Abstract
Objectives
We conducted a systematic literature review (SLR) to determine the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyps (CRSwNP) and to describe how the addition of biologics has affected outcomes for patients with CRSwNP.
Methods
The SLR adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Embase, MEDLINE, and Evidence-Based Medicine Reviews databases were searched using OVID. Relevant studies published between 1 January 2008 and 8 February 2019, for epidemiology, and 1 January 2008 and 16 February 2019, for clinical burden, and relevant conference abstracts from 1 January 2017 to 7 March 2019, for epidemiology and 1 January 2017–16 February 2019 for clinical burden were included.
Results
For the epidemiology and clinical burden SLR, 147 and 119 records, respectively, met the inclusion criteria. We found the prevalence of CRSwNP was 1–2.6% and was greater in men. Asthma, allergy, and allergic rhinitis were the most common comorbidities identified. Reported risk factors included asthma, gene polymorphisms, age, and eosinophilia. Studies indicated that dupilumab, mepolizumab, and omalizumab each improved different clinical outcomes. Non-biologics (drugs such as corticosteroids or antibiotics, surgery, or aspirin desensitization) improved clinical outcomes as well.
Conclusions
CRSwNP is fairly prevalent in the general population. Despite the significant efficacy of existing treatments, several unmet needs remain. The high burden of uncontrolled symptoms, frequent recurrence of nasal polyps after surgery, and long-term adverse effects of oral corticosteroids indicate that new therapies addressing these unmet needs should be developed. Although data on biologics from randomized controlled trials look promising, the efficacy of biologics in the real world has yet to be established.
The SLR of the epidemiology and clinical burden of CRSwNP revealed key gaps in the literature. There was a paucity of prevalence data across many geographic areas, and no prevalence projections could be determined. Studies showed varying efficacy of non-biologics and no studies directly compared biologics for efficacy. Data regarding clinical efficacy of agents for eosinophilic CRSwNP or severe CRSwNP were lacking, and these patient populations would be served by more trials.
Introduction
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a chronic inflammatory condition associated with significant morbidityCitation1. CRSwNP is estimated to affect 1–4% of the general population and 25–30% of patients with chronic rhinosinusitis (CRS)Citation1,Citation2. CRSwNP can cause long-term symptoms such as prominent nasal obstruction, post-nasal drip, loss of smell, and discharge, all of which negatively affect patients’ health-related quality of life (HRQOL)Citation1. Generally a condition of middle age with 42 years being an average age of onset, CRSwNP is typically diagnosed at 40–60 yearsCitation1.
The exact cause of nasal polyposis (NP) is unknown, but allergy, asthma, infection, and aspirin sensitivity have been associated with this complex refractory disease in adultsCitation1. Inflammatory mediators that may play roles in the development of NP include the cytokines IL-4, IL-5, and IL-13, and the chemokines CCL24 and CCL26Citation3,Citation4. Furthermore, microbial colonization contributes to the development of NP, including Alternaria species and Staphylococcus aureusCitation3. NP may also result from non-allergic disorders such as cystic fibrosisCitation1. CRSwNP is associated with more severe symptoms than for patients without NPCitation1.
Treatment options for patients with CRSwNP remain limited. According to US guidelines, both topical corticosteroids and nasal saline irrigations are recommended as initial medical therapiesCitation5. Intranasal corticosteroids (ICS) can decrease NP size, reduce sinonasal symptoms, and improve patient HRQOLCitation6,Citation7. Oral corticosteroids (OCS) can reduce polyp size and improve disease symptoms, but should be administered cautiously, given their association with serious systemic adverse effectsCitation8. Antibiotics may be useful in treating infectious exacerbations of CRSwNP, but information regarding clinically significant efficacy (i.e. NP shrinkage) from large, randomized trials is lackingCitation1. Patients with serious disease or who have failed medical management may be eligible for sinus surgery. Functional endoscopic sinus surgery (FESS) can improve sinonasal symptoms and inflammation, but NP may still recurCitation9, with rates as high as 50% (for patients observed over a period of 3 years)Citation10.
A systematic literature review (SLR) was conducted to understand the epidemiology, and the clinical, humanistic, and economic burdens of CRSwNP. The results of this SLR are published in two parts. This part covers the epidemiology and clinical burden of CRSwNP. A companion article in this journal presents the results of the SLR describing the humanistic and economic burdens of CRSwNP.
The authors’ overall objective was to determine the epidemiology and clinical burden of CRSwNP described in the literature by conducting an SLR. The objective of the epidemiology portion of the SLR was to summarize the literature on the following specific epidemiological aspects of CRSwNP: prevalence, incidence, mortality risk, comorbidities, symptoms, severity, surgery as a treatment option, most commonly used drugs for treatment, and risk factors. The objective of the clinical burden portion of the SLR was to review randomized controlled trials (RCTs) and real-world evidence (RWE) studies of therapies used for the treatment of patients with CRSwNP, to assess the efficacy of current treatment options, and to determine unmet needs for CRSwNP. In particular, the authors wanted to see how the addition of biologics has affected outcomes for patients with CRSwNP.
Methods
The SLR was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelinesCitation11. Databases searched via the OVID platform included Ovid MEDLINE, containing E-Pub Ahead of Print and In-Process & Other Non-Indexed Citations; Embase; and the following Evidence-Based Medicine Reviews (EBMR) databases, as applicable to each topic: the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, the Health Technology Assessment Database, the Cochrane Central Register of Controlled Trials, and the NHS Economic Evaluation Database. Details of the search strategies are provided in the Appendix (Supplementary Tables 1 and 2).
Available government websites, professional organization websites, and patient advocacy group websites were searched for the epidemiology SLR to validate the findings from published literature. The countries with demographic websites searched included the United States, Canada, France, Germany, Spain, the United Kingdom, and Japan. For the clinical burden SLR, results of the searches were validated and supplemented by searching the US National Library of Medicine’s ClinicalTrials.gov website and reviewing Cochrane systematic reviews. In addition, findings from published literature were validated against targeted literature searches and the bibliographies of select review articles.
Study selection
Epidemiology searches were performed 8 February 2019, while clinical burden searches were conducted 16 February 2019. Inclusion criteria were patients with CRSwNP ≥18 years old, any treatment for CRSwNP (clinical burden SLR only), outcomes relevant to epidemiology or clinical burden (as described in further detail below), and RCTs and non-RCTs/RWE studies (e.g. retrospective database analyses or chart reviews; case-control, cross-sectional survey, longitudinal, pilot, cohort, and single-arm studies). Exclusion criteria included pediatric studies (without adults), non-English articles, animal studies, commentaries, editorial reviews, expert-opinion articles, letters, articles published prior to 1 January 2008, and conference abstracts published prior to 1 January 2017. Detailed inclusion and exclusion criteria are shown in and .
Table 1. Detailed criteria for inclusion/exclusion of studies for epidemiology SLR.
Table 2. Detailed criteria for inclusion/exclusion of studies for clinical burden SLR.
Key epidemiology outcomes of interest included prevalence, incidence, mortality, comorbidities, risk factors, symptoms, and commonly used drugs for the treatment of CRSwNP. For clinical burden, studies reporting outcomes related to the current standard of care for CRSwNP, and the clinical outcomes of different therapies (e.g. non-biologics, surgery, and biologics) were included.
Review process
Study screening (title and abstract) was performed using the systematic review software DistillerSR (Evidence Partners, Ontario, Canada) and was conducted by two reviewers who assessed study eligibility based on the pre-defined PICOS criteria. Citations considered to be eligible at the title-and-abstract stage were then independently reviewed by two reviewers in full-text form to determine formal inclusion in the final review. Reasons for exclusion were documented at the full-text stage. Any disagreements during screening were resolved by a third independent reviewer.
Data extraction
Details for selected articles were collected using a standardized data extraction template in Microsoft Excel. For both portions of the SLR, data extractions were performed by a single reviewer and validated by a second reviewer.
Results
Epidemiology
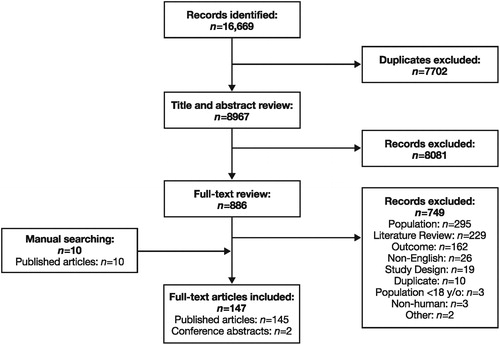
A total of 16,669 records were identified through the database searches. After de-duplication, 8967 records were screened at the title-and-abstract stage. After assessing all records based on title and abstract, 886 records were selected for full-text review. Full-text review and additional searches identified 147 studies that met the inclusion criteria for epidemiology studies. The SLR identified six studies reporting prevalence, one study reporting incidence, one study reporting mortality risk, 98 studies reporting comorbidities, 26 studies reporting symptoms, and 41 studies reporting risk factors. In addition, 16 studies reported surgery statistics, 23 studies reported previous treatment, 67 studies reported severity of CRSwNP, and 17 studies reported eosinophilia-related results. The results of each stage of the screening process are presented as a PRISMA diagram in . Note that data for some of the outcomes of interest (e.g. risk factors, severity, symptoms, and comorbidities) extracted from smaller studies with a total study population of fewer than 500 were not considered population-representative and are therefore not summarized herein.
Figure 1. PRISMA diagram: Epidemiology. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; y/o, years old.
Prevalence
The prevalence of CRSwNP in the general population was reported in six studies. In South Korea, the prevalence was 2.5–2.6% of the general populationCitation12–15, which was greater than that reported for the United States (1.1% of the general population)Citation16. CRSwNP was more prevalent for males (3.2–3.7%) than for females (2.0–3.3%), and the greatest prevalence was observed for the 60- to 69-year age groupCitation12,Citation17. The prevalence of NP was reported to be 6.1–31% of the population with CRS in the United States, and 24% of the population with CRS in DenmarkCitation18–21.
Uncontrolled (i.e. inadequately controlled) CRSwNP is defined as three or more of the following: nasal blockage present on most days of the week, rhinorrhea/postnasal drip on most days of the week, facial pain/pressure on most days of the week, impaired smell, sleep disturbance/fatigue, and nasal endoscopy with diseased mucosa; symptoms (as above) persist despite rescue treatment(s) in the last 6 monthsCitation2. The prevalence of uncontrolled CRSwNP among the general CRSwNP population is not reported in the literature. Only two studies from Belgium have reported 41.8% and 40% uncontrolled CRSwNP among the CRSwNP population who previously had surgery for NPCitation22,Citation23.
The prevalence of eosinophilic CRSwNP (eCRSwNP) varied by regionCitation24,Citation25. In Europe, the prevalence estimates for eCRSwNP in the CRSwNP population ranged from 84‒91%, with the lowest prevalences noted for Belgium, The Netherlands, and Luxembourg. The highest prevalence was reported for patients in Berlin, GermanyCitation25. In Asia, the estimated prevalence for eCRSwNP in the CRSwNP population ranged from 65% for Chengdu, China, to 89% for Tochigi, JapanCitation25. The prevalence of eCRSwNP in the CRSwNP population was estimated to be 91% for Adelaide, AustraliaCitation25.
Incidence
Our search of multiple medical-literature databases identified one report of the incidence of CRSwNP in community practice clinics and hospitals in a 31-county region of central and northeastern PennsylvaniaCitation26. Between 2007 and 2009, the reported average incidence of CRSwNP was 83 cases per 100,000 person-years in the region.
Mortality risk
Our search identified one report of mortality risk among 27,005 patients diagnosed with CRS in the Utah Population Database. Mortality risk for this population was greater for patients with CRSwNP compared with patients who had CRS without NP (CRSsNP) (hazard ratio [HR] = 1.38; 95% confidence interval [CI] 1.09–1.77)Citation18.
Comorbidities
Most studies reported comorbidities of CRSwNP from a US population. Asthma (percentage of patients, range 5–56%), allergy (12–77%), and allergic rhinitis (17–76%) were the most commonly reported comorbidities for patients with CRSwNP. Details of studies reporting prevalence of these comorbidities are summarized in .
Table 3. Most commonly reported comorbidities for patients with CRSwNPa.
Symptoms
Facial pain, nasal congestion, loss of smell, sneezing, and headache were the most commonly reported symptoms for patients with CRSwNP. Facial pain was reported for 60–92.2% of patients in North AmericaCitation16,Citation27, 31–45% of patients in EuropeCitation28,Citation29, and 19–100% of patients in AsiaCitation30–33. Nasal congestion was reported in 95–100% of patients with CRSwNP in North AmericaCitation17,Citation27, 73–88% of patients in EuropeCitation34,Citation35, and 76–81% of patients in AsiaCitation31,Citation36. Loss of smell was reported for 56–84% of patients with CRSwNP in North AmericaCitation16,Citation27, 35–90% of patients in EuropeCitation28,Citation34,Citation35,Citation37,Citation38, and 30–100% of patients in AsiaCitation30–32,Citation36,Citation39. Sneezing was reported for 88% of patients in North AmericaCitation26, 12–73% of patients in EuropeCitation28,Citation34,Citation35,Citation38, and 27–51% of patients in AsiaCitation31,Citation33. Headache was reported for 33–95.8% of patients in North AmericaCitation27,Citation40, 20–41% of patients in EuropeCitation33,Citation34, and 2–76% of patients in AsiaCitation33,Citation36.
Risk factors
Asthma, gene polymorphisms, and aging were the most commonly reported risk factors for developing CRSwNP. Other risk factors included male sex, smoking, allergy, presence of polyps, CRS-related symptoms, tobacco use, chronic bronchitis, aspirin intolerance, occupation (e.g. firefighter), high serum concentrations of cytokines IL-5 or IL-13, lower education levels, obesity, lower S100A8/9 (calprotectin) protein concentrations, first- or second-degree relatives with CRSwNP, greater Lund-Mackay score (LMS), and high tissue and blood eosinophil counts.
Asthma as a risk factor was reported in five studiesCitation12,Citation15,Citation41–43, with reported odds ratios (ORs) of 5.9 (95% CI 1.79–19.65)Citation42, 2.20 (95% CI 1.41–3.45)Citation12, and 5.85 (no 95% CI given)Citation41. Won et al.Citation15 reported relative risk of 3.18 (95% CI 1.8–5.6). Pearlman et al.Citation43 reported that the association was significant (p=.0019).
The extent to which gene polymorphisms are risk factors for developing CRSwNP varied by gene. Bernstein et al.Citation44 reported that an allele in a single nucleotide polymorphism located in the tumor necrosis factor-α gene was associated with developing CRSwNP (OR 1.86; 95% CI 1.14–3.09). Sitarek et al.Citation45 reported that the C/G genotype of the proto-oncogene c-met (hepatocyte growth factor receptor; OR 2.83; 95% CI 1.74–4.61) and the −765 G/C genotype of the cycloxygenase-2 gene (OR 7.79; 95% CI 4.88–12.4) were associated with developing CRSwNP. Zielinska-Blizniewska et al.Citation46 found that the A/G genotype of the lactoferrin gene (OR 4.78; 95% CI 3.07–7.24) and the C/G and G/G genotypes (OR 3.48; 95% CI 2.19–5.52; OR 16.45; 95% CI 6.71–40.30, respectively) of the osteoblast-specific factor-2 gene increased the likelihood of developing CRSwNP.
Age as a risk factor was reported in two studies. The first study reported cross-sectional data from more than 35,000 patients in the Korean National Health and Nutrition Examination Survey from 2008 through 2012Citation12. The second study was a case-control study of 367 patients in SwedenCitation38. Both studies reported an OR of 1.03 (95% CI 1.02–1.04).
Eosinophilia may be a risk factor for developing CRSwNP. Putman et al.Citation47 reported that an eosinophil count ≥240 cells/µL was a greater risk factor for CRSwNP than for CRSsNP (HR 1.39; 95% CI 1.22–1.58 versus 1.13; 95% CI 1.10–1.27). Chen et al.Citation48 found that peripheral eosinophils were a risk factor for NP (OR 2.02; 95% CI 1.08–3.72). Eosinophilia was associated with CRSwNP recurrence (RR 3.68; 95% CI 0.19–11.38)Citation49 and with recurrence of NP (RR 6.18; 95% CI 1.29–29.42)Citation50. Furthermore, eosinophilia was also associated with worse respiratory functionCitation37,Citation51–57, disease severityCitation32,Citation51,Citation58–61, and greater CRSwNP recurrence ratesCitation49,Citation62,Citation63. Guo et al.Citation62 reported that blood eosinophil counts of 0.25 × 103/µL (OR 3.63; 95% CI 0.73–18.10) or 2.6% blood eosinophils (OR 5.12; 95% CI 0.62–42.58) predicted multiple recurrences of CRSwNP after FESS.
Severity of CRSwNP
Severity as assessed by LMS was found to vary by geographic region and to depend on factors such as eosinophilia and comorbidities. In North America, LMS ranged from 12.0Citation64 to 26.0Citation65. In Europe, LMS ranged from 6.2Citation59 to 17.6Citation37 for patients with low mucosal eCRSwNP and CRSwNP patients with asthma, respectively. In Asia, the lowest LMS was 11.6 and the greatest LMS was 22, reported for a CRSwNP population without atopy and a CRSwNP population characterized by marked tissue eosinophiliaCitation66,Citation67. One Australian study reported a mean LMS of 16.79 for patients who underwent ESSCitation68.
Surgery for CRSwNP
Surgery is a common treatment option for patients with CRSwNP. The percentage of patients who had undergone surgery as reported in five studies varied by geographic region: United States, 43–52%; UK, 55%; Europe, 46%; and Belgium, 84%Citation16,Citation69–72. In five studies, 21–59% of patients with CRSwNP were reported to have undergone revision surgeryCitation20,Citation70,Citation73–75. Two studies reported that 6% and 6.5% of patients with CRSwNP had undergone two revision surgeries over study durations of 20 and 5 years, respectivelyCitation20,Citation70. Patients with CRSwNP were reported to undergo a mean number of 1.4–2.98 surgeries, with women found to have 1.2-times more surgeries than menCitation71,Citation73,Citation76.
Most commonly used drugs for CRSwNP
Corticosteroids (percentage of patients, intranasal: range 90–93%; oral: range 23–71%), antibiotics (29–55%), and antihistamines (34%) were the most commonly used drugs for CRSwNP, as reported in six studies. These studies are summarized in . A summary of evidence for the efficacy of these drugs, as well as for biologics, is included in the clinical burden results section.
Table 4. Drugs most commonly used for CRSwNPa.
Clinical burden
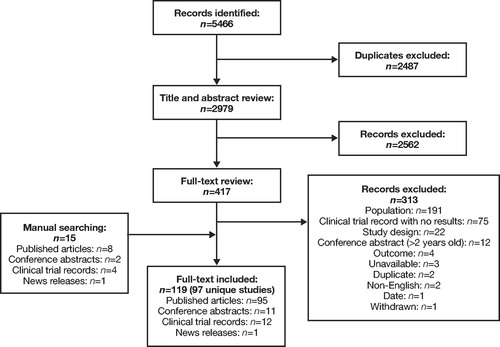
A total of 5466 records were identified for RCTs. After removal of duplicates, the titles and abstracts of 2979 records were examined. Of these, 417 were selected for full-text review. Full-text review and additional searches yielded 119 records, representing 97 unique RCTs. Treatments investigated in the RCTs included biologics, corticosteroids, antibiotics, antifungals, aspirin desensitization, surgical treatments, and alternative therapies. The PRISMA flow chart of record selection for RCTs is presented in .
Figure 2. PRISMA diagram for RCTs: Clinical Burden. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs, randomized clinical trials.
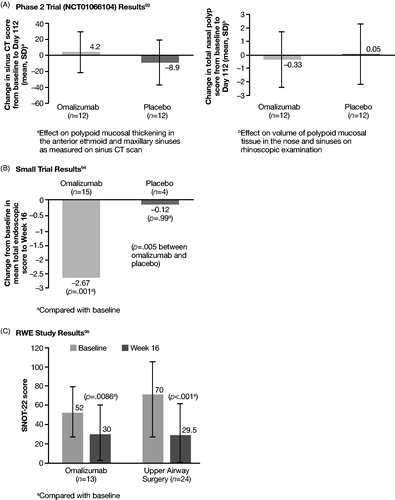
A total of 4117 records were identified for RWE studies. After removing duplicates, 2809 records were evaluated at the title-and-abstract stage. Of these, 404 were selected for full-text review. In all, 276 records representing 263 unique RWE studies that fulfilled the inclusion criteria were identified. Treatments investigated in the RWE studies included biologics, corticosteroids, aspirin desensitization, and ESS, among others. The PRISMA flow chart of record selection for RWE studies is presented in .
Results for non-biologics
Non-biologics for the treatment of CRSwNP included intranasal and oral corticosteroids; surgery, such as ESS; nasal irrigation; antibiotics, such as clarithromycin and doxycycline; antifungals; aspirin desensitization; and alternative therapies. RCTs demonstrated that non-biologics improved the following commonly reported clinical outcomes: Sino-Nasal Outcomes Test (SNOT)-22 scores (Supplementary Table 4), Lund-Mackay CT scores (Supplementary Table 6), nasal obstruction scores (Supplementary Table 8), total symptom scores by visual analog scale (VAS) (Supplementary Table 10), and total endoscopic NP scores (Supplementary Table 12). In addition to improvements in the above outcomes, RWE studies reported that non-biologics improved SNOT-22 scores, Lund-Kennedy CT and endoscopic scores, total symptom scores by VAS, and nasal polyp scores (Supplementary Tables 5, 7, 9, 11, 13, and 14). Four RCTsCitation77–80 and 4 RWE studiesCitation81–84 reported on non-biologics (corticosteroids and ESS) for patients with eCRSwNP. All eight studies demonstrated improvement in clinical outcomes. Despite clinical efficacy provided by non-biologics, these treatments do not remove polyps completely or eliminate disease symptoms. The burden of CRSwNP symptoms for patients, as measured by SNOT-22 and NP scores after treatment (Supplementary Tables 4, 8, 10, and 12), remains high. Moreover, although OCS are effective in improving symptoms and size of polyps in the short term and the adverse events associated with their use have been described in other disease, their long-term adverse effects have not been studied in this patient population.
Results for biologics
Seven RCTs with published results for three biologics were found: dupilumab (three studies), omalizumab (two studies), and mepolizumab (two studies). These studies are summarized in .
Table 5. RCTs and RWE studies investigating biologicsa.
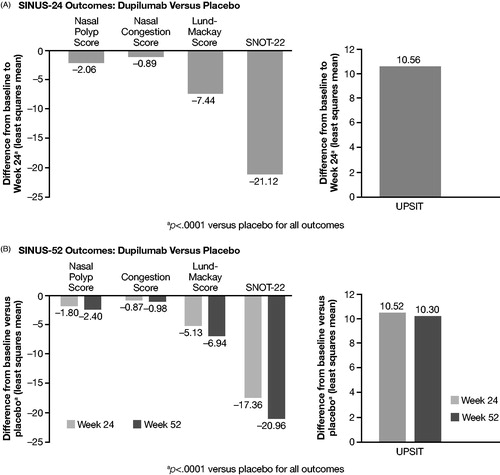
Three RCT studies for dupilumab, the Phase III SINUS-24Citation85 and SINUS-52Citation86 studies, and a Phase II studyCitation87 were retrieved. No RWE studies were found for dupilumab. In the SINUS-24 study, dupilumab significantly improved endoscopic nasal congestion, and NP, Lund-Mackay, University of Pennsylvania Smell Identification Test (UPSIT), and SNOT-22 scores relative to placebo for patients with severe CRSwNP ()Citation85. At 24 weeks, dupilumab reduced rescue treatment (systemic corticosteroids) or NP surgery by 73% versus placebo (p < .001)Citation88,Citation89. Also at 24 weeks, dupilumab significantly improved Asthma Control Questionnaire (ACQ)-6 scores (p < .0001) and forced expiratory volume in 1 second (FEV1) (p < .001) versus placebo for patients with severe CRSwNP plus comorbid asthmaCitation85. In the SINUS-52 trial, dupilumab significantly improved endoscopic nasal congestion and NP, Lund-Mackay, UPSIT, and SNOT-22 scores relative to placebo for patients with severe CRSwNP ()Citation86. At 52 weeks, dupilumab reduced rescue treatment (systemic corticosteroids) or NP surgery by 76% versus placebo (p < .0001)Citation88. Also at 52 weeks, dupilumab significantly improved both ACQ-6 scores (p < .0001) and FEV1 (p < .0001) versus placebo for patients with severe CRSwNP plus comorbid asthmaCitation86. The Phase II trial (NCT01920893) demonstrated that dupilumab significantly improved endoscopic nasal congestion and NP, Lund-Mackay, UPSIT, and SNOT-22 scores compared with placebo for patients with CRSwNP refractory to intranasal corticosteroidsCitation87.
Figure 4. Clinical outcomes scores for dupilumab versus placebo from SINUS-24 and SINUS-52 Phase III RCTs.
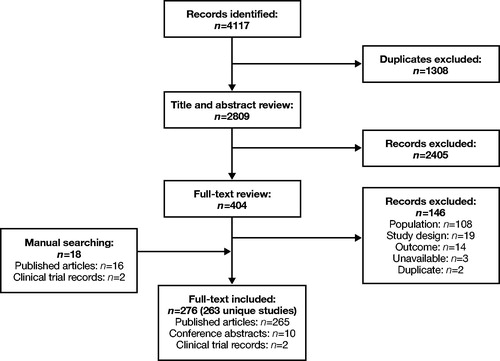
Searches for mepolizumab retrieved one Phase II study (NCT01362244)Citation90 and one study not specifying phaseCitation91. Bachert et al.Citation90 found that the NP severity VAS scores and mean individual symptom VAS scores (mucus in throat, loss of smell, rhinorrhea and nasal blockage) were significantly improved in the mepolizumab group compared with the placebo group at Week 25 (). In the second study, treatment difference reflected by the total polyp score demonstrated that mepolizumab resulted in improvement compared with placebo ()Citation91. In one RWE study of mepolizumab for patients with aspirin-exacerbated respiratory disease (AERD)Citation92, after three or more doses of mepolizumab, the SNOT-22 score decreased significantly by 17.7 pointsCitation92.
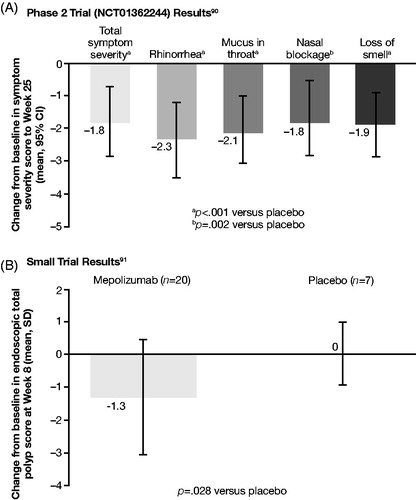
For omalizumab, one Phase II studyCitation93 and one study of unspecified phase were retrievedCitation94. In the Phase II trial (NCT01066104), omalizumab had no significant effect on polypoid mucosal thickening in the anterior ethmoid and maxillary sinuses, or on volume of polypoid mucosal tissue in the noses and sinuses of patients with CRSwNP ()Citation93. In a smaller clinical study of 24 patients (no phase specified), omalizumab significantly improved total endoscopic NP score compared with baseline and placebo for patients with CRSwNP and comorbid asthma ()Citation94. In one RWE study, omalizumab treatment significantly improved SNOT-22 scores after 4 and 16 weeks relative to baseline ()Citation95. ACQ-7 scores also improved significantly at Weeks 4 (p < .005) and 16 (p < .03)Citation95.
Discussion
A major strength of this SLR was that it adhered to best practices for the conduct and reporting of systematic reviews. Notably, the literature searches were performed and peer-reviewed by experienced information specialists. A limitation of this SLR was that it included studies restricted to the English language at the study-selection stage. This is likely a minor limitation, given that the majority of studies are published in English-language journals. This SLR included only RCTs and RWE studies with results available at the time of the literature searches. Results for ongoing Phase III trials for biologics may have become available after the completion of this review. As well, novel biologics, which will affect disease management for patients with CRSwNP, may be in future trials.
The epidemiology SLR found that CRSwNP affects approximately 1–3 of 100 people and is more common in men and older individualsCitation96. The prevalence varies across geographic regions. In particular, the prevalence of eCRSwNP within the CRSwNP population was reported to range from 65% in Asia to 91% in EuropeCitation24,Citation25. CRSwNP has a greater mortality risk compared with CRSsNP. Asthma, allergy, and allergic rhinitis were the most commonly reported comorbidities identified for CRSwNP. Common symptoms of CRSwNP were nasal congestion, loss of smell, sneezing, headache, and facial pain. Asthma, gene polymorphisms, and aging can increase the likelihood of developing CRSwNP.
Although Fokkens et al.Citation2 in the European Position Paper (EPOS) published in 2020 describe an association between asthma and CRS, eosinophilia and asthma are identified in the literature covered in this review as risk factors for CRSwNP and disease recurrence. Eosinophilia is also associated with worse respiratory function, and greater disease severity and recurrence rates. The most common treatments for CRSwNP were corticosteroids, followed by antibiotics and antihistamines.
Prevalence data were reported more often for CRS than for CRSwNP. Prevalence data for patients with CRSwNP were available only for South Korea and the United States in articles published from 2008 onward. The lack of prevalence data from Europe, South America, and other regions is a notable gap in the literature. In addition, there were few reports on incidence or mortality, and no projected prevalence data. Finally, few studies focusing on severe CRSwNP and eCRSwNP were retrieved.
Symptoms remain highly prevalent among CRSwNP patients, indicating a large percentage of patients with disease not well-controlled with current treatment options. A short course of OCS might be used for patients with CRSwNP who failed initial therapy. Despite the fact that long-term OCS adverse effects were not directly assessed in CRSwNP patients, the impact for patients with other conditions, such as asthma, is well-documentedCitation97. Therefore, the long-term deleterious effects of steroid use should be weighed against any gains in symptom relief.
Patients with disease refractory to medical intervention often have surgery. However, recurrence rates of symptoms and polyps are high. The GA2LEN study found that 59% of patients who had undergone surgery also later received revision surgery, and nearly 23% of patients experienced four or more revision surgeriesCitation98. Another study reported that within 18 months of surgery, 40% of patients reported recurrence of polyps and within 3–5 years of surgery, 80% of patients reported inadequately-controlled symptomsCitation99.
Three biologics had published literature attesting to efficacy, but the efficacy metrics varied. Of them, omalizumab had efficacy only in the more limited setting of patients with AERD. This implies that head-to-head trials are needed to determine potential superiority for one biologic over another.
Key data gaps identified by the clinical burden portion of the SLR included lack of head-to-head Phase III trials for biologics in CRSwNP. Moreover, there was a lack of evidence on the effects of prolonged treatment with biologics, and on the duration of efficacy after treatment with biologics. The efficacy and safety of biologics for patients with CRSwNP in the real-world setting requires further study. There was also a lack of RCTs and RWE studies of patients with eCRSwNP. Finally, there was variability in how outcomes were measured, both between RCTs and between RCTs and RWE studies. Such variation in data reporting made robust comparisons not feasible.
There was heterogeneity across the studies included in the review in terms of factors such as patient inclusion criteria, patient characteristics, and study setting (e.g. recruitment at secondary or tertiary clinics), which may have contributed to the wide ranges observed in the results. Future studies should clarify how these factors may have influenced the results.
Conclusions
CRSwNP is fairly prevalent in the general population. Despite the significant efficacy of existing treatments, several unmet needs remain. The high burden of uncontrolled symptoms, frequent recurrence of nasal polyps after surgery, and long-term adverse effects of OCS indicate that new therapies addressing these unmet needs should be developed. Although data on biologics from RCTs look promising, the efficacy of biologics for CRSwNP in the real world has yet to be established.
The SLR of the epidemiology and clinical burden of CRSwNP revealed key gaps in the literature. There was a paucity of prevalence data across many geographic areas, and no prevalence projections could be determined. Studies showed varying efficacy of non-biologics and no studies directly compared biologics for efficacy. Data regarding clinical efficacy of agents for eCRSwNP or severe CRSwNP were lacking, and these patient populations would be served by more trials.
Transparency
Declaration of financial/other relationships
SC and BE are employees of AstraZeneca. AZ, KT, and HG are employees of EVERSANA. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work. One of these peer reviewers discloses receiving a NIHR grant for chronic rhinosinusitis. The peer reviewers have no other relevant financial relationships to disclose.
19_SLR_CRSwNP_Supplemental_Material_07072020_Final.docx
Download MS Word (342.1 KB)Acknowledgements
Medical writing and editorial support were provided by Beverly E. Barton, PhD, of Kay Square Scientific, Newtown Square, PA, USA, and Michael A. Nissen, ELS, of AstraZeneca, Gaithersburg, MD, USA.
Additional information
Funding
References
- Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4(4):565–572.
- Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on chronic rhinosinusitis and nasal polyps 2020. Rhin. 2020;58(1):1–464.
- Hulse KE, Stevens WW, Tan BK, et al. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45(2):328–346.
- De Greve G, Hellings PW, Fokkens WJ, et al. Endotype-driven treatment in chronic upper airway diseases. Clin Transl Allergy. 2017;7:22.
- Peters AT, Spector S, Hsu J, Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014;113(4):347–385.
- Lund VJ, Flood J, Sykes AP, et al. Effect of fluticasone in severe polyposis. Arch Otolaryngol Head Neck Surg. 1998;124(5):513–518.
- Rudmik L, Smith TL. Olfactory improvement after endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):29–32.
- Poetker DM, Jakubowski LA, Lal D, et al. Oral corticosteroids in the management of adult chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013;3(2):104–120.
- Young J, Frenkiel S, Tewfik MA, et al. Long-term outcome analysis of endoscopic sinus surgery for chronic sinusitis. Am J Rhinol. 2007;21(6):743–747.
- Kim J, Naclerio R. Therapeutic potential of dupilumab in the treatment of chronic rhinosinusitis with nasal polyps: evidence to date. Ther Clin Risk Manag. 2020;16:31–37.
- Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLOS Med. 2009;6(7):e1000097.
- Ahn JC, Kim JW, Lee CH, et al. Prevalence and risk factors of chronic rhinosinusitus, allergic rhinitis, and nasal septal deviation: results of the Korean National Health and Nutrition Survey 2008–2012. JAMA Otolaryngol Head Neck Surg. 2016;142(2):162–167.
- Cho YS, Choi SH, Park KH, et al. Prevalence of otolaryngologic diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008. Clin Exp Otorhinolaryngol. 2010;3(4):183–193.
- Hong SN, Lee WH, Lee SH, et al. Chronic rhinosinusitis with nasal polyps is associated with chronic otitis media in the elderly. Eur Arch Otorhinolaryngol. 2017;274(3):1463–1470.
- Won HK, Kim YC, Kang MG, et al. Age-related prevalence of chronic rhinosinusitis and nasal polyps and their relationships with asthma onset. Ann Allergy Asthma Immunol. 2018;120(4):389–394.
- Palmer JN, Messina JC, Biletch R, et al. A cross-sectional, population-based survey of U.S. adults with symptoms of chronic rhinosinusitis. Allergy Asthma Proc. 2019;40(1):48–56.
- Kim DH, Han K, Kim SW. Effect of chronic rhinosinusitis with or without nasal polyp on quality of life in South Korea: 5th Korea National Health and Nutrition Examination Survey Korean. Clin Exp Otorhinolaryngol. 2016;9(2):150–156.
- Alt JA, Thomas AJ, Curtin K, et al. Mortality risk in patients with chronic rhinosinusitis and its association to asthma. Int Forum Allergy Rhinol. 2017;7(6):591–599.
- Mahdavinia M, Benhammuda M, Codispoti CD, et al. African American patients with chronic rhinosinusitis have a distinct phenotype of polyposis associated with increased asthma hospitalization. J Allergy Clin Immunol Pract. 2016;4(4):658–664.
- Smith KA, Orlandi RR, Oakley G, et al. Long-term revision rates for endoscopic sinus surgery. Int Forum Allergy Rhinol. 2019;9(4):402–408.
- Lange B, Holst R, Thilsing T, et al. Quality of life and associated factors in persons with chronic rhinosinusitis in the general population: a prospective questionnaire and clinical cross‐sectional study. Clin Otolaryngol. 2013;38(6):474–480.
- Calus L, Van Bruaene N, Bosteels C, et al. Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin Transl Allergy. 2019;9(1):30.
- van der Veen J, Seys SF, Timmermans M, et al. Real-life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy. 2017;72(2):282–290.
- Wang W, Gao Y, Zhu Z, et al. Changes in the clinical and histological characteristics of Chinese chronic rhinosinusitis with nasal polyps over 11 years. Int Forum Allergy Rhinol. 2019;9(2):149–157.
- Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138(5):1344–1353.
- Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131(5):1350–1360.
- Racette SD, Wijewickrama RC, Jayaprakash V, et al. Correlation of symptoms, clinical signs, and biomarkers of inflammation in postsurgical chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2017;126(6):455–462.
- Abdalla S, Alreefy H, Hopkins C. Prevalence of sinonasal outcome test (SNOT-22) symptoms in patients undergoing surgery for chronic rhinosinusitis in the England and Wales National prospective audit. Clin Otolaryngol. 2012;37(4):276–282.
- Costa C, Garzaro M, Boggio V, et al. Detection of herpesviruses 1-6 and community-acquired respiratory viruses in patients with chronic rhinosinusitis with nasal polyposis. Intervirology. 2014;57(2):101–105.
- Khairuddin NK, Salina H, Gendeh BS, et al. Quality of life and recurrence of disease in patients with eosinophilic and non-eosinophilic 1 chronic rhinosinusitis with nasal polyposis. Med J Malaysia. 2018;73(1):1–6.
- Amal Al Abdulla M, Darwish A, Bella MH. Chronic eosinophilic rhinosinusitis clinical implications. BMB. 2017;39(2):92–95.
- Sreeparvathi A, Kalyanikuttyamma LK, Kumar M, et al. Significance of blood eosinophil count in patients with chronic rhinosinusitis with nasal polyposis. J Clin Diagn Res. 2017;11(2):MC08–MC11.
- Han DH, Kim SW, Cho SH, et al. Predictors of bronchial hyperresponsiveness in chronic rhinosinusitis with nasal polyp. Allergy. 2009;64(1):118–122.
- Daval M, Picard H, Bequignon E, et al. Chronic otitis media with effusion in chronic sinusitis with polyps. Ear Nose Throat J. 2018;97(8):E13–E18.
- Davila I, Rondon C, Navarro A, et al. Aeroallergen sensitization influences quality of life and comorbidities in patients with nasal polyposis. Am J Rhinol Allergy. 2012;26(5):e126–e31.
- Jain S, Das S, Gupta N, et al. Frequency of fungal isolation and antifungal susceptibility pattern of the fungal isolates from nasal polyps of chronic rhinosinusitis patients at a tertiary care centre in north India. Med Mycol. 2013;51(2):164–169.
- Staikūnienė J, Vaitkus S, Japertienė L, et al. Association of chronic rhinosinusitis with nasal polyps and asthma: clinical and radiological features, allergy and inflammation markers. Medicina. 2008;44(4):257–265.
- Bohman A, Oscarsson M, Holmberg K, et al. Relative frequencies of symptoms and risk factors among patients with chronic rhinosinusitis with nasal polyps using a case-control study. Acta Otolaryngol. 2018;138(1):46–49.
- Yoshimura K, Kawata R, Haruna S, et al. Clinical epidemiological study of 553 patients with chronic rhinosinusitis in Japan. Allergol Intern. 2011;60(4):491–496.
- Wu D, Gray ST, Holbrook EH, et al. SNOT-22 score patterns strongly negatively predict chronic rhinosinusitis in patients with headache. Int Forum Allergy Rhinol. 2019;9(1):9–15.
- Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013;123(Suppl 7):S1–S11.
- Hakansson K, Thomsen SF, Konge L, et al. A comparative and descriptive study of asthma in chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28(5):383–387.
- Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23(2):145–148.
- Bernstein JM, Anon JB, Rontal M, et al. Genetic polymorphisms in chronic hyperplastic sinusitis with nasal polyposis. Laryngoscope. 2009;119(7):1258–1264.
- Sitarek P, Zielinska-Blizniewska H, Dziki L, et al. Association of the -14C/G MET and the -765G/C COX-2 gene polymorphisms with the risk of chronic rhinosinusitis with nasal polyps in a Polish population. DNA Cell Biol. 2012;31(7):1258–1266.
- Zielinska-Blizniewska H, Sitarek P, Milonski J, et al. Association of the -33C/G OSF-2 and the 140A/G LF gene polymorphisms with the risk of chronic rhinosinusitis with nasal polyps in a Polish population. Mol Biol Rep. 2012;39(5):5449–5457.
- Putman B, Zeig-Owens R, Singh A, et al. Risk factors for post-9/11 chronic rhinosinusitis in Fire Department of the City of New York workers. Occup Environ Med. 2018;75(12):884–889.
- Chen F, Wen L, Qiao L, et al. Impact of allergy and eosinophils on the morbidity of chronic rhinosinusitis with nasal polyps in Northwest China. Int Arch Allergy Immunol. 2019;179(3):209–214.
- Brescia G, Marioni G, Franchella S, et al. Can a panel of clinical, laboratory, and pathological variables pinpoint patients with sinonasal polyposis at higher risk of recurrence after surgery? Am J Otolaryngol. 2015;36(4):554–558.
- Ottaviano G, Cappellesso R, Mylonakis I, et al. Endoglin (CD105) expression in sinonasal polyposis. Eur Arch Otorhinolaryngol. 2015;272(11):3367–3373.
- Lou H, Meng Y, Piao Y, et al. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54(2):150–159.
- Guida G, Rolla G, Badiu I, et al. Determinants of exhaled nitric oxide in chronic rhinosinusitis. Chest. 2010;137(3):658–664.
- Mori E, Matsuwaki Y, Mitsuyama C, et al. Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auris Nasus Larynx. 2013;40(5):465–469.
- Tanaka S, Hirota T, Kamijo A, et al. Lung functions of Japanese patients with chronic rhinosinusitis who underwent endoscopic sinus surgery. Allergol Intern. 2014;63(1):27–35.
- Kambara R, Minami T, Akazawa H, et al. Lower airway inflammation in eosinophilic chronic rhinosinusitis as determined by exhaled nitric oxide. Int Arch Allergy Immunol. 2017;173(4):225–232.
- Uraguchi K, Kariya S, Makihara S, et al. Pulmonary function in patients with eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2018;45(3):476–481.
- Morse JC, Shilts MH, Ely KA, et al. Patterns of olfactory dysfunction in chronic rhinosinusitis identified by hierarchical cluster analysis and machine learning algorithms. Int Forum Allergy Rhinol. 2019;9(3):255–264.
- Ikeda K, Shiozawa A, Ono N, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013;123(11):E1–E9.
- Aslan F, Altun E, Paksoy S, et al. Could Eosinophilia predict clinical severity in nasal polyps? Multidiscip Respir Med. 2017;12(1):21.
- Czerny MS, Namin A, Gratton MA, et al. Histopathological and clinical analysis of chronic rhinosinusitis by subtype. Int Forum Allergy Rhinol. 2014;4(6):463–469.
- Soler ZM, Sauer DA, Mace J, et al. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(4):454–461.
- Guo M, Alasousi F, Okpaleke C, et al. Prognosis of chronic rhinosinusitis with nasal polyps using preoperative eosinophil/basophil levels and treatment compliance. Am J Rhinol Allergy. 2018;32(5):440–446.
- Brescia G, Barion U, Pedruzzi B, et al. Sinonasal polyposis in the elderly. Am J Rhinol Allergy. 2016;30(5):153–156.
- Weibman AR, Huang JH, Stevens WW, et al. A prospective analysis evaluating tissue biopsy location and its clinical relevance in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2017;7(11):1058–1064.
- Mady LJ, Schwarzbach HL, Moore JA, et al. The association of air pollutants and allergic and nonallergic rhinitis in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(3):369–376.
- Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126(5):962–968.
- Liu C, Zheng M, He F, et al. Role of exhaled nasal nitric oxide in distinguishing between chronic rhinosinusitis with and without nasal polyps. Am J Rhinol Allergy. 2017;31(6):389–394.
- Bassiouni A, Wormald PJ. Role of frontal sinus surgery in nasal polyp recurrence. Laryngoscope. 2013;123(1):36–41.
- Bhattacharyya N, Villeneuve S, Joish VN, et al. Cost burden and resource utilization in patients with chronic rhinosinusitis and nasal polyps. Laryngoscope. 2019;129(9):1969–1975.
- Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN) Rhinosinusitis Cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57(1):32–42.
- Philpott C, Hopkins C, Erskine S, et al. The burden of revision sinonasal surgery in the UK-data from the Chronic Rhinosinusitis Epidemiology Study (CRES): a cross-sectional study. BMJ Open. 2015;5(4):e006680–e006680.
- De Schryver E, Derycke L, Campo P, et al. Alcohol hyper-responsiveness in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2017;47(2):245–253.
- Stevens WW, Peters AT, Suh L, et al. A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immun Inflamm Dis. 2015;3(1):14–22.
- Hunter TD, DeConde AS, Manes RP. Disease-related cost burden in patients undergoing sinus surgery for chronic rhinosinusitis: a claims-based analysis. Value Health. 2017;20(9):A644.
- Hopkins C, Slack R, Lund V, et al. Long-term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009;119(12):2459–2465.
- Benjamin MR, Stevens WW, Li N, et al. Clinical characteristics of patients with chronic rhinosinusitis without nasal polyps in an academic setting. J Allergy Clin Immunol Pract. 2019;7(3):1010–1016.
- Shen KH, Wang YH, Hsu TW, et al. Differential effects of postoperative oral corticosteroid on eosinophilic vs. non-eosinophilic CRSwNP subtypes. Am J Otolaryngol. 2019;40(1):22–29.
- Kobayashi Y, Yasuba H, Asako M, et al. HFA-BDP metered-dose inhaler exhaled through the nose improves eosinophilic chronic rhinosinusitis with bronchial asthma: A blinded, placebo-controlled study. Front Immunol. 2018;9:2192.
- Wang C, Lou H, Wang X, et al. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2015;135(4):922–929.
- Zeng M, Wang H, Liao B, et al. Comparison of efficacy of fluticasone propionate versus clarithromycin for postoperative treatment of different phenotypic chronic rhinosinusitis: a randomized controlled trial. Rhinology. 2019;57(2):101–109.
- Ikeda K, Ito S, Hibiya R, et al. Postoperative management of eosinophilic chronic rhinosinusitis with nasal polyps: Impact of high-dose corticosteroid nasal spray. Int Arch Otorhinolaryngol. 2019;23(1):101–103.
- Liu S, Che N, Fan K, et al. Impact of genetic variants of GLCCI1 on operational therapy in Chinese chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2018;8(11):1356–1362.
- Yelverton JC, Holmes TW, Johnson CM, et al. Effectiveness of leukotriene receptor antagonism in the postoperative management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(3):243–247.
- Jang DW, Comer BT, Lachanas VA, et al. Aspirin sensitivity does not compromise quality-of-life outcomes in patients with Samter's triad. Laryngoscope. 2014;124(1):34–37.
- Han JK, Bachert C, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with chronic rhinosinusitis with nasal polyps: results from the randomized Phase 3 SINUS-24 study. J Allergy Clin Immunol. 2019;143(2):AB422.
- Bachert C, DesRosiers M, Mullol J, et al. A randomized phase 3 study, SINUS-52, evaluating the efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2019;143(2):AB433.
- Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469–479.
- Regeneron. Positive results presented from two phase 3 trials of Dupixent (dupilumab) in severe chronic rhinosinusitis with nasal polyps (CRSwNP) [updated 2019 Feb 25; cited 2020 May 18]. Available from: https://investor.regeneron.com/news-releases/news-release-details/positive-results-presented-two-phase-3-trials-dupixentr.
- U.S. National Library of Medicine. A controlled clinical study of dupilumab in patients with bilateral nasal polyps (SINUS-24). ClinicalTrials.gov. identifier: NCT02912468 [updated 2019 Jul 25; cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT02912468.
- Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol. 2017;140(4):1024–1031.
- Gevaert P, Bruaene NV, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128(5):989–995.
- Tuttle KL, Buchheit KM, Schneider T, et al. A pragmatic analysis of mepolizumab in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2018;141(2):AB168.
- U.S. Library of National Medicine. Subcutaneous omalizumab for treatment of chronic rhinosinusitis with nasal polyposis. ClinicalTrials.gov. identifier: NCT01066104. [updated 2017 June 14; cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT01066104.
- Gevaert P, Calus L, Zele TV, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(1):110–116.
- Bidder T, Sahota J, Rennie C, et al. Omalizumab treats chronic rhinosinusitis with nasal polyps and asthma together-a real life study. Rhinology. 2018;56(1):42–45.
- Busaba NY. The impact of a patient’s age on the clinical presentation of inflammatory paranasal sinus disease. Am J Otolaryngol. 2013;34(5):449–453.
- Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. JAA. 2018;11:193–204.
- Vandeplas G, Khan A, Huynh TMT, et al. The ‘GA2LEN Sinusitis Cohort’: an introduction. Clin Transl Allergy. 2015;5(S4):O1.
- DeConde AS, Mace JC, Levy JM, et al. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550–555.