especially when studies included a cost element. This not only suggests that procedural should be incorporated into decision-making considerations at the regulatory level, it also suggests that there is a lack of congruence between physicians and patients. Finding alignment between physicians and patients can lead to patient-centric prescribing and can shift the focus on driving patient-valueCitation64. In turn, improved alignment through shared decision-making has been shown to positively affect treatment outcomes through increased adherence ratesCitation65. The results of this review can stimulate communication of preferences between patients and physicians. The most important attributes identified in this review can potentially be integrated into patient-decision aids, which have also been shown to be effective at increasing patient’s knowledge and satisfactionCitation66.
Lastly, given that patient and physician preferences are indeed heterogenous, participant subgroups must be distinguished in order to allow regulators the opportunity to adapt their decisions to the appropriate population groups in questionCitation19. The current review only briefly addresses the subgroup analysis performed by the studies included, however in doing so we were able to identify that there is significant variability in the preferences of patient subgroups. Of note, age, disease severity, and quality-of-life impact (as measured by the DLQI) are significant observable characteristics that clinicians should consider when deciding upon the best treatment course.
This review includes certain limitations that are worth mentioning. Firstly, our review complemented the PREFS checklist with four items from the ISPOR checklist. In doing so, we did not adequately evaluate the data-collection plan and statistical analysis executed in the studies included. As noted above, we highlighted gaps in the reporting of results in certain studies. Thus, a more in-depth analysis could have unearthed more limitations in the results reported. Furthermore, it is important to note that using the range method to quantify the relative importance of attributes is highly dependent on the range of levels chosen to define any given attribute. This emphasizes the importance of setting realistic (clinically-relevant) levels for each attribute identified. Another limitation of this study is that although we consulted with an expert in the field of psoriasis to assist with the categorization of all attributes, it is possible that other authors may opt to categorize attributes using language more consistent with their local context. This study does however provide transparency regarding the categorization process executed (Appendices B–E). Lastly, it was decided to occlude conference publications from this review. However, 12 conference abstracts were identified that evaluated patient-preferences in a DCE format. This again points to the growing relevance of DCE studies as being a preferred method to study patient preferences, but it also highlights the importance of updating the results of this review once new evidence becomes available.
Conclusion
In this systematic review of DCEs investigating physician and patient preferences for psoriasis treatment, it was determined that both patient and physicians place the greatest level of importance on efficacy-specific outcome measures such as response rates (especially PASI 90) when making decisions regarding treatment choice. In general, efficacy, safety, and process attributes were all deemed important by patients and physicians, whereas physicians placed more weight on safety attributes and patients on process attributes. To facilitate shared-decision making, clinicians must take into consideration diverse treatment attributes and become accustomed to individual variability in preferences. The highly-important attributes identified in this review can serve to design patient-decision aids and may provide clinicians with the starting point to facilitate these conversations. Lastly our review confirms that process attributes in addition to efficacy and safety attributes deserve further consideration at the regulatory level.
Transparency
Declaration of funding
No funding was received for the conduct of this study.
Declaration of financial/other relationships
NS was registered as a student at Maastricht University at the time this analysis was performed and has after study completion become an employee of UCB Pharma. DW is a registered (external) PhD student at Maastricht University and employee of UCB Pharma. MC is an employee of UCB Pharma. MH declares no conflicts of interest. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
MH and NS were involved in the conception and design of the study. NS was involved in the analysis and interpretation of the data, whereas DW supported analysis and provided quality control checking. All authors were involved in the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published; and that all authors agree to be accountable for all aspects of the work.
Acknowledgements
None.
References
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Griffiths CEM, van der Walt JM, Ashcroft DM, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017;177(1):e4–e7.
- Bhosle MJ, Kulkarni A, Feldman SR, et al. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35.
- de Arruda LH, De Moraes AP. The impact of psoriasis on quality of life. Br. J. Dermatol. 2001;144(Suppl 58):33–36.
- Umar N, Schaarschmidt M, Schmieder A, et al. Matching physicians’ treatment recommendations to patients’ treatment preferences is associated with improvement in treatment satisfaction. J Eur Acad Dermatol Venereol. 2013;27(6):763–770.
- Thorneloe RJ, Bundy C, Griffiths CE, et al. Nonadherence to psoriasis medication as an outcome of limited coping resources and conflicting goals: findings from a qualitative interview study with people with psoriasis. Br J Dermatol. 2017;176(3):667–676.
- Jevtic T, Bukumiric Z, Jankovic SM. Effects of treatment adherence on clinical and economic outcomes in patients with psoriasis. Med Glas (Zenica). 2013;10(1):106–112.
- Kitchen H, Cordingley L, Young H, et al. Patient-reported outcome measures in psoriasis: the good, the bad and the missing!. Br J Dermatol. 2015;172(5):1210–1221.
- Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319.
- Bridges J, Onukwugha E, Johnson F, et al. Patient preference methods—a patient centered evaluation paradigm. ISPOR Connections. 2007;13(6):4–7.
- Bolt T, Mahlich J, Nakamura Y, et al. Hematologists’ preferences for first-line therapy characteristics for multiple myeloma in Japan: attribute rating and discrete choice experiment . Clin Ther. 2018;40(2):296–308.e2.
- Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–2993.
- Underwood G. NICE to research patient preferences in HTA. 2016; [cited 2020 Jun 2]. Available from: http://www.pharmatimes.com/news/nice_to_research_patient_preferences_in_hta_1033029.
- van Overbeeke E, Whichello C, Janssens R, et al. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: a literature review. Drug Discov Today. 2019;24(1):57–68.
- Mangham LJ, Hanson K, McPake B. How to do (or not to do). Designing a discrete choice experiment for application in a low-income country. Health Policy Plan. 2009;24(2):151–158.
- Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320(7248):1530–1533.
- Alcusky M, Lee S, Lau G, et al. Dermatologist and Patient Preferences in Choosing Treatments for Moderate to Severe Psoriasis. Dermatol Ther (Heidelb). 2017;7(4):463–483.
- Reed SD, Lavezzari G. International experiences in quantitative benefit-risk analysis to support regulatory decisions. Value Health. 2016;19(6):727–729.
- Vass CM, Payne K. Using discrete choice experiments to inform the benefit-risk assessment of medicines: are we ready yet? Pharmacoeconomics. 2017;35(9):859–866.
- Schaarschmidt M-L, Schmieder A, Umar N, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011;147(11):1285–1294.
- Kauf TL, Yang J-C, Kimball AB, et al. Psoriasis patients’ willingness to accept side-effect risks for improved treatment efficacy. J Dermatolog Treat. 2015;26(6):507–513.
- Schaarschmidt M-L, Kromer C, Herr R, et al. Patient preferences for biologicals in psoriasis: top priority of safety for cardiovascular patients. PLoS One. 2015;10(12):e0144335.
- Gonzalez JM. Evaluating risk tolerance from a systematic review of preferences: the case of patients with psoriasis. The patient-patient-centered outcomes research. Patient. 2018;11(3):285–300.
- Fink A. Conducting research literature reviews: from the Internet to paper. Los Angeles: Sage Publications; 2019.
- Yu T, Enkh-Amgalan N, Zorigt G. Methods to perform systematic reviews of patient preferences: a literature survey. BMC Med Res Methodol. 2017;17(1):166.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- Umar N, Yamamoto S, Loerbroks A, et al. Elicitation and use of patients’ preferences in the treatment of psoriasis: a systematic review. Acta Derm Venereol. 2012;92(4):341–347.
- Kleij K-S, Tangermann U, Amelung VE, et al. Patients’ preferences for primary health care – a systematic literature review of discrete choice experiments. BMC Health Serv Res. 2017;17(1):476.
- Papaioannou D, Sutton A, Carroll C, et al. Literature searching for social science systematic reviews: consideration of a range of search techniques. Health Info Libr J. 2010;27(2):114–122.
- Cuchacovich R, Perez-Alamino R, Garcia-Valladares I, et al. Steps in the management of psoriatic arthritis: a guide for clinicians. Ther Adv Chronic Dis. 2012;3(6):259–269.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health-a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413.
- Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13.
- Joy SM, Little E, Maruthur NM, et al. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31(10):877–892.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315.
- Kromer C, Schaarschmidt M-L, Schmieder A, et al. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS One. 2015;10(6):e0129120.
- Rothery C, Bojke L, Richardson G, et al. A discrete choice experiment to explore patients’ willingness to risk disease relapse from treatment withdrawal in psoriatic arthritis. Clin Rheumatol. 2016;35(12):2967–2974.
- Eliasson L, Bewley AP, Mughal F, et al. Evaluation of psoriasis patients’ attitudes toward benefit-risk and therapeutic trade-offs in their choice of treatments . Patient Prefer Adherence. 2017;11:353–362.
- Fairchild AO, Reed SD, Johnson FR, et al. What is clearance worth? Patients’ stated risk tolerance for psoriasis treatments. J Dermatolog Treat. 2017;28(8):709–715.
- Guevara B, Gonzales N, Visitacion L. patient preference on psoriasis treatment in a Philippine Tertiary Hospital: a conjoint analysis: P127. J Eur Acad Dermatol Venereol. 2017;30:62.
- Gonzalez J, Johnson F, McAteer H, et al. Comparing preferences for outcomes of psoriasis treatments among patients and dermatologists in the U.K.: results from a discrete-choice experiment. Br J Dermatol. 2017;176(3):777–785.
- Bolt T, Kobayashi H, Mahlich J. Patient and physician preferences for therapy characteristics for psoriasis: a discrete choice experiment in Japan. PharmacoEconomics-open. 2018;3:255–264.
- Schaarschmidt M-L, Herr R, Gutknecht M, et al. Patients’ and physicians’ preferences for systemic psoriasis treatments: a nationwide comparative discrete choice experiment (PsoCompare). Acta Derm Venereol. 2018;98(2):200–205.
- Xu Y, Sudharshan L, Hsu M-A, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11(8):408.
- Feldman SR, Regnier SA, Chirilov A, et al. Patient-reported outcomes are important elements of psoriasis treatment decision making: a discrete choice experiment survey of dermatologists in the United States. J Am Acad Dermatol. 2019;80(6):1650–1657.
- Tada Y, Ishii K, Kimura J, et al. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46(6):466–477.
- Ashcroft D, Seston E, Griffiths C. Trade-offs between the benefits and risks of drug treatment for psoriasis: a discrete choice experiment with U.K. dermatologists. Br J Dermatol. 2006;155(6):1236–1241.
- Seston EM, Ashcroft DM, Griffiths CE. Balancing the benefits and risks of drug treatment: a stated-preference, discrete choice experiment with patients with psoriasis. Arch Dermatol. 2007;143(9):1175–1179.
- Hauber AB, Gonzalez JM, Schenkel B, et al. The value to patients of reducing lesion severity in plaque psoriasis. J Dermatolog Treat. 2011;22(5):266–275.
- Schmieder A, Schaarschmidt M-L, Umar N, et al. Comorbidities significantly impact patients’ preferences for psoriasis treatments. J Am Acad Dermatol. 2012;67(3):363–372.
- Umar N, Schöllgen I, Terris DD. It is not always about gains: utilities and disutilities associated with treatment features in patients with moderate-to-severe psoriasis. Patient Preference and Adherence. 2012;6:187.
- Schaarschmidt ML, Umar N, Schmieder A, et al. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27(2):187–198.
- Torbica A, Fattore G, Ayala F. Eliciting preferences to inform patient-centred policies: the case of psoriasis. Pharmacoeconomics. 2014;32(2):209–223.
- Kromer C, Peitsch WK, Herr R, et al. Treatment preferences for biologicals in psoriasis: experienced patients appreciate sustainability. J Dtsch Dermatol Ges. 2017;15(2):189–200.
- Rigopoulos D, Ioannides D, Chaidemenos G, et al. Patient preference study for different characteristics of systemic psoriasis treatments (Protimisis). Dermatol Ther. 2018;31(3):e12592
- The World Bank. World Bank Country and Lending Groups. 2019.
- Bien DR, Danner M, Vennedey V, et al. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient. 2017;10(5):553–565.
- National Institute for Health and Care Excellence. NICE provides first scientific advice on patient preference study design. 2019; [cited 2020 Jun 2]. Available from: https://www.nice.org.uk/news/article/nice-provides-first-scientific-advice-on-patient-preference-study-design.
- Johnson FR, Zhou M. Patient preferences in regulatory benefit-risk assessments: a US perspective. Value Health. 2016;19(6):741–745.
- Klein AV, Hardy S, Lim R, et al. Regulatory decision making in Canada-exploring new frontiers in patient involvement. Value Health. 2016;19(6):730–733.
- Mott DJ. Incorporating quantitative patient preference data into healthcare decision making processes: is hta falling behind? Singapore: Springer; 2018.
- Mühlbacher AC, Juhnke C, Beyer AR, et al. Patient-focused benefit-risk analysis to inform regulatory decisions: the European Union perspective. Value Health. 2016;19(6):734–740.
- Addelman S. Symmetrical and asymmetrical fractional factorial plans. Technometrics. 1962;4(1):47–58.
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–648.
- Mühlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163–180.
- Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566–577.
- Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews. 2017;4(4):CD001431.
Appendix A. Frequency of general characteristics of studies included by category
Appendix B. Quality overview of all the DCE studies included. This graph denotes the percentage of studies that achieved an acceptable score (score = 1) in each of the PREFS and ISPOR checklist items
Appendix C. The distribution of all attributes by category for each of the 25 DCE studies included in this review
Appendix D. All efficacy-specific outcome attributes extracted from all 25 DCE studies
Appendix E. All safety-specific outcome attributes extracted from all 25 DCE studies
Appendix F. All process attributes extracted from all 25 DCE studies
Appendix G. All cost-related attributes extracted from all 25 DCE studies