Abstract
Objective
Two similarly designed studies compared user experiences with a second-generation extra-thin-wall, 5-bevel 32 G × 4 mm pen needle (PN) with redesigned hub versus four thinner commercially available PNs.
Methods
Adults (18–75 years old) with type 1 or type 2 diabetes and ≥3 months of experience with pen injectors qualified for single-visit, single-blinded randomized studies. The investigational 32 G PN was compared with three 33–34 G × 3.5–4 mm PNs in Study 1 and one 34 G × 4 mm PN in Study 2. Participants completed 12 abdominal injections of 0.3 mL sterile saline using insulin pens in 6 pairs, each comprising one investigational 32 G PN and one comparator PN in random order. After each injection pair, participants compared injection pain via relative 150 mm visual analog scale (VAS) and perceived dose delivery force via relative 5 point Likert scale. Adjusted models tested injection pain scores (primary endpoint) for noninferiority and, if met, then for superiority. ClinicalTrials.gov identifiers: NCT03878758 and NCT03878745.
Results
The investigational 32 G PN met noninferiority as well as superiority criteria for less injection pain vs. each comparator (p < .01), with adjusted mean relative VAS scores 9.1–17.6 in Study 1 (n = 154) and 7.3 in Study 2 (n = 55). The investigational 32 G PN was also superior vs. each comparator PN in requiring less relative perceived force to deliver the dose (p < .01).
Conclusions
The investigational 32 G PN was associated with less participant-reported injection pain and less perceived dose delivery force compared with four thinner PNs, suggesting no additional pain reduction or force reduction benefit conferred by the thinner PNs.
Introduction
Pen injectors and pen needles (PNs) were developed as alternatives to syringes and vials for patients with type 1 and type 2 diabetes mellitus who require insulinCitation1. Patients with diabetes may prefer pens/PNs and find them easier to use and more convenient than drawing insulin from a vial with a syringeCitation2,Citation3. Recognizing such patient preferences, together with reducing injection-related anxiety, may improve the patient experience and promote adherence and persistence with insulin therapyCitation4–6. Moreover, pens/PNs can provide better accuracy in insulin dose deliveryCitation7–11.
Manufacturers are continually modifying PN designs to improve the injection experience and insulin delivery into the subcutaneous (SC) tissue for consistent insulin absorption and actionCitation1. Over the years, PNs have become shorter (4 mm and 3.5 mm) and thinner (34 gauge [34 G]). Compared with larger PNs, such as 31 G × 8 mm PNs, the shorter and thinner PNs are preferred by patients, and they provide similar glycemic controlCitation12. Shorter length needles also reduce the risk of inadvertent intramuscular (IM) injection, which can impact the rate of insulin absorption, putting patients at an increased risk of developing hypoglycemiaCitation1,Citation13–16. Ultrasound measurements of the usual insulin injection sites have demonstrated that 4 mm PNs are long enough to penetrate the SC tissue while reducing IM injection risk in both pediatric and adult populationsCitation13,Citation15. There is a belief that longer needles are required with obese patients, but this idea is not supported by clinical dataCitation17. Studies with obese patients have demonstrated that 4 to 6 mm PNs, compared with longer PNs (8 mm), provide equivalent glycemic controlCitation18–20. Moreover, shorter needles (4 to 5 mm) simplify the injection experience, because they can be inserted without a pinch-up to tent the skin, a step recommended with longer needles in order to reduce the risk of unintended IM injections.
Pen needle tips vary in terms of bevel design, e.g. the number and angularity of the tip facets. Most commercially available insulin PNs have 3 bevelsCitation21. A study using a 32 G PN with 5-bevel tip geometry found that the 5-bevel needle tip contributes to less penetration force and less perceived injection painCitation21.
Many insulin pen manufacturers recommend that the PN should remain in the body for up to 10 s after fully depressing the pen thumb buttonCitation1. This is because sufficient time is needed for the pen to fully deliver the insulin; otherwise, with premature needle removal from the body, insulin may leak from the needle tip and from the injection site, reducing the delivered insulin doseCitation1,Citation22.
Reports have shown that PNs with thinner outer diameters, such as 33 G and 34 G, are noninferior to a comparator 32 G PN for efficacy, safety, glycemic outcomes and glycemic variabilityCitation23,Citation24. Thinner needles are intended to reduce injection pain, but the subsequent decrease in inner diameter of these needles may compromise other aspects of the patient experience, resulting in more force required to deliver the dose, insulin leakage from the PN tip or injection site, and potentially more frequent bending or breaking.
For these reasons, a 32 G extra-thin-wall, 5-bevel cannula PN has been re-engineered with a contoured hub with expanded surface area to improve the injection experience and injection depth consistencyCitation21,Citation25–27. The aim of this investigation, conducted as two separate studies, was to compare user experiences with this redesigned, second generation 32 G × 4 mm PN (investigational BD 32 G PN: BD NanoFootnotei 2nd Gen, also known as BD Nano PRO) versus four thinner and similar length, commercially available PNs (33 G × 4 mm, 34 G × 4 mm, and 34 G × 3.5 mm). The primary objective was to compare the investigational BD 32 G PN with each of the other four PNs for participant-reported relative injection pain. Secondary objectives included comparisons of 1) perceived force to deliver the dose, 2) amount of leakage from the needle tip and injection site, and 3) PN bending.
Methods
Both studies were designed as prospective, randomized, partially single-blinded trials to compare the investigational BD 32 G × 4 mm PN with thinner gauge PNs during a single 60–120 minute site visit. Study 1 was conducted from 8 February through 30 April 2019, at three sites in the US (TKL Research Inc., Fair Lawn, NJ; East-West Medical Research Institute, Honolulu, HI; and Mills-Peninsula Medical Center, San Mateo, CA; ClinicalTrials.gov identifier NCT03878758). Study 2 was conducted from 8 February through 14 June 2019, at a single site (East-West Medical Research Institute; ClinicalTrials.gov identifier NCT03878745).
The protocol was approved by the appropriate Institutional Review Board for each study site, and written informed consent was obtained from each participant in accordance with international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines.
Participants
For both studies, adults with type 1 or type 2 diabetes, 18–75 years of age, who had used a pen injector to self-administer insulin at doses ≥10 units and/or liraglutide at least once daily for at least 3 months were eligible for study participation after demonstrating injection proficiency. No restrictions were placed on gauge or length of current PNs. Adults who were taking antiplatelet or anticoagulant therapy (other than aspirin up to 162 mg/day) were excluded from both studies. Other key exclusion criteria were a history of a bleeding disorder, recurrent dermatological conditions or skin disorder, gross skin abnormalities on or close to the abdominal injection sites, unwillingness to inject into the abdomen, a history of symptomatic low blood pressure or fainting during hypodermic injections, and use of any analgesic medication within 24 h before and during the study (except aspirin at ≤162 mg/day). Women who were pregnant were also excluded. Study 2 had the additional inclusion criterion of Japanese descent – namely, either being born in Japan or having at least one parent or one grandparent born in Japan. The comparator PN used in Study 2 (Terumo NanopassFootnoteii 34 G × 4 mm PN) is currently available in Japan; to minimize potential bias regarding population differences, the intent was to ethnically mimic the intended users of the Terumo 34 G PN vs. the investigational BD 32 G PN.
Potential participants were required to demonstrate injection proficiency by performing three proper mock injections (up to 10 attempts permitted) into an injection pad with a saline pen prepared by study staff. Eligible participants who successfully completed the proficiency test were randomly assigned to PN order and abdominal injection sites (described below) after signing written informed consent.
Investigational and comparator pen needles
In Study 1, the investigational BD 32 G PN was compared with three PNs (). In Study 2, the investigational BD 32 G PN was compared with one PN.
Table 1. Pen needles used in Studies 1 and 2.
Study procedures
In both studies, the study staff prepared six pairs of reusable insulin pens (ClikSTARFootnoteiii) with sterile saline cartridges for each participant. To each pair of insulin pens, staff attached one investigational BD 32 G PN and one comparator PN. In Study 1, with three comparator PNs, the six pairs thus included six investigational BD 32 G PNs and two of each comparator PN. In Study 2, with one comparator PN, six pairs of injections were completed with each pair including the investigational plus the one comparator PN.
While maintaining blinding for participants, study staff attached each PN to a pen, removed the PN outer cover and inner needle shield, primed the pen with saline equivalent of two insulin units, and set the pen to deliver 0.3 mL saline (the 30 unit mark on the pen). While the study staff were not blinded to PN type, participants were not aware of PN brand, length or gauge; however, they could potentially have been aware of differences between the comparators and the investigational BD 32 G PN because of the latter’s distinctive hub design (). Therefore, we considered the studies to be partially single-blinded.
Figure 1. Hub design differences between the investigational BD 32 G pen needle and the comparator pen needles.
From left to right: investigational BD 32 G PN, Insupen 33 G PN, Comfort EZ 33 G PN, Insupen 34 G PN, Terumo 34 G PN.

Because all the study PNs had lengths of 4 mm or less, regarding injection technique, participants were instructed only to insert the PNs “straight in” (perpendicular) to the skin (i.e. no pinch up was instructed or required). They then performed the six pairs of injections into the abdomen. Specific abdominal sites were randomized, and the order of the PNs was also randomized within each pair of injections. An injection site diagram was provided to assist with adherence to injection site randomization.
After each pair of injections, participants completed two rating scales for relative injection pain and relative perceived injection force. Injection pain was rated using a 150 mm visual analogue scale (VAS) in response to the question “Which injection was less painful?” as depicted in . Perceived dose delivery force was assessed using a 5 point Likert scale ranging from 1 to 5 ().
Figure 2. Relative injection pain 150 mm visual analogue scale (VAS). For the analyses, the VAS scale was converted to a uniform range from −75 mm (much less pain with the comparator) to +75 mm (much less pain with the investigational BD 32 G PN).
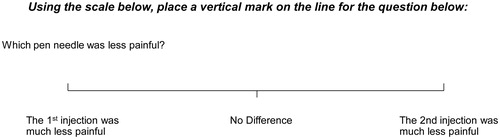
Table 2. The delivery force Likert scale used in the studies.
During each injection, study staff recorded the times from initiation to full compression of the pen injector button (dose delivery time) and from full button depression to removal of the PN from the skin (dwell time). After each injection, staff measured any leakage upon removal of the PN and noted any bleeding. Leakage from the needle tip and the injection site after removal of the PN was measured (only in the absence of bleeding) by collecting fluid (wet), if present, from skin and needle tip using a preweighed (dry) absorbent cellulose spear. The spear was weighed using a calibrated scale accurate to 0.001 g, and dry and wet weights were recorded. In addition, staff inspected each PN for needle bend and for breakage at the patient end. If bending of the patient end of the needle after removal from the body was identified visually, study staff measured and documented the extent of bending using a provided diagram. The degree of bend was scored in 10° increments as 0 (no bend), 1 (>0° to 10° bend), 2 (>10° to 20°), 3 (>20° to 30°), 4 (>30° to 40°) and 5 (>40°).
After completion of all injections, participants completed a four-question survey regarding their usual (in-home use) injection experience based on a 5 point Likert scale (1, disagree; 2, somewhat disagree; 3, neutral; 4, somewhat agree; and 5, disagree): (1) injection pain affects my level of satisfaction with my treatment, (2) thumb pressure needed to deliver the dose affects my level of satisfaction with my treatment, (3) post-injection leakage increases my level of concern that I may not be receiving my full dose of medication, and (4) a bent needle increases my level of concern about the reliability of my injection.
Endpoints
The primary endpoint of each study was to demonstrate noninferiority of the investigational BD 32 G × 4 mm PN for participant-reported injection pain compared with each of the 33 G and 34 G comparator PNs. For this analysis, the 150 mm VAS scale for relative injection pain was converted to a uniform scale for each pair of injections whereby −75 mm indicated much less pain with the comparator and +75 mm indicated much less pain with the investigational BD 32 G PN (see ). If the primary endpoint met the noninferiority criterion of −10 mm, then the 32 G PN was evaluated for superiority vs. each comparator.
The secondary endpoints all tested superiority of the investigational BD 32 G × 4 mm PN vs. the comparator PNs. Participant-reported perceived force to deliver the dose was evaluated via the 5 point Likert scale. The responses were converted to a uniform −2 to +2 scale of force needed to deliver medication, with negative scores (−) defined as less thumb force needed for the comparator PN and positive scores (+) defined as less thumb force needed for the investigational BD 32 G PN (see ).
The occurrence of leakage from the needle tip and the injection site (measurements combined) was defined as wet weight equivalent to ≥5% of the injection volume, thus equivalent to ≥0.015 g (0.015 mL). Needle bend was defined as being present if the bend rating was ≥2 (>10°).
Exploratory objectives, not reported in this paper, included comparisons of the investigational BD 32 G PN vs. comparator PNs for dose delivery time, dwell time, total injection time (dose delivery plus dwell time) and breakage of the patient end of the needle. In addition, participant responses to the final four-question survey were summarized.
Occurrence of any adverse events was evaluated, recorded and followed up as required.
Statistical analyses
Baseline demographic and diabetes-related characteristics of study participants were summarized with the mean (SD) or median (range) to describe continuous variables, as appropriate, and count and percentage were calculated for the categorical variables.
The average rating for injection pain was calculated with 95% confidence intervals (CIs) in a modeling approach adjusting for the effects of pair order, order within pair for the investigational BD 32 G PN (because of the often-observed bias favoring the second PN used in a pair), abdominal site and random participant effect. Results were tested for noninferiority (lower 95% CI bound > −10 mm) and, if met, then for superiority (lower 95% CI bound of >0 mm).
A similar modeling approach, testing only for superiority, was used to evaluate the 5 point scale for participant-perceived dose delivery force.
The average difference in percentage of occurrence of leakage and needle bending score between the 32 G PN and comparators, with 95% CI, was calculated with the score method.
For the primary endpoint of participant-reported injection pain in both studies, we calculated that a sample size from 266–300 pairs per comparator was required to provide >90% power for the noninferiority criterion of −10 mm, using an SD for relative VAS of 50 mm from a prior studyCitation27 and assuming no true difference in pain (based on a two-sided 95% CI for the mean).
With regards to leakage, for Study 1, the same sample size would generate data from 798 paired investigational PNs and 266 of each paired comparator PN and would provide >90% power to demonstrate superiority with the investigational PN. For Study 2, the sample size would generate comparison data for 300 investigational-comparator PN pairs and provide 90% power to demonstrate significantly less frequent leakage with the investigational PN.
All statistical tests were two-sided with a significance level of 5%, and adjustments were made for multiple comparisons when appropriate. Analyses were carried out using the R language for statistical computing (version 3.5.1; https://www.r-project.org/).
Results
Participants
Study 1
Of 168 individuals initially enrolled, 10 did not meet eligibility criteria, and 1 withdrew consent. The remaining 157 participants completed the study and were evaluated for safety, while 154 (98%) were included in the outcome analyses after exclusion of 3 participants not meeting protocol criteria.
Study 1 participants ranged in age from 22 to 75 years (mean age 56 years) and included similar percentages of men and women. Additional demographic data, diabetes history, and history of pen and PN use in the prior month are summarized in . The most common injection site used in the prior month was the abdomen (87%), followed by the arm (6%), thigh (4%) and buttocks (3%). Overall, 59 participants (38%) reported reuse of PNs. Of those who reused PNs, 41% most commonly reused twice, and 24% reused five times or more.
Table 3. Demographic and diabetes-related characteristics of participants.
Study 2
In the second study, 55 individuals were enrolled. All of them met study eligibility criteria, completed the study, and were included in both safety and outcome analyses.
These 55 participants ranged in age from 34 to 75 years (mean age 60 years) and included 56% men. All were of Japanese descent. Additional demographic data, diabetes history, and history of pen and PN use in the prior month are summarized in . The most common injection site used in the prior month was the abdomen (89%), followed by the thigh (7%) and arm (4%). Overall, 26% reported reusing PNs, most commonly twice (29%), or five times or more (29%).
Endpoints
Study 1
For less participant-reported relative injection pain (primary endpoint, noninferiority; secondary endpoint, superiority), the investigational BD 32 G PN met the noninferiority criterion (lower bound of the 95% CI > −10), as well as the superiority criterion (lower bound of the 95% CI >0) compared with each of the comparator PNs ().
Figure 3. Adjusted mean differences for perceived (A) injection pain and (B) delivery force between the investigational BD 32 G × 4 mm pen needle vs. each of three comparator pen needles in Study 1 and the Terumo 34 G in Study 2.
Lower bound 95% CI >0 indicates investigational BD 32 G PN perceived as having significantly less injection pain and requiring significantly less dose delivery force.
Visual analogue scale (VAS) scores ranged from -75 mm (much less pain with the comparator) to +75 mm (much less pain with the investigational BD 32 G PN).
Likert scale scores for perceived dose delivery force ranged from -2 (less thumb force needed for the comparator pen needle) to +2 (less thumb force needed for the investigational BD 32 G pen needle).
Abbreviations. CI, Confidence interval; G, Gauge.
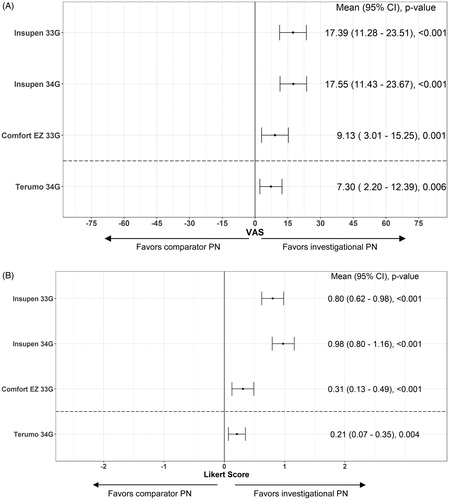
The investigational BD 32 G PN was superior vs. each comparator PN in requiring less relative perceived force to deliver the dose (secondary endpoint; ). For each of the three PN comparisons, more participants perceived that less dose delivery force was needed with the investigational BD 32 G vs. the Insupen 33 G, Comfort EZ 33 G, and Insupen 34 G, respectively ().
Figure 4. Perceived injection force: percentage of participants in Study 1 and Study 2 selecting the comparator pen needle or the investigational BD 32 G pen needle as requiring less thumb force for the injection, or reporting no difference between the two.
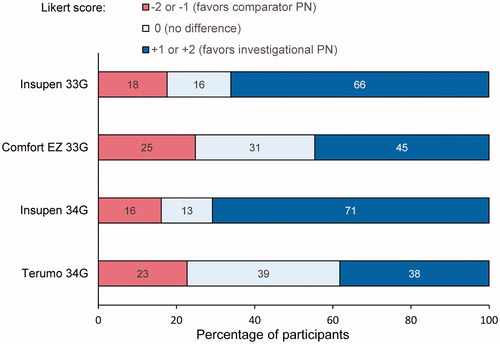
For leakage (secondary endpoint), the investigational BD 32 G PN demonstrated significantly less frequent occurrence of leakage compared with the Insupen 33 G PN (p < .001) and Insupen 34 G PN (p = .026), and there was no statistical difference between the investigational BD 32 G PN and the Comfort EZ 33 G PN. Leakage ≥0.015 g (0.015 mL, equivalent to 1.5 units of insulin) occurred with 0.4% of the investigational BD 32 G PNs, 6.2% of the Insupen 33 G PNs, 0.6% of the Comfort EZ 33 G PNs, and 18.8% of the Insupen 34 G PNs. Significant clinical site effects were present for one of the PNs for leakage (Insupen 34 G PN); therefore, unadjusted results are presented, and observed leakage volume is depicted graphically by PN and study site in .
Figure 5. Observed leakage weight for each pen needle by study site in Study 1 and Study 2. The solid horizontal line indicates predefined leakage threshold from injection site and/or needle tip of ≥0.015 g (equivalent to 1.5 U of U-100 insulin).
The percentage of needle bending showed no statistical difference between the investigational BD 32 G PN and each of the three comparators (unadjusted results, all sites combined, because of significant site effect for the investigational BD 32 G PN). The observed percentage of bent needles for the four study PNs ranged from 1.3% to 2.3%.
Study 2
For less participant-reported relative injection pain (primary endpoint – noninferiority, secondary endpoint – superiority), the investigational BD 32 G PN met the noninferiority criterion (lower bound of the 95% CI > −10) as well as the superiority criterion (lower bound of the 95% CI >0) vs. the comparator Terumo 34 G PN ().
More participants perceived less dose delivery force was needed with the investigational BD 32 G vs. the Terumo 34 G PN (), and the investigational BD 32 G PN was significantly superior vs. the Terumo 34 G PN in requiring less relative perceived force to deliver the dose (secondary endpoint; ).
Leakage occurred with 0.3% of the investigational BD 32 G PNs and 1.8% of the Terumo 34 G PNs but demonstrated no statistical difference. Observed leakage volume for the two PNs is depicted graphically in . The occurrence of needle bending was not significantly different between the two PNs, with an observed percentage of bent needles of 0.6% with each PN.
Safety evaluations
No adverse events were reported in either study.
Discussion
The results of these two similarly designed prospective, randomized, partially single-blinded studies indicate that participants perceived the investigational BD 32 G PN, compared with each of the four thinner, commercially available PNs, as resulting in significantly less injection pain and requiring less force to deliver a dose. These results were consistent in both Study 1 with ethnically diverse participants and Study 2 with participants of Japanese ethnicity. In Study 1, occurrence of leakage from the needle tip and injection site was also significantly less frequent compared with two of the three comparators, while there was no difference in leakage occurrence vs. the third comparator PN or vs. the comparator PN in Study 2. There were no significant differences in occurrence of needle bending for any PN comparisons.
In a prior study, the investigational BD 32 G × 4 mm PN was compared with PNs of similar gauge and was rated as being more comfortable, less painful and easier to use, and preferred overallCitation27. In our investigation, the investigational BD 32 G PN was compared with PNs of thinner gauge and similar or shorter length, and our findings of significantly less participant-reported injection pain and force to deliver the dose were in line with those prior study results.
There could be several reasons why a wider gauge PN was associated with less injection pain in this study. Besides gauge, other factors associated with PN design, such as needle hub and 5-bevel tip geometry, have been shown to contribute to less perceived injection pain. In both Study 1 and Study 2, the investigational BD 32 G PN had a 5-bevel tip while the comparators had a 3-bevel tip. In a prior study, the 5-bevel 32 G needle tip required less penetration force than similar-length 3-bevel tipsCitation21. Patients in the latter study also reported significantly more comfort, easier insertion, more preference, and less pain during home use of the 5-bevel PN compared with their usual 3-bevel PNsCitation21. Moreover, in the present two studies, the investigational BD 32 G PN was of a re-engineered design with a contoured hub and an expanded surface area vs. the comparator PNs with cylindrical posted hubs (). The intent of the re-engineered hub with the investigational BD 32 G PNs is to distribute insertion forces across a wider skin surface with the aim of minimizing the clinical impact of variable patient injection forcesCitation27.
Patients with insulin-requiring diabetes self-administer insulin injections one to four times or more per day. Consequently, minimizing injection-related pain and discomfort are patient concerns and serve as the impetus for insulin syringe and PN manufacturers to modify PN design. While an initial study with the 32 G PN demonstrated significantly less perceived injection pain compared with larger gauge PNs (31 G, 5 mm and 8 mm)Citation12, an unresolved question remained as to whether injection pain would continue to be reduced with progressive evolution of needle gauge to even thinner 33–34 G PNs. The data from the present two studies indicated that the investigational BD 32 G × 4 mm PNs were perceived to have less associated injection pain than each of the thinner comparator PNs – Insupen 33 G × 4 mm, Comfort EZ 33 G × 4 mm, Insupen 34 G × 3.5 mm and Terumo 34 G × 4 mm – suggesting that the 5-bevel needle and re-engineered hub design, in addition to needle gauge, all contribute to reduced injection pain.
Needles with thinner outer diameters (gauge) often have narrower inner diameters. As needles become narrower, more force or time is needed to deliver similar doses. The amount of force required to deliver doses may also be associated with injection painCitation28. With insulin pens, the amount of force applied to the thumb button to deliver the dose may also be transferred to the needle insertion into the body. The required force to deliver medication is substantially affected by internal PN diameter. When needle gauges are made thinner, unless the needle wall thickness is also made thinner, then the internal diameter also becomes narrower. The BD 32 G PN is an extra-thin-wall cannula designed to have the same outer diameter as regular or thin wall needles of the same gauge but with a thinner wall, resulting in 30% wider inner diameter than the earlier BD 32 G PN version. In a prior study the extra-thin-wall PN was rated by patients as requiring less time and less thumb force for injections than their usual PNs of 31 G or 32 G (4–8 mm)Citation25. In the present studies, the investigational BD 32 G PN was perceived as needing less delivery force by 3.7, 1.8, 4.4 and 1.7 times as many study participants compared with, respectively, the Insupen 33 G PN, the Comfort 33 G PN, the Insupen 34 G PN and the Terumo 34 G PN.
With insulin pens, time to deliver the dose (delivery time) and the time during which patients keep the PN in their body (dwell time) may impact the volume of insulin delivered. If patients remove the PN from their body too quickly, then insulin may leak from the injection site and/or needle tip, resulting in injecting less insulin than intended. To compensate for this phenomenon, most insulin pens recommend keeping the PN in the body for up to 10 s after pushing the pen’s thumb button to zero. Insulin leakage may have clinical consequences. Significantly less frequent leakage, defined as equivalent to ≥5% of the intended 0.3 mL injection volume (≥0.015 mL), occurred with the investigational BD 32 G PN vs. two of the four comparator PNs. In these two studies, the intended saline dose of 0.3 mL was delivered without subsequent leakage more often with the BD 32 G PN vs. either of the thinner Insupen PNs (both 33 G and 34 G). Since insulin was not used, the impact of these differences in leakage on glycemic variability could not be determined in this study.
Needle bending was also investigated to determine whether thinner 33/34 G PNs were less robust than the 32 G PN. The PNs were used only once and no significant differences in the amount of bending were observed among the study PNs. We note that needle bending is potentially more likely with needle reuse, which is not recommended and was not assessed in this investigation, although 38% and 26% of participants in Study 1 and Study 2, respectively, reported needle reuse in the past.
The strengths of these studies include the randomized assignment of PN order, consistent use of abdominal injection sites (almost 90% of participants reported the abdomen as their usual injection site), and the large numbers of PNs tested under standardized conditions. Individuals participating in the study were required to have a minimum of 3 months’ daily experience using insulin and/or liraglutide pens, thereby ensuring a basic understanding of pen use and the ability to evaluate the study PNs. The primary endpoint of relative injection pain and secondary study endpoint of perceived force to deliver the dose were both participant-perceived measures, which were considered to be important outcomes to evaluate in light of the associations of patient perceptions and preferences with adherence to therapy and long-term persistence with therapyCitation4,Citation6,Citation29.
Several limitations should also be considered when evaluating our findings. The study was conducted under supervision, which could limit the generalizability of results to patients with diabetes using insulin and/or liraglutide pens in unsupervised settings. Participants were blinded insofar as they were not told the identity of the pen needles under investigation. The investigators, some of whom are authors of the manuscript, did indeed know the identity of the studied products. Because of packaging and differences in pen needle appearance, it would have been challenging – if not impossible – to conduct a double-blinded study using these comparators and investigational product. Therefore, we considered this to be a partially single-blinded study (meaning the participants were unaware of the PN brand but could see the design differences between the investigational PN and the comparator PNs).
To improve generalizability, we allowed participants to utilize their own injection techniques rather than requiring standardized injection hold times. Assessments were limited to abdominal injection sites; findings could potentially have differed had other injection sites been used. Moreover, it is uncertain whether participants’ usual dose volumes would have had an impact on relative comparisons. Finally, in Study 1, statistically significant clinical site effects for one PN were present for two secondary endpoints (leakage and needle bending); therefore, we reported the unadjusted results.
Further research is needed to determine whether the administration of different volumes would affect relative pain, force or leakage, and whether potential differences in leakage have an impact on glycemic variability for patients self-administering insulin or other drugs. In addition, the study of larger patient populations using different PNs and insulin pens, ideally in both supervised and unsupervised settings, would be of interest. A specific pen, the FlexTouchFootnoteiv, which diminishes delivery force differences between PNsCitation30, should be studied to determine whether the investigational BD 32 G PN continues to provide a better patient experience vs. thinner PNs. Additional studies may be considered for specific populations, such as children, obese patients and people with reduced dexterity; however, there is no evident reason why the investigational BD 32 G PN would not be appropriate for all patients.
Conclusions
In summary, the findings of these two prospective, randomized studies demonstrated that the investigational BD 32 G × 4 mm PN was associated with less participant-reported injection pain and less force required for dose delivery vs. the thinner Insupen 33 G × 4 mm, Comfort EZ 33 G × 4 mm, Insupen 34 G × 3.5 mm and Terumo 34 G × 4 mm PNs. Advances in PN manufacturing technology have enabled the production of very thin needles with the primary intent of reducing injection pain; however, the results of these studies suggest no additional pain reduction benefit or force reduction benefit conferred by these thinner PNs when compared with the investigational BD 32 G PN.
Transparency
Declaration of funding
This study was funded by Becton, Dickinson and Company (BD), Franklin Lakes, NJ, USA. The study sponsor participated in the study design, data collection, data review, data analysis, writing of the report and decision to submit the article for publication.
Declaration of financial/other relationships
M.A.G., S.W. and B.L. have disclosed that they were full-time employees of BD at the time of the study. W.Y. and S.G. have disclosed that they are full-time employees and stock-holders of BD. D.F.-P. has disclosed that he has received research funding from BD, AstraZeneca, Novo Nordisk, Sanofi Aventis, Lilly, and LifeScan. D.C.K. has disclosed that he is a consultant to Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche and Thirdwayv. CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no relevant financial or other relationships to disclose.
Author contributions
M.A.G., S.W. and B.L. were involved in the conception and design of the study; D.F.-P. and D.C.K. were involved in the data collection and W.Y. in the data analysis. All authors were involved in the interpretation of the data, the drafting of the paper and revising it critically for intellectual content, and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Previous presentation
Study results were presented in part as abstract IP36, AADE19 (American Association of Diabetes Educators Conference); 2019 Aug 9–12; Houston, TX.
Acknowledgements
We thank all the study participants and the trial investigators and staff at TKL Research Inc., Fair Lawn, NJ; East-West Medical Research Institute, Honolulu, HI; and Mills-Peninsula Medical Center, San Mateo, CA, for their contributions. We gratefully acknowledge the assistance of BD’s Global Clinical Development team. Medical writing and editorial assistance was provided by Elizabeth V. Hillyer DVM, funded by BD.
Notes
i BD Nano is a trade name of Becton, Dickinson and Company, Franklin Lakes, NJ, USA.
ii Nanopass is a registered trade name of Terumo Corporation, Tokyo, Japan.
iii ClikSTAR is a registered trade name of sanofi-aventis, Paris, France.
iv FlexTouch is a registered trade name of Novo Nordisk.
References
- Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231–1255.
- Molife C, Lee LJ, Shi L, et al. Assessment of patient-reported outcomes of insulin pen devices versus conventional vial and syringe. Diabetes Technol Ther. 2009;11(8):529–538.
- Lasalvia P, Barahona-Correa JE, Romero-Alvernia DM, et al. Pen devices for insulin self-administration compared with needle and vial: systematic review of the literature and meta-analysis. J Diabetes Sci Technol. 2016;10(4):959–966.
- Peyrot M, Perez-Nieves M, Ivanova J, et al. Correlates of basal insulin persistence among insulin-naïve people with type 2 diabetes: results from a multinational survey. Curr Med Res Opin. 2017;33(10):1843–1851.
- Aronson R. The role of comfort and discomfort in insulin therapy. Diabetes Technol Ther. 2012;14(8):741–747.
- Peyrot M, Rubin RR, Kruger DF, et al. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–245.
- Lteif AN, Schwenk WF. Accuracy of pen injectors versus insulin syringes in children with type 1 diabetes. Diabetes Care. 1999;22(1):137–140.
- Korytkowski M, Bell D, Jacobsen C, et al. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836–2848.
- Bailey TS, Edelman SV. Insulin pen use for type 2 diabetes – a clinical perspective. Diabetes Technol Ther. 2010;12 (Suppl 1):S86–S90.
- Luijf YM, DeVries JH. Dosing accuracy of insulin pens versus conventional syringes and vials. Diabetes Technol Ther. 2010;12 (Suppl 1):S73–S77.
- Krzywon M, van der Burg T, Fuhr U, et al. Study on the dosing accuracy of commonly used disposable insulin pens. Diabetes Technol Ther. 2012;14(9):804–809.
- Hirsch LJ, Gibney MA, Albanese J, et al. Comparative glycemic control, safety and patient ratings for a new 4 mm × 32G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26(6):1531–1541.
- Gibney MA, Arce CH, Byron KJ, et al. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519–1530.
- Hirsch L, Byron K, Gibney M. Intramuscular risk at insulin injection sites – measurement of the distance from skin to muscle and rationale for shorter-length needles for subcutaneous insulin therapy. Diabetes Technol Ther. 2014;16(12):867–873.
- Lo Presti D, Ingegnosi C, Strauss K. Skin and subcutaneous thickness at injecting sites in children with diabetes: ultrasound findings and recommendations for giving injection. Pediatr Diabetes. 2012;13(7):525–533.
- Vaag A, Handberg A, Lauritzen M, et al. Variation in absorption of NPH insulin due to intramuscular injection. Diabetes Care. 1990;13(1):74–76.
- Bergenstal RM, Strock ES, Peremislov D, et al. Safety and efficacy of insulin therapy delivered via a 4mm pen needle in obese patients with diabetes. Mayo Clin Proc. 2015;90(3):329–338.
- Kreugel G, Keers JC, Kerstens MN, et al. Randomized trial on the influence of the length of two insulin pen needles on glycemic control and patient preference in obese patients with diabetes. Diabetes Technol Ther. 2011;13(7):737–741.
- Hirsch LJ, Gibney MA, Li L, et al. Glycemic control, reported pain and leakage with a 4 mm × 32 G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28(8):1305–1311.
- Schwartz S, Hassman D, Shelmet J, et al. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge × 6 mm needle versus a 29 gauge × 12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26(10):1663–1678.
- Hirsch L, Gibney M, Berube J, et al. Impact of a modified needle tip geometry on penetration force as well as acceptability, preference, and perceived pain in subjects with diabetes. J Diabetes Sci Technol. 2012;6(2):328–335.
- Joubert M, Haddouche A, Morera J, et al. Potential insulin underdelivery from prefilled and reusable insulin pens in cases of premature needle withdrawal: a laboratory evaluation. Diabetes Technol Ther. 2015;17(10):712–716.
- De Berardis G, Scardapane M, Lucisano G, et al. Efficacy, safety and acceptability of the new pen needle 34G × 3.5 mm: a crossover randomized non-inferiority trial; AGO 02 study. Curr Med Res Opin. 2018;34(9):1699–1704.
- Valentini M, Scardapane M, Bondanini F, et al. Efficacy, safety and acceptability of the new pen needle 33G × 4 mm. AGO 01 study. Curr Med Res Opin. 2015;31(3):487–492.
- Aronson R, Gibney MA, Oza K, et al. Insulin pen needles: effects of extra-thin wall needle technology on preference, confidence, and other patient ratings. Clin Ther. 2013;35(7):923–933.
- Rini C, Roberts BC, Morel D, et al. Evaluating the impact of human factors and pen needle design on insulin pen injection. J Diabetes Sci Technol. 2019;13(3):533–545.
- Whooley S, Briskin T, Gibney MA, et al. Evaluating the user performance and experience with a re-engineered 4 mm × 32g pen needle: a randomized trial with similar length/gauge needles. Diabetes Ther. 2019;10(2):697–712.
- Praestmark KA, Jensen ML, Madsen NB, et al. Pen needle design influences ease of insertion, pain, and skin trauma in subjects with type 2 diabetes. BMJ Open Diab Res Care. 2016;4(1):e000266.
- Garnero TL, Davis NJ, Perez-Nieves M, et al. Insulin non-persistence among people with type 2 diabetes: how to get your patients to stay on insulin therapy. Postgrad Med. 2018;130(4):394–401.
- Hemmingsen H, Niemeyer M, Hansen MR, et al. A prefilled insulin pen with a novel injection mechanism and a lower injection force than other prefilled insulin pens. Diabetes Technol Ther. 2011;13(12):1207–1211.
