Abstract
Objective
Migraine is a chronic, disabling neurological disease affecting >1 billion people worldwide. Migraine remains undertreated in Asia, including Taiwan. Galcanezumab is a humanized monoclonal antibody that selectively binds calcitonin gene-related peptide, a peptide firmly established in the pathophysiology of migraine, with demonstrated efficacy and safety in patients with episodic or chronic migraine. Our objective was to evaluate the efficacy and safety of galcanezumab in Taiwanese patients with episodic or chronic migraine.
Methods
We conducted a sub-group analysis of the Taiwanese cohort from two double-blind, placebo-controlled, Phase 3 clinical trials of galcanezumab in the prevention of episodic and chronic migraine, EVOLVE-2 (NCT02614196) and REGAIN (NCT02614261), respectively. During the EVOLVE-2 and REGAIN double-blind periods, 2092 patients were randomly assigned to receive monthly injections of either placebo, 120 mg galcanezumab (240 mg loading dose), or 240 mg galcanezumab. In REGAIN, a 9-month open-label period followed. Post-hoc analysis on the Taiwanese population across both trials included 106 patients, 45 of whom continued into the open-label period in REGAIN.
Results
Our findings show that galcanezumab has similar efficacy and safety in the Taiwanese population, as compared to the “All Patients” population included in the study. Galcanezumab treatment reduced the number of monthly migraine headache days, determined a higher percentage of patients with a ≥ 50% response, and positively impacted quality of life.
Conclusion
Galcanezumab is a promising therapeutic for the preventive treatment of migraine in the Taiwanese population.
Introduction
Migraine is a chronic neurological disease characterized by recurrent episodes of headache lasting 4–72 h that can manifest with or without additional symptomsCitation1. Migraine is diagnosed according to the most current version of the International Classification of Headache Disorders (ICHD-3), which specifies the disabling symptoms that accompany headache pain during the attackCitation1. For example, migraine with or without aura can be accompanied by nausea, vomiting, photophobia and/or phonophobia, whereas visual, sensory, speech and/or motor disturbances can occur as part of migraine with aura. Migraine can be episodic or chronic, depending on the number of migraine headache days experienced by an individual. The occurrence of ≤14 headache days a month is classified as episodic, whereas chronic migraine is characterized by ≥15 headache days a month, where at least eight of those days meet the ICHD-3 criteria for migraineCitation1. Migraine can be very disabling also due to the associated symptoms, such as autonomic symptoms and frequent comorbiditiesCitation2–4. These associated symptoms and disorders contribute to the significant negative impact of migraine on a patient’s quality of life.
Migraine is the leading cause of disability in persons under 50 years of ageCitation5 and affects 11.6% of the global population, or approximately 1 billion people worldwideCitation6. It has a two- to three-fold higher prevalence in women compared with menCitation6,Citation7. The median prevalence of migraine in the Asia-Pacific region is 9.1%, ranging from 1.5% in Hong Kong to 22.8% in IndiaCitation8. In Taiwan specifically, prevalence is 9.1%, with only 18% of those affected having received a correct diagnosisCitation9. Studies in Taiwan have reported that migraine is associated with high levels of disability, comorbidity, lower quality of life, greater health care resource utilization and loss of productivityCitation10–12. The consequence of migraine on the employed labor force in Taiwan is high absenteeism and as a result, significant economic lossCitation13.
Preventive treatment has the potential to improve a patient’s ability to function, reduce migraine-associated disability, and enhance the response to acute treatments by reducing the frequency and severity of migraine attacks. As a result, preventive treatment can improve a patient’s quality of life significantlyCitation14. A survey across eight countries in Asia, including Taiwan, reported that 29.2% of patients with migraine were taking preventive medicationCitation15. Of the patients not taking preventive treatment, 68.2% were considered eligible for such treatment by neurologistsCitation15. Hence, there is a recognized unmet need for Taiwanese as well as other Asian patients with migraine, concerning diagnosis and treatmentCitation15. Current guidelines in Taiwan for preventive treatment of migraine suggest beta-blockers, anti-depressants, calcium channel blockers, anticonvulsants, non-steroidal anti-inflammatory drugs, onabotulinumtoxinA and miscellaneous medicationsCitation16. However, these medications were not initially developed as preventive treatments for migraine and the majority are associated with high discontinuation rates, largely due to reasons surrounding efficacy and tolerabilityCitation17,Citation18. Therefore, the Taiwanese population with migraine needs new treatment options with improved efficacy and tolerability.
A preventive therapy with demonstrated efficacy in episodic and chronic migraine is galcanezumab, a humanized IgG4 monoclonal antibody that binds calcitonin gene-related peptide (CGRP) and prevents its biological activity without blocking the CGRP receptorCitation19–25. CGRP is a molecular mediator of neurogenic inflammation and migraine pathophysiologyCitation26–28. Targeting CGRP signaling has demonstrated promising results in the preventive treatment of migraineCitation29–31. Galcanezumab is approved by the FDA for the prevention of migraine in adults along with EMA approval for the prophylaxis of migraine in patients with at least four migraine headache days per monthCitation32–34. Phase 3 randomized, double-blind, placebo-controlled studies in patients with episodic migraine (EVOLVE-1 and EVOLVE-2)Citation19,Citation20 and chronic migraine (REGAIN)Citation21 demonstrated significant reductions in the number of monthly migraine headache days following treatment with galcanezumab, compared with placebo. In addition, significantly more patients achieved ≥50% response with galcanezumab compared with placebo in all the three studiesCitation19–21.
Both EVOLVE-2 and REGAIN trials included a Taiwanese cohort. In the current study, we investigated the efficacy and safety of galcanezumab for the preventive treatment of migraine in Taiwanese patients, by analyzing the data from EVOLVE-2 and REGAIN. In addition, efficacy outcomes for the entire East Asian population in EVOLVE-2 are presented, to further support the results from the Taiwanese population.
Methods
Patients and study design
EVOLVE-2 and REGAIN were Phase 3, multi-center, randomized, double-blind, placebo-controlled studies. In EVOLVE-2, patients with episodic migraine were treated with either placebo, 120 mg galcanezumab, or 240 mg galcanezumab for 6 months. In REGAIN, patients with chronic migraine were treated with either placebo, 120 mg galcanezumab, or 240 mg galcanezumab for 3 months, followed by a 9-month open-label extension (OLE) period. Patients randomized to the 120 mg dose during the double-blind periods in EVOLVE-2 and REGAIN received a loading dose of 240 mg at the first injection only. During the 9-month open-label period in REGAIN, irrespective of previous treatment assigned, all patients received an initial loading dose of 240 mg galcanezumab in the first month and 120 mg in the following month. Thereafter, patients received either 120 mg or 240 mg galcanezumab monthly at the discretion of the investigator (). International Headache Society (IHS) International Classification of Headache Disorders–3rd edition (ICHD-3) beta version definitions of migraine were used.
Figure 1. Study designs for EVOLVE-2 and REGAIN. *Eligibility period determined between a minimum of 30 days and a maximum of 40 days. Investigators may have up to 5 additional days (beyond the 40 days) if needed to schedule patients’ Visit 3 appointment. aPatients randomized to the 120 mg dose will receive a loading dose of 240 mg at the first injection only (Visit 3). bAt visit 7, all patients who enter the open-label extension will receive galcanezumab at a dose of 240 mg. cAt visit 8, all patients will receive galcanezumab at a dose of 120 mg. dStarting at visit 9, dosing will be flexible (galcanezumab 120 or 240 mg) at the discretion of the investigator.
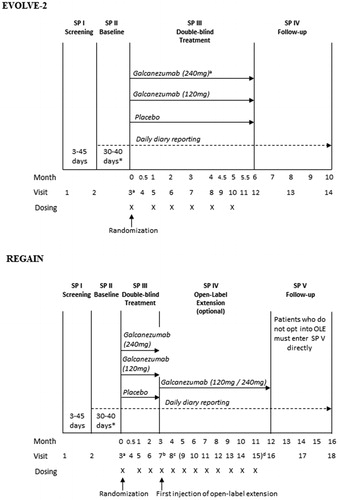
According to the protocols for both EVOLVE-2 and REGAIN the concomitant use of acute medications to treat migraine was allowed, inclusive of the following: acetaminophen (paracetamol), NSAIDs; triptans; ergotamine and derivatives; isometheptene mucate, dichloralphenazone and acetaminophen combination (Midrin); or combinations thereof. Use of opioid- and barbiturate-containing medications was restricted to no more than 3 days per month, and a single dose of injectable steroids was allowed only once during the study, in an emergency setting.
Patients were 18–65 years of age and had migraine onset prior to age 50 years. Patients were excluded if prior treatment with three or more adequately dosed preventive treatments from different specified drug classes had failed, if they had taken a therapeutic antibody in the 12 months prior to the study, and/or if they had any medical or psychiatric illness that would preclude study participation. Detailed inclusion and exclusion criteria for both trials have been previously reportedCitation20,Citation21.
Ethics approval and consent to participate
Study protocols were reviewed and approved by appropriate institutional review boards for each of the study sites. The clinical trials were conducted according to the “Good Clinical Practice” and “The Declaration of Helsinki” guidelines. All patients provided written informed consent before undergoing the study procedure. The studies are registered at ClinicalTrials.gov (NCT02614196 and NCT02614261).
Outcome measures
The primary objective was to compare the overall mean change from baseline in the number of monthly migraine headache days during the double-blind treatment period, with galcanezumab versus placebo. A key secondary outcome measure was ≥50% response rate, the percentage of patients with ≥50% reduction from baseline in monthly migraine headache days. Another secondary outcome measure was the change from baseline in the Migraine-Specific Quality of life (MSQ v2.1) Role Function-Restrictive (RF-R) score. MSQ results of both trials have already been reportedCitation20,Citation21. Key efficacy and safety outcomes for the subpopulation of Taiwanese patients in EVOLVE-2 and REGAIN are presented in the current study, as well as efficacy outcomes for the greater East Asian population in EVOLVE-2, which included patients from Taiwan and Korea. Results for the Taiwanese subpopulation are compared to the “All Patients” population, which includes Taiwanese patients, for reference only as the regulatory submission was based upon this population. The percentages of Taiwanese patients in the “All Patients” populations in EVOLVE-2 and REGAIN were 5.9% (58 Taiwanese patients/979 “All Patients”) and 4.3% (48 Taiwanese patients/1113 “All Patients”), respectively ().
Table 1. Patient demographics and baseline disease characteristics (EVOLVE-2 and REGAIN).
Statistical analysis
All efficacy analyses were conducted among the subgroup of Taiwanese or East Asian patients on the intent-to-treat population, which included randomized patients who received at least one dose of study drug. Change from baseline in monthly migraine headache days was analyzed using a mixed models repeated measures (MMRM) approach with terms for treatment, month, treatment-by-month, baseline and baseline-by-month. For the EVOLVE-2 East Asian and “All Patients” populations, a term for pooled region/country was also included. For the REGAIN analysis of Taiwanese and “All Patients” populations, a term for pooled country was added to the above model as well as concurrent prophylaxis, consistent with the clinical study report. The same models were used for MSQ total and domain scores except that for EVOLVE-2, Taiwan, East Asian, and “All Patients” populations, an additional term for baseline monthly migraine headache days category was included, and for REGAIN a term for pooled country was added for analysis of the “All Patients” population but not for the Taiwanese population. Percentage of patients with ≥50% reduction from baseline in the number of monthly migraine headache days was analyzed with a categorical, pseudo-likelihood-based repeated measures model for binary outcomes with terms for treatment, month, treatment-by-month and baseline monthly migraine headache days. For REGAIN, the covariates of baseline medication overuse and concurrent prophylaxis were also included. An unstructured covariance structure was used to model within-patient errors except for the REGAIN Taiwanese population analyses of ≥50% response rate and MSQ role function-restrictive, for which heterogenous autoregressive and heterogenous Toeplitz covariance structures were used, respectively. Analysis of adverse events was conducted on the safety population, which analyzed patients according to the modal dose of treatment received. Adverse event incidence rates were compared between treatment groups using Fisher’s exact test. Treatment effects were evaluated based upon a two-sided, .05 significance level. Results were not adjusted for multiple testing as these analyses were post-hoc and exploratory in nature. Treatment effects for MMRM models are presented using mean difference compared with placebo and 95% confidence intervals, whereas for binary outcomes, they are presented using odds ratios and 95% confidence intervals. All statistical analyses were performed with the use of SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC).
Results
Patient disposition and baseline characteristics
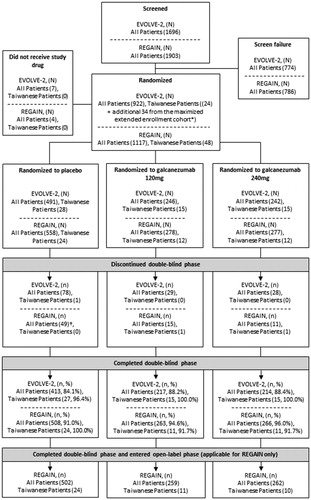
A total of 1696 and 1903 patients entered study screening and 922 and 1117 patients, inclusive of 24 and 48 Taiwanese patients, were randomized in EVOLVE-2 and REGAIN respectively (). Additional patients from Taiwan and Korea entered EVOLVE-2 at a later stage through the maximized extended enrollment provision, including 34 randomized Taiwanese patients, bringing the total number of Taiwanese patients in the intent-to-treat population to 58 and 48 in EVOLVE-2 and REGAIN, respectively. The total number of “All Patients” in this population, following maximized extended enrollment, was 979 and 1113 in EVOLVE-2 and REGAIN, respectively. More than 84% of the patients in each treatment group completed the double-blind treatment period in EVOLVE-2, for both “All Patients” and Taiwanese patients. More than 90% of the patients in each treatment group completed the double-blind treatment period in REGAIN, for both “All Patients” and Taiwanese patients ().
Figure 2. Flowchart of patient disposition throughout EVOLVE-2 and REGAIN. The “All Patients” population is inclusive of Taiwanese patients. *EVOLVE-2 was designed to allow maximized extended enrollment. This provision enabled patient enrollment to continue in Taiwan and Korea, if the required pre-specified number of patients for these countries was not reached when EVOLVE-2 had met its planned total sample size for the primary analysis. Maximized extended enrollment was not implemented in REGAIN. †One patient randomized to placebo discontinued early, but because the discontinuation date was listed after the data cutoff date, the patient is not counted among the 49 discontinuations.
Baseline demographics and disease characteristics for the subgroup of Taiwanese patients and the “All Patients” population are shown in . The number of Taiwanese patients that participated in EVOLVE-2 and REGAIN was 58 and 48 respectively. Across both EVOLVE-2 and REGAIN, the Taiwanese population was generally similar to the “All Patients” population in measures such as age, gender, body mass index, migraine headache days per month, migraine attacks per month, migraine headache days with acute medication use per month, and prior preventive treatment. Regarding the duration of migraine disease and migraine headache days category ≥8, these were noticeably less in Taiwanese patient’s in EVOLVE-2 compared with “All Patients”, as was failure with ≥1 or ≥2 prior preventive treatments across both EVOLVE-2 and REGAIN.
Primary and secondary efficacy measures
Reductions in monthly migraine headache days during the double-blind treatment period
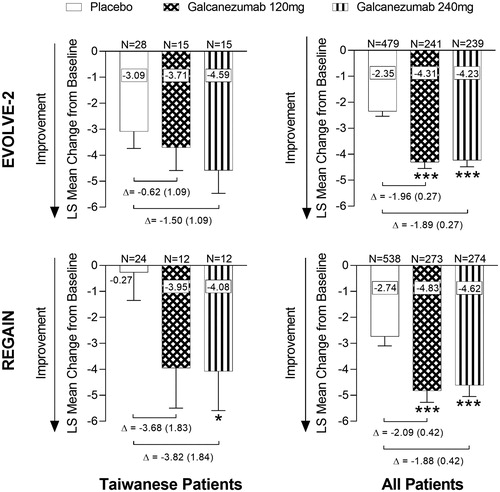
For Taiwanese patients in both EVOLVE-2 and REGAIN trials, treatment with galcanezumab resulted in numerically, but not statistically significantly, greater reductions in the overall mean number of monthly migraine headache days from baseline compared with placebo, with the exception of the 240 mg versus placebo comparison in REGAIN, which was statistically significant (Least squares (LS) mean change difference vs placebo [SE]; EVOLVE-2, Galcanezumab 120 mg = −.62 [1.09], Galcanezumab 240 mg = −1.50 [1.09]; REGAIN, Galcanezumab 120 mg = −3.68 [1.83], Galcanezumab 240 mg = −3.82 [1.84], p < .05) (). As previously reported for “All Patients”, a population with a much larger number of patients, statistically significant differences were found for galcanezumab 120 mg and 240 mg compared with placebo (LS mean change difference vs placebo [SE]; EVOLVE-2, Galcanezumab 120 mg = −1.96 [0.27], Galcanezumab 240 mg = −1.89 [0.27]; REGAIN, Galcanezumab 120 mg = −2.09 [0.42], Galcanezumab 240 mg = −1.88 [0.42], all p < .001) () Citation20,Citation21. As shown, the placebo effect was somewhat greater in Taiwanese patients compared with “All Patients” in EVOLVE-2, whereas in REGAIN the placebo effect was much less in Taiwanese patients compared with “All Patients” ().
Figure 3. Overall mean change from baseline in the number of monthly migraine headache days during the double-blind periods (EVOLVE-2 and REGAIN). Overall mean change in monthly migraine headache days during the double-blind treatment periods for EVOLVE-2 and REGAIN clinical trials (Months 1–6 for EVOLVE-2, and Months 1–3 for REGAIN). Abbreviations. LS, Least Squares; N, Number of intent-to-treat patients who had non-missing baseline and at least 1 post-baseline value. Data are represented as mean ± standard error (SE); *p < .05, ***p < .001 vs placebo. Difference between group means (SE) is shown by Δ.
Percentage of patients with ≥50% reduction in migraine headache days during the double-blind treatment period
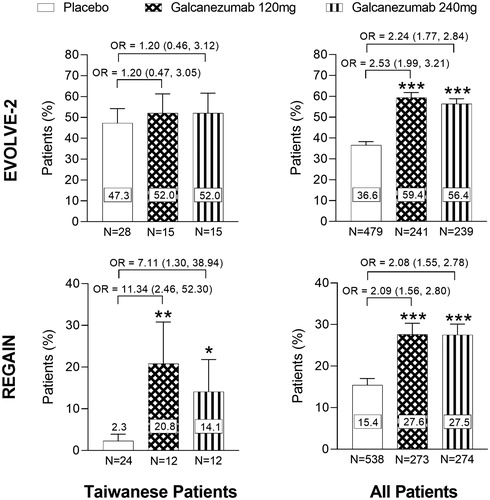
For Taiwanese patients in EVOLVE-2, the overall mean percentage of patients with ≥50% reduction from baseline in monthly migraine headache days was numerically higher, but not statistically significant, with galcanezumab compared with placebo (Odds ratio vs placebo [95% Confidence Interval (CI)]; Galcanezumab 120 mg = 1.20 [0.47, 3.05], Galcanezumab 240 mg = 1.20 [0.46, 3.12]) (). In REGAIN, there was a statistically significant increase in the overall mean percentage of Taiwanese patients with ≥50% reduction from baseline in monthly migraine headache days with both galcanezumab dose groups compared with placebo (Odds ratio vs placebo [95% CI]; Galcanezumab 120 mg = 11.34 [2.46, 52.30] p < .01, Galcanezumab 240 mg = 7.11 [1.30, 38.94] p < .05) (). As previously reported, the percentage of patients achieving a ≥ 50% response was statistically significantly higher in both galcanezumab dose groups compared with placebo for the “All Patients” population (Odds ratio vs placebo [95% CI]; EVOLVE-2, Galcanezumab 120 mg = 2.53 [1.99, 3.21], Galcanezumab 240 mg = 2.24 [1.77, 2.84]; REGAIN, Galcanezumab 120 mg = 2.09 [1.56, 2.80], Galcanezumab 240 mg = 2.08 [1.55, 2.78], all p < .001) ()Citation20,Citation21. As shown, the placebo effect was slightly greater in Taiwanese patients compared with “All Patients” in EVOLVE-2, whereas in REGAIN the placebo effect was much less in Taiwanese patients compared with “All Patients” ().
Figure 4. Overall mean percentage of patients with ≥50% reduction from baseline in the number of monthly migraine headache days during the double-blind periods (EVOLVE-2 and REGAIN). Overall mean percentage of patients with ≥50% reduction from baseline in the number of migraine headache days during the double-blind treatment periods for EVOLVE-2 and REGAIN clinical trials (Months 1–6 for EVOLVE-2, and Months 1–3 for REGAIN). Abbreviations. OR, Odds Ratio; N, Number of intent-to-treat patients who had non-missing baseline and at least 1 post-baseline value. Data are represented as mean ± SE; *p < .05, **p < .01, ***p < .001 vs placebo. OR (95% Confidence Interval) between groups are shown.
Migraine-Specific Quality of Life Questionnaire (MSQ)
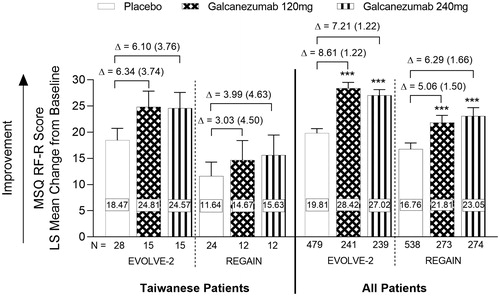
The mean changes from baseline for the MSQ role function-restrictive (RF-R) scores for the Taiwanese and “All Patients” population for months 4–6 of EVOLVE-2 and month 3 of REGAIN are shown in . Following treatment with galcanezumab in the Taiwanese population in both studies, there was a numerically greater, but not statistically significant, improvement from baseline in the RF-R domain score compared with placebo, indicating a reduction in migraine-related functional impairment (LS mean change difference vs placebo [SE]; EVOLVE-2, Galcanezumab 120 mg = 6.34 [3.74], Galcanezumab 240 mg = 6.10 [3.76]; REGAIN, Galcanezumab 120 mg = 3.03 [4.50], Galcanezumab 240 mg = 3.99 [4.63]) (). For the “All Patients” population in EVOLVE-2 and REGAIN, treatment with either 120 mg or 240 mg galcanezumab resulted in statistically significant reductions of migraine-associated functional impairment, as measured in the RF-R, compared with placebo (LS mean change difference vs placebo [SE]; EVOLVE-2, Galcanezumab 120 mg = 8.61 [1.22], Galcanezumab 240 mg = 7.21 [1.22]; REGAIN, Galcanezumab 120 mg = 5.06 [1.50], Galcanezumab 240 mg = 6.29 [1.66], all p < .001) (). As shown, similar effect sizes were found in Taiwanese patients and “All Patients”, between galcanezumab groups and placebo ().
Figure 5. Summary of migraine-specific quality of life role function-restrictive scores during the double-blind periods (EVOLVE-2 and REGAIN). MSQ RF-R scores in the Taiwanese and “All Patients” populations (Months 4–6 for EVOLVE-2 and Month 3 for REGAIN). Abbreviations. N, Number of intent-to-treat patients who had a non-missing baseline and at least 1 post-baseline value; MSQ, Migraine-specific Quality of Life; RF-R, Role-Function-Restrictive; LS, Least Square. Data are represented as mean ± SE; ***p < .001 vs placebo. Difference between group means (SE) is shown by Δ.
Results from the open-label period during REGAIN
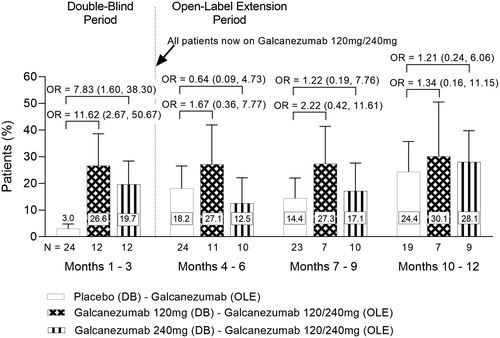
Forty-five Taiwanese patients continued from the double-blind to the open-label period in REGAIN. During the open-label period, those patients that were previously on galcanezumab during the double-blind period maintained reductions in the number of monthly migraine headache days (). In addition, the proportion of patients achieving ≥50% response on galcanezumab during the double-blind period improved or was maintained during the open-label period (). For patients taking placebo during the double-blind period, a reduction in the number of monthly migraine headache days was observed during the open-label period when they received galcanezumab () as well as an increase in the proportion achieving ≥50% response (). For the “All Patients” population that received galcanezumab, mean reductions from baseline in the number of monthly migraine headache days reached a maximum of −7.3 at month 6, and −9.0 at month 12Citation35. The overall mean percentage of the “All Patients” population with ≥50% response following treatment with galcanezumab, reached a maximum of 45.6% at month 6 and 56.9% at month 12Citation35.
Figure 6. Mean change from baseline in the number of monthly migraine headache days in Taiwanese patients during the double-blind and open-label periods in REGAIN. Mean change in migraine headache days for Taiwanese patients who received 120 mg or 240 mg galcanezumab during the open-label treatment period in REGAIN. aAll patients received a loading dose of 240 mg galcanezumab at Month 3, bfollowed by a 120 mg galcanezumab dose at Month 4. cStarting at month 5, dosing is flexible (Galcanezumab120/240 mg) at the discretion of the investigator. Abbreviations. LS, Least Squares; N, Number of intent-to-treat patients who had a non-missing baseline value and a non-missing value for the specified month; DB, Double-Blind; OLE, Open-Label Extension. Data are represented as mean ± SE; *p < .05 vs placebo.
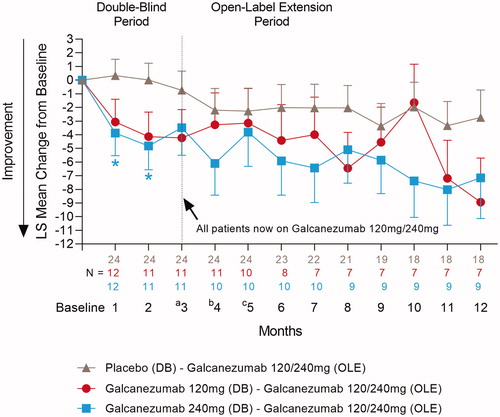
Figure 7. Proportion of Taiwanese patients with ≥50% response in the reduction of monthly migraine headache days during the double-blind and open-label periods in REGAIN. Overall mean proportion of Taiwanese patients with ≥50% response in the reduction of the number of migraine headache days during REGAIN. Patients received 120 mg or 240 mg galcanezumab throughout the open-label period. Abbreviations. N, Number of intent-to-treat patients with a non-missing baseline value and a non-missing value within the specific month interval; DB, Double-Blind; OLE, Open-Label Extension; OR, Odds Ratio. Data are represented as mean ± SE. OR (95% Confidence Interval) between groups are shown.
Primary and secondary efficacy measures in the East Asian population
We wished to study the efficacy results from the Taiwanese population in the context of a greater East Asian population. This was possible for EVOLVE-2 but not REGAIN, as Taiwan was the only participating East Asian country in REGAIN. The following results are for East Asian patients from the 6-month double-blind period of EVOLVE-2 (Months 1–6 for monthly migraine headache days and ≥50% response, Months 4–6 for MSQ RF-R). There were 156 East Asian patients enrolled in EVOLVE-2, inclusive of the 58 Taiwanese patients reported on in the current study. In placebo, galcanezumab 120 mg, and galcanezumab 240 mg groups there were 76, 39, and 41 East Asian patients respectively. Treatment with galcanezumab 120 mg or 240 mg resulted in numerically greater reductions in the overall mean number of monthly migraine headache days from baseline, compared with placebo. The difference between galcanezumab 240 mg and placebo was statistically significant (LS mean change difference vs placebo [SE; N]; Galcanezumab 120 mg = −1.47 [0.75; 39], Galcanezumab 240 mg = −2.16 [0.74; 40] p < .05). The overall mean percentage of patients with ≥50% reduction from baseline in monthly migraine headache days was statistically significantly higher, for both galcanezumab 120 mg and 240 mg, compared with placebo (Odds ratio vs placebo [95% CI; N]; Galcanezumab 120 mg = 1.856 [1.029, 3.350; 39], Galcanezumab 240 mg = 1.918 [1.061, 3.465; 40], both p < .05). Following treatment with galcanezumab, there were statistically significant improvements from baseline in the MSQ RF-R domain score for both galcanezumab 120 mg and 240 mg compared with placebo, indicating a significant reduction in migraine-related functional impairment (LS mean change difference vs placebo [SE; N]; Galcanezumab 120 mg = 7.59 [2.78; 38], Galcanezumab 240 mg = 6.40 [2.74; 40], both p < .05).
Safety measures
A summary of adverse events (AEs) in Taiwanese patients and “All Patients” during the double-blind treatment periods of EVOLVE-2 and REGAIN are shown in . No deaths were reported during either study. Acute pancreatitis and nasal septum deviation were reported as serious adverse events (SAE) in one patient each among Taiwanese patients in both studies. Across EVOLVE-2 and REGAIN, the frequency of treatment emergent adverse events (TEAEs) with both galcanezumab dose groups was comparable for Taiwanese and “All Patients” populations, while the frequency of TEAEs with placebo was slightly lower in the Taiwanese population compared with “All Patients” ().
Table 2. Overview of adverse events during double-blind treatment period (EVOLVE-2 and REGAIN).
A summary of the individual TEAEs reported in the Taiwanese population during EVOLVE-2 and REGAIN is shown in . The most common TEAE reported in the galcanezumab groups was upper respiratory tract infection. Insomnia, hypoaesthesia, contusion, weight decrease, menstrual disorder, vaginal infection, vulvovaginitis and injection site pruritus/rash/reaction/swelling were reported in galcanezumab dose groups only. With the exception of upper respiratory infection, few patients (no more than two) in either galcanezumab group reported other individual adverse events.
Table 3. Common treatment emergent adverse events in Taiwanese patients during EVOLVE-2 and REGAIN (≥5% and greater than placebo).
Discussion
There is currently an unmet need for adequate treatment of migraine in the Taiwanese populationCitation15, despite the high prevalence and burden of diseaseCitation9–13. This study presents galcanezumab as an effective and safe preventive treatment for Taiwanese patients with migraine, through a post-hoc analysis of two phase 3 clinical trials in episodic and chronic migraine, EVOLVE-2 and REGAINCitation20,Citation21.
In EVOLVE −2 and REGAIN studies at baseline, Taiwanese and “All Patients” were generally similar across the characteristics of age, gender, body mass index (BMI), migraine headache days & migraine attacks per month, acute medication use, and prior preventive treatment use. The duration of migraine disease and migraine headache days category ≥8 was lower in Taiwanese patients compared with “All Patients” in EVOLVE-2 (). Taiwanese patients can access medical centers without the need of a referral from a physician and can easily visit headache specialists to participate in a clinical trialCitation36. Thus, Taiwanese patients being recruited into clinical trials at a relatively earlier stage of the disease might explain these differences. These factors could in turn explain why the percentage of patients who failed ≥1 and ≥2 prior preventives was numerically lower in Taiwanese patients compared with “All Patients”, especially for REGAIN ().
Taiwanese patients with episodic or chronic migraine who received galcanezumab experienced a larger reduction in the number of monthly migraine headache days, and higher percentages of patients with ≥50% reduction in monthly migraine headache days compared with placebo ( and ). In REGAIN, statistically significant differences compared with placebo were found in the galcanezumab 240 mg group for monthly migraine headache days reduction (), and in both galcanezumab dose groups for ≥50% reduction in monthly migraine headache days (). No statistically significant treatment differences were found in Taiwanese patients in EVOLVE-2 likely due to the small sample size and corresponding low statistical power, and/or greater placebo effects than those found in “All Patients” ( and ). When monthly migraine headache days, ≥50% response, and MSQ RF-R were assessed in a greater East Asian population in EVOLVE-2, inclusive of Taiwanese patients, significant differences were found for both galcanezumab dose groups compared with placebo, except for galcanezumab 120 mg in monthly migraine headache days (Results; Primary and Secondary Efficacy Measures in the East Asian population). In both EVOLVE-2 and REGAIN for “All Patients”, statistically significant differences for both doses of galcanezumab compared with placebo were observed for overall mean reduction from baseline in monthly migraine headache days () and overall mean percentage of patients with a ≥ 50% reduction in monthly migraine headache days ().
For monthly migraine headache day reduction and ≥50% response rates in REGAIN, a much smaller placebo response was observed in Taiwanese patients compared with “All Patients”. As this difference was not observed in EVOLVE-2, where a slightly stronger placebo response was observed in Taiwanese patients ( and ), we do not have a clear explanation for this but believe it to be likely related to the small sample size. MSQ results demonstrate that treatment with galcanezumab positively impacts quality of life for Taiwanese patients with episodic or chronic migraine, as demonstrated by an increase in MSQ RF-R score () during the double-blind periods in EVOLVE-2 and REGAIN. A similar result for RF-R domain scores was observed for the East Asian population in EVOLVE-2 (Results; Primary and Secondary Efficacy Measures in the East Asian population). Taiwanese patients in REGAIN who were previously taking galcanezumab during the double-blind period maintained both reductions in the number of monthly migraine headache days and a ≥ 50% response during the open-label period ( and ). Those taking placebo during the double-blind period reported reductions in the number of monthly migraine headache days and had a higher likelihood of achieving a ≥ 50% response during the open-label period.
Across EVOLVE-2 and REGAIN the frequency of adverse events in galcanezumab dose groups was comparable between the Taiwanese and “All Patients” populations (). Within the Taiwanese population, the most common TEAE was upper respiratory tract infection (). Injection site reactions were reported in galcanezumab dose groups (), as was reported in previous publications for “All Patients”Citation20,Citation21.
Some limitations of this study should be noted. Although the data were gathered prospectively, this was a post-hoc analysis of a subset of Taiwanese patients from two multi-center phase 3 clinical trials, EVOLVE-2 and REGAIN and no adjustments were made for multiplicity or multiple comparisons. Therefore, results are exploratory and should be interpreted with this in mind. Although, an effect size similar to that seen in the “All Patients” population was found in a majority of the efficacy analyses on the Taiwanese subpopulation, some results were not statistically significant due to the small sample size. This was the case for EVOLVE-2 in particular. The low number of Taiwanese patients is likely to have reduced the power of the statistical tests used in our analyses. In support of this, when efficacy analyses were conducted in a greater East Asian population in EVOLVE-2, inclusive of patients in Taiwan, additional statistically significant differences were observed for galcanezumab dose groups compared with placebo, that were not significant in the Taiwanese-only population.
Conclusion
In conclusion, preventive treatment of migraine in the Taiwanese population with galcanezumab was efficacious and safe, and generally comparable with the results from “All Patients” population.
Transparency
Declaration of funding
This study was funded by Eli Lilly and Company.
Declaration of financial/other relationships
CFL, GDA, and HPH are full-time employees of Eli Lilly and Company. All are minor stockholders of Eli Lilly and Company. CPY is part of speaker bureaus at Eli Lilly and Company. SJW has served on the advisory boards of Eli Lilly and Company, Daiichi-Sankyo, and Taiwan Novartis. He has received honoraria as a moderator from Allergan, Pfizer, Eli Lilly and Company, Bayer, and Eisai. He has received research grants from the Brain Research Center, National Yang-Ming University, Taipei Veterans General Hospital, Taiwan Headache Society, and Taiwan Minister of Technology and Science. SL is a full-time employee of Syneos Health. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
CFL, GDA and HPH contributed to conception and/or study design. SJW contributed to acquisition of the data. CFL and SL contributed to analysis of the data. All authors contributed to interpretation of the data and critical revision of the manuscript for intellectual content. All authors have approved and agreed to be accountable for all aspects of the work.
Acknowledgements
The authors thank all of the study participants, site investigators, and personnel involved in the EVOLVE-2 and REGAIN clinical trials. They also thank Sarah Roche, PhD, and Sreelatha Akkala, employees of Eli Lilly and Company, who provided writing assistance.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808.
- Chen P-K, Wang S-J. Non-headache symptoms in migraine patients. F1000Res. 2018;7:188–188.
- Yang CP, Wang SJ. Sleep in patients with chronic migraine. Curr Pain Headache Rep. 2017;21(9):39.
- Wang S-J, Chen P-K, Fuh J-L. Comorbidities of migraine. Front Neurol. 2010;1:16–16.
- Steiner TJ, Stovner LJ, Vos T, et al. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain. 2018;19(1):17–17.
- Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. 2017;372:307–315.
- Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16(1):76–87.
- Peng KP, Wang SJ. Epidemiology of headache disorders in the Asia-Pacific region. Headache. 2014;54(4):610–618.
- Wang SJ, Fuh JL, Young YH, et al. Prevalence of migraine in Taipei, Taiwan: a population-based survey. Cephalalgia. 2000;20(6):566–572.
- Wang SJ, Wang PJ, Fuh JL, et al. Comparisons of disability, quality of life, and resource use between chronic and episodic migraineurs: a clinic-based study in Taiwan. Cephalalgia. 2013;33(3):171–181.
- Hung PH, Fuh JL, Wang SJ. Validity, reliability and application of the Taiwan version of the migraine disability assessment questionnaire. Journal of the Formosan Medical Association = Taiwan yi Zhi. 2006;105(7):563–568.
- Chen YC, Tang CH, Ng K, et al. Comorbidity profiles of chronic migraine sufferers in a national database in Taiwan. J Headache Pain. 2012;13(4):311–319.
- Fuh JL, Wang SJ, Lu SR. Impact of migraine on the employed labor force in Taiwan. J Chin Med Assoc. 2008;71(2):74–78.
- Silberstein SD. Preventive migraine treatment. Continuum. 2015;21(4 Headache):973–989.
- Wang SJ, Chung CS, Chankrachang S, et al. Migraine disability awareness campaign in Asia: migraine assessment for prophylaxis. Headache. 2008;48(9):1356–1365.
- Huang T-C, Lai T-H, Treatment Guideline Subcommittee of Taiwan Headache Society Taiwan Headache Society. Medical treatment guidelines for preventive treatment of migraine. Acta Neurol Taiwan. 2017;26(1):33–53.
- Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–655.
- Berger A, Bloudek LM, Varon SF, et al. Adherence with migraine prophylaxis in clinical practice. Pain Pract. 2012;12(7):541–549.
- Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–1088.
- Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–1454.
- Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–e2221.
- Silberstein SD, Stauffer VL, Day KA, et al. Galcanezumab in episodic migraine: subgroup analyses of efficacy by high versus low frequency of migraine headaches in phase 3 studies (EVOLVE-1 & EVOLVE-2). J Headache Pain. 2019;20(1):118.
- Yang Y, Wang Z, Gao B, et al. Different doses of galcanezumab versus placebo in patients with migraine and cluster headache: a meta-analysis of randomized controlled trials. J Headache Pain. 2020;21(1):14.
- Raffaelli B, Mussetto V, Israel H, et al. Erenumab and galcanezumab in chronic migraine prevention: effects after treatment termination. J Headache Pain. 2019;20(1):66.
- Forderreuther S, Zhang Q, Stauffer VL, et al. Preventive effects of galcanezumab in adult patients with episodic or chronic migraine are persistent: data from the phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN studies. J Headache Pain. 2018;19(1):121.
- Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8.
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6(10):573–582.
- Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61.
- Giamberardino MA, Affaitati G, Curto M, et al. Anti-CGRP monoclonal antibodies in migraine: current perspectives. Intern Emerg Med. 2016;11(8):1045–1057.
- Ong JJY, Wei DY, Goadsby PJ. Recent Advances in pharmacotherapy for migraine prevention: from pathophysiology to new drugs. Drugs. 2018;78(4):411–437.
- Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6.
- Lamb YN. Galcanezumab: first global approval. Drugs. 2018;78(16):1769–1775.
- US Food and Drug Administration. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761063.
- European Medicines Agency. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/emgality.
- Detke H, Pozo-Rosich P, Reuter U, et al. One-year treatment with galcanezumab in patients with chronic migraine: results from the open-label phase of the REGAIN Study (P2.10-010). Neurology. 2019;92(15 Supplement):P2.10–010.
- Wu T-Y, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. London J Prim Care. 2010;3(2):115–119.
