Abstract
Objective
To explore current recommendations for intravenous (IV) iron use in clinical guidelines for iron deficiency anemia (IDA) across different therapeutic areas and identify recommendations, if any, for the treatment of IDA.
Methods
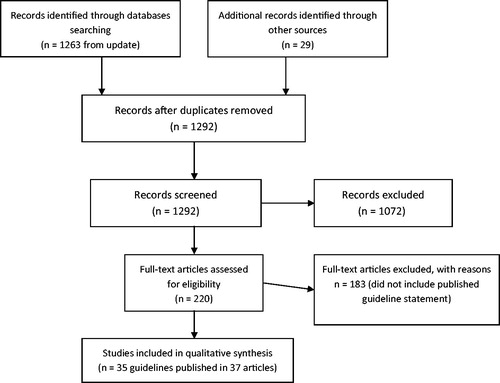
A literature search was conducted in Medline, EMBASE, BIOSIS, Cochrane Collaboration, and on websites of relevant professional associations. Searches were limited to English publications. 1292 citations were identified, 219 papers were assessed, and 35 guidelines were identified for inclusion.
Results
The guidelines covered a variety of geographies: United States (US; n = 10); Europe (n = 11); “Rest-of-World” (n = 9); and “Other” organizations (n = 5). These covered a variety of specialties. Guidelines defined iron deficiency and IDA generally by serum ferritin and transferrin saturation levels. One-fifth of the reviewed guidelines (7 of 35) included no mention or recommendation regarding parenteral iron’s utility in the management of IDA. Fifteen guidelines recommended using parenteral iron in the management of IDA. Fewer US guidelines included recommendations around IV iron than in Europe or the rest of the world. Approximately 60% of the guidelines have not been updated in ≥5 years and consequently do not reflect current evidence on the safety and efficacy of IV iron.
Conclusions
While national and international guidelines for management of IDA exist, many are outdated and do not reflect current evidence including, but not limited to, parenteral iron use. Urgent consideration should be given to updating and clarifying management guidelines for IDA using the latest treatment modalities and options, particularly in the US.
Introduction
The World Health Organization (WHO) defines anemia as “a condition in which the number of red blood cells or the hemoglobin concentration within them is lower than normal.” It is the most commonly encountered hematological abnormality and is caused by a reduction in the concentration of erythrocytes or hemoglobin (Hb) in blood.Citation1 The WHO estimates that over 1.6 billion people are affected by anemia globally, equating to 24.8% of the global population.Citation1
The main cause of anemia is iron deficiency,Citation2 which is suspected as an underlying cause in 50% of all cases,Citation3 although anemia can be caused by other factors such as blood loss, nutritional deficiencies, hemolysis, myelosuppression, renal insufficiency, inflammation, or infection.Citation4,Citation5 Iron plays an essential role in Hb formation and productive erythropoiesis.Citation6 If untreated, iron deficiency can lead to iron deficiency anemia (IDA). The WHO definition of iron deficiency varies according to age, sex and presence or absence of infection. In otherwise healthy individuals, serum ferritin (SFer) levels <15 μg/ml (or <12 μg/ml in pre-school children) are indicative of iron depletion, while levels below 30 μg/ml in pre-school children indicate iron depletion in the presence of infection.Citation7 Notably, ferritin is an acute phase reactant and can be increased in the presence of inflammatory conditions, therefore, SFer level alone may not be an ideal diagnostic parameter. Thus SFer, percent transferrin saturation (TSAT), and Hb together are generally regarded as the parameters which correlate best with iron status.Citation8 TSAT is calculated either as the ratio of iron to transferrin or iron to total iron-binding capacity.Citation9 Other measures may be used, including hypochromic red cellsCitation10 where blood cells are pale and relatively colorless due to a disproportionate reduction of red cell Hb and reticulocyte Hb content. As reticulocytes are only 1–2 days old, the result is reflective of the iron available in the bone marrow for erythropoiesis.Citation11 This approach may help confirm the diagnosis of IDA since it more accurately reflects the presence of anemia than SFer alone. Many organizations and professional bodies produce their own guidelines for the definition and diagnosis of IDA, and the variability of recommendations reflects the need to carefully consider characteristics of the underlying condition and its effect on the parameters used to identify IDA.Citation12 Iron deficiency, with or without anemia, may have significant consequences in many conditions, including impact upon quality of life (QOL),Citation13–15 increased length of hospital stays,Citation16 and increased mortalityCitation17,Citation18 and morbidity.Citation19,Citation20 In the most recent update to the Global Burden of Disease Study, IDA was ranked as the fourth highest cause of years lived with disability from a total of 328 diseases and conditions and the fifth most prevalent condition with 1.24 billion cases (95% uncertainty intervals 1.21–1.28 billion).Citation21
Iron supplementation is the most commonly prescribed treatment for anemia, although other options exist, including erythropoeitic stimulating agents (ESAs) and blood transfusions.Citation19 Iron supplementation can be either oral or parenteral. Ever since the introduction of low-molecular-weight intravenous (IV) iron formulations, many of the adverse events traditionally associated with parenteral iron administration have declined.Citation22 IV iron complexes have been engineered to allow administration of high doses of iron in a relatively short time. IV iron complexes must be stable, non-reactive, and non-toxic. These features are achieved with third generation IV irons, which include ferumoxytol, iron isomaltoside and ferric carboxymaltose in which carbohydrate-stabilized polynuclear Fe (III)-oxyhydroxide/oxide nanoparticles are formulated as colloidal solutions. The third generation IV irons can be delivered in one or two doses and have higher levels of stability which contributes to an improved safety profile compared to previous parenteral formulations.Citation23 Accordingly, IV iron complexes are polymers, not small molecules as most pharmaceuticals, and comprise mixtures of similar but not identical macromolecules. Therefore, they belong to the class of non-biological complex drugs.Citation24
Although there has been no previously published review regarding guidelines for treatment of IDA, there has been a systematic review of diagnosis and treatment of iron deficiencyCitation12 evaluating 29 guidelines providing recommendations. Oral iron was recommended in many guidelines, but the authors found significant heterogeneity on the management (diagnosis and treatment) of iron deficiency across indications. They further noted that new options (e.g. IV iron) were emerging at that time and therefore did not feature prominently in the then-current guidelines.
The objective of this systematic review is to explore the current global and United States (US) recommendations for IV iron use in clinical guidelines for IDA across different therapeutic areas and to see what, if any, specific recommendations exist for the treatment of IDA. We also intended to evaluate differences in guideline recommendations, particularly with respect to IV iron use, between the US and rest of the world.
Methods
A literature search was conducted in Medline, EMBASE, BIOSIS and the Cochrane Collaboration. Additionally, the websites of relevant professional association including those of the WHO and the National Institute for Health and Care Excellence (NICE) were also assessed. The search string used for Medline, EMBASE and BIOSIS was “guideline* AND iron deficiency” and was limited to publications in English since 1 January 1990. The search was conducted on 24 January 2020. The Cochrane Collaborations was also searched on the same date using the term “iron AND deficiency AND guideline.” Additional free text searching of the internet using the term “iron-deficiency anemia/anaemia” was conducted.
The authors screened retrieved citations and abstracts to select titles for the full-text article. All citations whose abstract or full text was not available were excluded. Reference lists of included articles were checked to find articles of interest that may not have been found in the search.
Studies were included if they included guidelines from any country, organization or consensus meeting and had a focus on the diagnosis and/or management of anemia and its use in healthy or ill patients (both adults and children). The search was limited to results in the English language and only the last update of a guideline was included. If available, information on the diagnosis and management of iron deficiency and IDA was extracted from each included article.
Results
A total of 1263 citations were identified on Medline, EMBASE and BIOSIS and an additional two in the Cochrane Collaboration. Web searches and/or checking the reference lists of those articles identified 29 additional papers (). A total of 1,292 citations were assessed by reviewing the title and/or the abstract. A total of 220 papers were reviewed in full, and of these 35 guidelines were identified for inclusion in the systematic review.
Overview
The guidelines came from a variety of countries. There were ten from the US,Citation4,Citation25–33 6 were pan-European,Citation34–40 as well as five from Canada,Citation41–46 four from the United Kingdom (UK),Citation47–50 two from India,Citation51,Citation52 and one each from AustraliaCitation53 and Japan.Citation54 There were also five “other” guidelines identified (e.g. those designed to applied anywhere in the world rather than limited to a specific geography).Citation1,Citation55–59 These included one set from the WHO and two from independent expert groups (the IRON CORE study group and an International Consensus Statement on the peri-operative management of anemia and iron deficiency).Citation55,Citation59 We have included the latter two studies since they offer guidance on the treatment of iron deficiency, although as they were published by independent expert groups, the guidelines may not have undergone the same development process as guidelines from national or professional bodies. Additionally we have included two papers discussing the use of Patient Blood Management (PBM) in anemia, which has been endorsed by the World Health Assembly of the WHO.Citation60 The guidelines covered a diverse range of indications including heart failure (HF),Citation25,Citation33,Citation35,Citation45 pediatrics,Citation26,Citation28,Citation46 oncology,Citation29,Citation30,Citation39 chronic kidney disease (CKD),Citation31,Citation37,Citation41–43,Citation47,Citation50,Citation54,Citation58 obstetric and gynecological (OB/GYN),Citation4,Citation49,Citation52 adults,Citation32 gastrointestinal (including inflammatory bowel disease [IBD]),Citation27,Citation38,Citation40,Citation48,Citation53 general anemia,Citation1,Citation44,Citation51 surgery,Citation34,Citation36,Citation56,Citation57,Citation59 and inflammatory diseases (including CKD, congestive heart failure [CHF], and IBD).Citation55 We have categorized the guidelines by four geographical regions (US, Europe [including pan-Europe and the UK], “Rest-of-World” and “Other”) and by specialty (cardiology, pediatrics, gastroenterology, oncology, OB/GYN, adults, general anemia, surgery, and one guideline which covered multiple inflammatory conditions noted above) ().Citation55 While all the guidelines included in this review cover the management of the underlying conditions in the following sections we have chosen to describe guidelines as either “iron deficiency guidelines” or “IDA guidelines” based upon whether or not they defined either term.
Figure 2. Guidelines for anemia, iron deficiency and/or iron deficiency anemia by therapy area and geographical region. *including CHF, CKD and IBD. Abbreviation. CHF, congestive heart failure; CKD, chronic kidney disease; IBD, inflammatory bowel disease; OB/GYN, obstetrics and gynecology; US, United States.
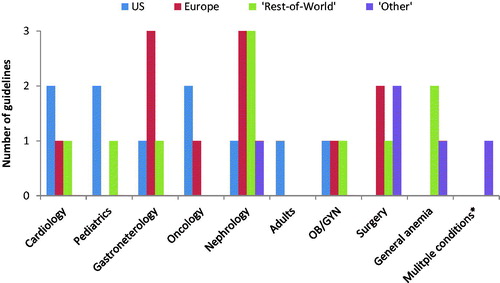
Of the 35 guidelines identified, the single specialty with the most guidelines was nephrology with 8 (24% of all guidelines), followed by gastroenterology (6) surgery and cardiology (4 each). The US has more guidelines in the disease areas of cardiology, oncology and pediatrics than any other region, and was the only region where guidelines for adult anemia were identified. However, all but one of these guidelines (American Academy of Family Practitioners [AAFP]) referred to “iron deficiency” rather than “IDA.” Nearly half of European iron deficiency guidelines covered just two specialties (gastroenterology and nephrology) and unlike the US, there were IDA guidelines in surgery but no iron deficiency guidelines in pediatric or adult patients. “Rest-of-World” guidelines, as in Europe, were most frequently in nephrology (one in IDA and one in iron deficiency), but there were no identified guidelines for either iron deficiency or IDA in surgery or oncology. “Other” IDA guidelines covered surgery and nephrology with iron deficiency guidelines in both general anemia and inflammatory conditions. Iron deficiency guidelines were developed between 2001 and 2019 and IDA guidelines between 2011 and 2018. There were 19 iron deficiency guidelines identified which also appeared in a previous published review of anemia.Citation12 Of these guidelines, only 8 have been updated; those from the National Comprehensive Cancer Network (NCCN),Citation29 the Japanese Society for Dialysis Therapy (JSDT),Citation54 NICE,Citation47 AAFP,Citation25 the Gastroenterological Society of Australia (GSA),Citation53 the British Society of Haematology (BSH),Citation49 the European Society of Medical Oncology (ESMO),Citation39 and the American Society of Clinical Oncology/American Society of Hematology (ASCO/ASH).Citation30 This equates to 30% of US guidelines and European guidelines updated in the last 5 years as compared to 20% of the “Rest-of-World” guidelines.
United States
US guidelines were identified from the American College of Physicians (ACP),Citation25 American Academy of Pediatrics (AAP),Citation26 the Crohn’s and Colitis Foundation,Citation27 US Preventive Services Task Force (USPSTF),Citation28 NCCN,Citation29 ASCO/ASH,Citation30 the American College of Obstetricians and Gynecologists (ACOG),Citation4 AAFP,Citation32 the American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA),Citation33 as well as a US commentary on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.Citation31
Diagnosis
Of these guidelines, 5 (AAP, Crohn’s & Colitis Foundation, NCCN, ACOG, and AAFP) provided defined measurements (in terms of Hb) to assess the presence of anemia, 4 of which included definitions of iron deficiency (Crohn’s & Colitis Foundation, NCCN, ACOG, and ACC/AHA). Only the AAFP guideline provided a clear definition of IDA.
Anemia was generally defined in reference only to measures of Hb, with exception of ACOG who also defined it in terms of hematocrit (Hct) levels. AAP defined anemia (for children aged between 12 and 25 months) as Hb level <11.0 g/dL while the Crohn’s & Colitis foundation uses Hb <12 g/dL for women and <13 g/dL for men. NCCN defined it as Hb <11 g/dL or ≥2 g/dL below the individual’s baseline. ACOG’s definition of anemia varied by trimester from Hb <11 g/dL and Hct percentage as 33% in the first trimester to Hb <10.5 g/dL to Hct as 32% in the second, and Hb <11 g/dL and Hct percentage as 33% in the third trimester. AAFP’s measures varied by age from <13 g/dL in adult males to <11 g/dL in infants.
The presence of iron deficiency was confirmed using measures of SFer and TSAT in all guidelines with the exception of ACOG who used the parameter of change in Hb level. Iron deficiency was defined by SFer <100 ng/mL or SFer >100 ng/mL with TSAT <20% by the Crohn’s and Colitis Foundation, SFer 30–800 ng/mL and TSAT <20–50% by NCCN. ACOG measured increase in Hb concentration of ≥1 g/dL following iron treatment, while ACC used SFer <100 ng/mL or 100–300 ng/mL if TSAT <20%.
Only AAFP guidelines defined “IDA” (as opposed to “iron deficiency”), which was defined as mean corpuscular volume (MCV) <95 μm3.
Management
The use of IV iron was recommended in most of the guidelines (7 of 10). The exceptions were from ACP, who felt the evidence was not yet strong enough to recommend its use but did make clear that it showed the most benefit in patients with New York Heart Association (NYHA) class III HF and low ferritin; AAP, who did not mention the use of IV iron; and USPSTF, who specifically excluded the use of IV iron as a means of iron supplementation (not unexpected, given their guidelines were to cover infants aged 6–24 months). However, none of the guidelines that included IV iron as a management option made clear whether this was to treat iron deficiency or IDA specifically, and most advised its use be restricted to those patients intolerant to oral therapy. Full details of the definitions and measurements can be found in .
Table 1. Definition and measurement of anemia.
Europe
We identified the following European Guidelines: Network for the Advancement of Transfusion Alternatives (NATA);Citation34 NICE;Citation47 European Society of Cardiology (ESC);Citation35 European Board of Anaesthesiology (EBA);Citation36 Anemia Working Group of European Renal Best Practice (ERBP);Citation37 British Society of Gastroenterology (BSG);Citation48 an International Working Group on Inflammatory Bowel Diseases;Citation38 BSH;Citation49,Citation50 ESMO,Citation39 and the European Crohn’s & Colitis Organisation (ECCO).Citation40
Diagnosis
All organizations and groups provided a clear definition of anemia with the single exception of the BSH guidelines,Citation50 which were specific to the diagnosis of functional iron deficiency in cases of anemia of chronic diseases and CKD. Six of the guidelines (NICE, ECCO, ERBP, BSH [both guidelines] and the International Working Group on Inflammatory Bowel Diseases) all provided cut-off values to identify iron deficiency, and four provided definitions to characterize IDA (NATA, ESC, ECCO and ESMO).
In all the guidelines which defined anemia, Hb level was used. The Hb levels used were as follows: NATA, ESC, EBA and BSG (<12 g/dL for females and <13 g/dL for males); NICE (<10.5 g/dL aged under 2 otherwise <11 g/dL); ERBP (<12 g/dL for females and <13.5 g/dL for males). As in ACOG guidelines, BSH guidelines in anemia in pregnancy varied the levels used based on trimester (<11 g/dL in first and third trimesters, <10.5 g/dL in second trimester and <10 g/dL post-partum). ESMO guidelines defined anemia as Hb levels of 10–11 g/dL. ECCO used Hb according to WHO guidelines ranging from <13 g/dL in adult males to <11 g/dL in pregnant women and very young (aged 0.6–5 years) children.
Iron deficiency was defined by using both SFer and TSAT by NICE, ESMO and ERBP (<100μg/L and <20% respectively) or by SFer alone: ERBP (<100μg/L); BSH (<30 μg/L in pregnancy and <200 μg/L–<800 μg/L in CKD) and ECCO (<30 μg/L). IDA was defined in similar terms using a combination of SFer and TSAT by NATA, ESC, ECCO and ESMO (SFer <10 g/dL and TSAT <20% in most cases).
Management
Only one of the eleven guidelines (ERBP) did not mention the use of IV iron in the treatment of iron deficiency, and five of the guidelines (NICE, BSH [guidelines on anemia in pregnancy], ECCO, ESMO and the International Working Group on Inflammatory Bowel Diseases) all had specific recommendations for the use of IV iron in cases of IDA. Full details of the definitions and measurements used in European guidelines can be found in .
“Rest-of-World”
The “Rest-of-World” guidelines were produced by JSDT,Citation54 the Canadian Agency for Drugs and Technology in Healthcare (CADTH), the Canadian Society of Nephrology (CSN),Citation42,Citation43 the Indian Ministry of Health,Citation51 GSA,Citation62 the Ministry of Health for British Colombia,Citation44 the Canadian Cardiovascular Society (CCS),Citation45 the Canadian Pediatric Surveillance Program (CPSP),Citation46 and the Federation of Obstetrics and Gynaecological Societies of India (FOGSI).Citation52
Diagnosis
There were three guidelines (JSDT, CSN and Government of India [GOI]) which provided values to assess the presence of anemia, with four providing clear definitions as to what constitutes iron deficiency (CSN, GSA, Ministry of Health for British Colombia, and CCS). JDST defined anemia as Hb <11–13 g/dL for men and <10.5–11.5 g/dL depending upon age and gender. CSN also used Hb values (<13.5 g/dL for men and <12 g/dL for women). The GOI guidelines followed a similar age/gender pattern as JSDT with Hb values <13 g/dL for men and <11–12 g/dL for women depending upon age and pregnancy.
CSN defines iron deficiency by Hb level (<11 g/dL), SFer (>100 ng/mL), and TSAT (>20%). The GSA uses a more complex measure combining SFer and reduced MCV with ion-binding capacity and TSAT. The Ministry of Health for British Colombia used SFer (varying by age) from <12 to 15 μg/mL, while the CCS used SFer <10 g/dL of SFer 10–30 g/dL with TSAT <20%. IDA was defined by SFer and TSAT by JSDT, whereas CPSP used Hb level (<11 g/dL) in combination with another “measure of poor iron status.”
Management
Two guidelines defined IDA (JSDT and CPSP) with seven mentioning the role of IV iron in cases where there is iron deficiency. CADTH discusses the specific use of IV ferumoxytol in IDA, noting that “IV ferumoxytol appears to be non-inferior to iron sucrose with regard to its efficacy in both dialysis-dependent and non-dialysis-dependent patients with or without CKD who experienced IDA. In addition, the safety profiles of ferumoxytol and iron sucrose are similar, though careful observation for potential rare and severe anaphylactic reactions has been suggested post-infusion”.Citation41 See for more detail.
“Other”
There were five guidelines from other independent bodies/groups: those from an expert group funded by Vifor Pharma (IRON CORE);Citation55 WHO;Citation1 KDIGO;Citation58 PBMCitation56,Citation57and an International Consensus Statement on the peri-operative management of anemia and iron deficiency.Citation59
Diagnosis
Of the five guidelines, four defined the measurement of anemia (WHO, KDIGO, PBM and the International Consensus Statement), and two clearly outlined the parameters to indicate a diagnosis of iron deficiency (IRON CORE, WHO). Anemia, as defined by the WHO using Hb (varying by age/gender) was also used by KDIGO and the PBM guidelines, although the International Consensus Statement defined anemia as Hb <13 g/dL for both sexes (this guideline only covered peri-operative anemia). SFer was used to define iron deficiency by WHO, whereas IRON CORE and KDIGO used a combination of SFer and TSAT. The International Consensus Statement defined IDA using SFer and TSAT or C-reactive protein levels. Measures to assess IDA were clearly specified within the KDIGO guidelines and the International Consensus Statement.
Management
Four of the five guidelines recommended the use of IV iron in the treatment of iron deficiency, with only the WHO guidelines omitting any mention. Since the WHO guidelines were focused largely on prevention rather than treatment, this omission is understandable. There were specific recommendations on the use of IV in the treatment of IDA contained in the guidelines from IRON CORE, KDIGO, PBM and the International Consensus Statement. Details of the guidelines and their recommendations can be found in .
IV Iron treatment
Although IV iron is recommended in almost all the guidelines, depending upon the condition, it is often restricted in its use to those intolerant to, or non-compliant with, oral iron. This is despite evidence that oral iron-replacement therapy is often poorly tolerated or ineffective and that, based on the evidence of five studies, an Hb response <1.0 g/dL at day 14 of oral iron can be used to identify subjects with iron-deficiency anemia who should be transitioned to IV iron supplementation.Citation63
Those organizations which specifically recommend the use of IV iron to treat IDA included the JSDT, IRON CORE (CHF, CKD, IBD), where IV iron is the iron deficiency/IDA treatment of choice in CHF, active/advanced IBD, and patients with CKD undergoing dialysis, and is an option (alongside oral iron) in cases of IDA in non-dialysis CKD patients; CADTH (CKD); NICE (CKD); ESC (heart failure with reduced ejection fraction); European Working Group (IBD); ECCO (IBD); KDIGO (CKD); AAFP (if intolerant to oral iron); BSH (if intolerant/non-compliant to oral iron in pregnancy or if a rapid response is needed and in CKD); the US commentary on the KDIGO guidelines (CKD); FOGSI (in people who are intolerant/non-compliant); International Consensus Statement (front-line therapy in patients who do not respond to oral iron or are not able to tolerate it, or if surgery is planned for <6 weeks after the diagnosis of peri-operative anemia); EBA (IV iron therapy should be considered particularly if rapid iron repletion is required [e.g. <2 months to none deferrable surgery]) and PBM in pre-operative surgery (with or without ESA use), although they do note it has potential in other non-surgical settings.Citation57
Those who recommend IV iron in cases of iron deficiency included NATA (if intolerant to oral iron) Crohn’s & Colitis Foundation (in cases where there is active IBD). CSN guidelines list IV iron as the preferred treatment option in CKD patients undergoing dialysis whilst it is an option (alongside oral iron) for the treatment of non-dialysis or peritoneal dialysis patients. Others include NCCN (in iron deficiency in cancer patients); BSG (when the patient is intolerant to oral iron); GSA (alongside dietary advice, oral iron and blood transfusion); Ministry of Health, British Colombia (cases where there is an inadequate response to oral iron, oral iron is not tolerated or there is ongoing blood loss); ESMO (functional iron deficiency or ongoing chemotherapy); ACP (NYHA class III HF and low ferritin levels); CCS (as an alternative to oral iron); ACOG (parenteral iron is used in the rare patient who cannot tolerate or will not take modest doses of oral iron. Patients with a malabsorption syndrome and severe iron deficiency anemia may benefit from parenteral therapy); AHA (NYHA class II and III HF and iron deficiency IV iron replacement might be reasonable to improve functional status and QOL). Further detail on each guideline’s recommendations can be found in and more information on IV iron use specifically is contained in Supplemental Table 1.
Table 2. Guidelines and IV iron recommendations.
Those guidelines which did not contain any recommendation for the use of IV iron included AAP (pediatrics); USPSTF (where studies on IV iron use were explicitly excluded from consideration; hence, no guidance on its use was included); GOI (which included intramuscular but not IV iron); WHO (whose guidelines only cover anemia); ASCO/ASH (where the guidelines refer only to treatment using ESAs); CPSP (children); and ERBP (CKD).
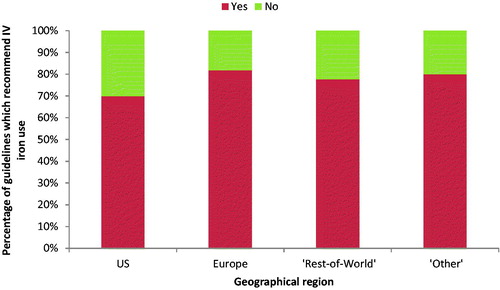
This is similar to the previous review in 2015Citation12 where 21 of 29 guidelines recommended IV iron, although there is considerable variation by geography: 30% of US guidelines compared to 10% of European, 22% of “Rest-of-World,” and 20% of “Other” guidelines had no recommendation for IV iron use (). It is also notable that only one US guideline (US Commentary on the KDIGO guidelines) had IV iron as first-line treatment.
Figure 3. Percentage of guidelines for anemia, iron deficiency and/or iron deficiency anemia which include any recommendations for the use of IV iron. Abbreviation. IV, intravenous; US, United States.
Relatively few guidelines discuss specific IV iron formulations, and where they do, they do not recommend one over another. The ESC guidelines are an exception to this and recommend the use of IV ferric carboxymaltose (FCM).Citation35
Discussion
Iron deficiency has been identified as the leading cause of anemia, a condition which causes death and morbidity for large numbers of people worldwide, as noted in the introduction the latest version of the Global Burden of Disease Study has IDA as one of the leading causes of disability worldwide. The objective of our review was to explore the current recommendations for IV iron use in clinical guidelines for iron deficiency across different therapeutic areas and evaluate the absence or presence of specific recommendations for the treatment of IDA. We also intended to see if there were differences in guideline recommendations, particularly with respect to IV iron use, between the US and other regions.
Our results have shown that whilst anemia itself is well-defined, generally agreeing in many cases with the Hb values recommended by the WHO (although it should be noted that recently there has been increasing discussion about the appropriateness of the gender distinctions in the WHO valuesCitation64) the concepts of iron deficiency and IDA are generally much vaguer, with many of the guidelines using the terms almost interchangeably. This is reflected in the fact that many guidelines fail to give clear recommendations for treatment, particularly with regards to the use of parenteral iron in management in cases of IDA. This is largely consistent with the results of the previous systematic review by Peyrin-Boulet et al.,Citation12 however, where they seemed optimistic that the then emerging IV iron treatments would be included in future iterations of the guidelines, we have found relatively little evidence that this has happened. Of the 19 guidelines which were reviewed in both studies, only eight had been updated within the last five years, meaning that many guidelines were still over a decade old and may no longer reflect the best available practice in their specialty area. It is interesting to note that many of the guidelines which consider the use of IV iron come from inflammatory conditions such as CHF and nephrology where the absorption of oral iron might be problematic. On the other hand, there is a noticeable lack of identified guidelines in the area of pediatric care, despite much recent research pointing to the potential role of IV iron in children.Citation65–67 There was also considerable variation by geography, with US guidelines on the whole having fewer that included recommendations around IV iron. The issue of the lack of updating of guidelines means that many of the guidelines are not reflecting the current evidence on the efficacy and safety of IV iron. See Auerbach and DeLougheryCitation68 which reported that “although oral iron is often viewed as front-line therapy, extensive published evidence has accumulated that IV iron is superior, in both efficacy and safety, to oral iron in many clinical situations and should be introduced much sooner in the treatment paradigm of iron-deficient patients.” Furthermore, the recent review by DeLougheryCitation69 notes that “physicians’” treatment practices may be based on old and out-of-date understanding and information, especially with regard to the safety of oral and IV iron therapy and that “the preponderance of the data reinforces the safety and low reaction rates of IV iron.” This is also supported by other recent studies, including an e-Delphi survey for IDA in gastrointestinal bleeding concluded “that current use of iron therapy by experts in the field is driven by clinical and cost-orientated considerations, rather than by clinical assessment and therapeutic targets or treatment thresholds”.Citation70 A recent meta-analysis of the safety of IV iron compounds in IBD treatments found that FCM was associated with fewer adverse events, although, its statistical significance remains unproven due to the lack of data from randomized controlled trials.Citation71 A cost-effectiveness study in the Nordic countries suggested that implementing the ESC guidelines for the treatment of iron deficiency in heart failure using FCM would improve both health-related QOL and reduce healthcare costs.Citation72 In addition to a lack of consideration of the cost-effectiveness of different treatment options, many of the guidelines fail to clarify the clinical criteria that indicate oral vs IV iron replacement beyond cases where patients might be intolerant to oral iron, and almost none recommended preparations of iron which should be used, given the differences in their efficacy/safety profiles. There was also a lack of consideration about the trigger and target iron and Hb levels, and about the timing allowed to obtain such target values based on a patient’s status and hemodynamic conditions.
The principle strength of our study is that it uses a well-defined and accepted methodology (i.e. that of a systematic review) to identify suitable studies for inclusion. It has also built upon a previous systematic review by Peryin-Biroulet et al.Citation12 to identify both a larger number of guidelines and to ensure that the latest available guidelines are now assessed, since the previous review was published in 2015. This study is subject to several limitations. First, as the literature review has shown many guidelines are either not published in peer-reviewed journals, or, since IDA is not the main focus of many of the guidelines (it is a consequence of other conditions and so is treated as a sub-section of most guidelines), it is not mentioned in the title/abstract of a paper. They are consequently difficult to identify. Therefore, some relevant guidelines may have been omitted for these reasons. Second, we have restricted our search to guidelines published in English, which may have excluded some guidelines (although this is likely to disproportionately affect guidelines from non-US countries/regions).
This review has shown that diagnosis and treatment of iron deficiency/IDA is still subject to heterogeneity and that many of the guidelines urgently need to be updated to reflect growing evidence for the benefits of IV iron, which would encourage changes in clinical practice to ensure the optimal management of patients with IDA.
Conclusions
While many national and international guidelines exist regarding anemia, there remains a lack of clarity on the diagnosis of iron deficiency and around the management modality of IDA, despite the recognition of the worldwide burden that this condition imposes. Many of the guidelines are outdated and do not reflect the current evidence available for the use of IV iron. Urgent consideration should be given to updating and clarifying guidelines around IDA. This is particularly true for the US (the region with the highest percentage of guidelines making no recommendations on IV use) where the only professional organization to endorse the use of IV iron in IDA currently is the AAFP.
Transparency
Declaration of funding
This study was sponsored by American Regent.
Declaration of financial/other relationships
SN and KK are both employees of American Regent. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
SN and KK were involved in the conception, design, analysis and interpretation of the data; revised it critically for intellectual content; and have given final approval of the version to be published. Both authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (44.3 KB)Acknowledgements
The authors would like to thank Maria Aziz of American Regent for her comments on the manuscript. Editorial support was provided by Keith Evans and Chris Radel of inScience Communications, Springer Healthcare (Chester, UK and Philadelphia, PA, USA), with funding from American Regent.
Data availability statement
There is no data set for this review. All guidelines are available online.
References
- World Health Organization. Iron deficiency anaemia: assessment prevention and control. 2001. [cited 2019 Nov 6]. Available from: https://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf.
- Annaloro C, Lambertenghi Deliliers D, Oriani A, et al. Clinical topics in iron deficiency. Internista. 1997;5(3):155–162.
- Api O, Breyman C, Cetiner M, et al. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: iron deficiency anemia working group consensus report. TJOD. 2015;12(3):173–181.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201–207.
- Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med. 2020;287(2):153–170.
- Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013;3(4):a011601.
- World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. 2011. [cited 2020 July 9]. Available from: https://www.who.int/vmnis/indicators/serum_ferritin.pdf
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: Systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16–23.
- Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(Suppl 6):1606S–1614S.
- Schoorl M, Schoorl M, van Pelt J, et al. Application of innovative hemocytometric parameters and algorithms for improvement of microcytic anemia discrimination. Hematol Rep. 2015;7(2):5843.
- Enders HM. Evaluating iron status in hemodialysis patients. Nephrol Nurs J. 2002;29(4):366–370.
- Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102(6):1585–1594.
- Biswas S, Simmons J, Myszor M, et al. Management of iron deficiency anaemia in the outpatient inflammatory bowel disease cohort. Gut. 2014;63(Suppl 1):A173.2–A174.
- Bokemeyer C, Aapro MS, Courdi A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43(2):258–270.
- Bou-Fakhredin R, Halawi R, Roumi J, et al. Insights into the diagnosis and management of iron deficiency in inflammatory bowel disease. Expert Rev Hematol. 2017;10(9):801–808.
- Shander A, Goodnough LT, Javidroozi M, et al. Iron deficiency anemia-bridging the knowledge and practice gap. Transfus Med Rev. 2014;28(3):156–166.
- Blosser CD, Bloom RD. Posttransplant anemia in solid organ recipients. Transplant Rev (Orlando). 2010;24(2):89–98.
- Daru J, Sobhy S, Pavord S. Revisiting the basis for haemoglobin screening in pregnancy. Curr Opin Obstet Gynecol. 2019;31(6):388–392.
- Busti F, Marchi G, Ugolini S, et al. Anemia and iron deficiency in cancer patients: role of iron replacement therapy. Pharmaceuticals (Basel). 2018;11(4):94.
- Cases Amenos A, Ojeda Lopez R, Portoles Perez JM, et al. Heart failure in patients with kidney disease and iron deficiency; the role of iron therapy. Nefrologia. 2017;37(6):587–591.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259.
- Clark SF. Iron deficiency anemia: diagnosis and management. Curr Opin Gastroenterol. 2009;25(2):122–128.
- Girelli D, Ugolini S, Busti F, et al. Modern iron replacement therapy: clinical and pathophysiological insights. Int J Hematol. 2018;107(1):16–30.
- Martin-Malo A, Borchard G, Fluhmann B, et al. Differences between intravenous iron products: focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail. 2019;6(2):241–253.
- Qaseem A, Humphrey LL, Fitterman N, et al. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(11):770–779.
- Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics. 2010;126(5):1040–1050.
- Hou JK, Gasche C, Drazin NZ, et al. Assessment of gaps in care and the development of a care pathway for anemia in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23(1):35–43.
- McDonagh M, Blazina I, Dana T. Routine iron supplementation and screening for iron deficiency anemia in children ages 6 to 24 months: a systematic review to update the U.S. Preventive Services Task Force Recommendation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. (Evidence Syntheses, No. 122.).
- National Comprehensive Cancer Network. Management of Cancer and chemotherapy-induced anemia. 2019. [cited 2019 November 6]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf
- Bohlius J, Bohlke K, Castelli R, et al. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH Clinical Practice Guideline Update. J Cin Oncol. 2019;37(15):1336–1351.
- Kliger AS, Foley RN, Goldfarb DS, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis. 2013;62(5):849–859.
- Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87(2):98–104.
- Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–e161.
- Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975.
- European Board of Anaesthesiology. European Board of Anaesthesiology (EBA) recommendations for Preoperative anaemia and Patient Blood Management. 2013. [cited 2019 Nov 6]. Available from: http://www.eba-uems.eu/resources/PDFS/safety-guidelines/EBA–Preop-anaemia-recommend.pdf
- Locatelli F, Covic A, Eckardt KU, et al. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2008;24(2):348–354.
- Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(12):1545–1553.
- Aapro M, Beguin Y, Bokemeyer C, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(Suppl 4):iv271.
- Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9(3):211–222.
- Canadian Agency for Drugs and Technolgies in Health. Ferumoxytol versus other intravenous iron therapies for anemia: a review of the clinical and cost-effectiveness and guidelines – an update. 2014. [cited 2019 Nov 6]. Available from: https://www.cadth.ca/sites/default/files/pdf/htis/nov-2014/RC0608%20Ferumoxytol%20Final.pdf
- Madore F, White CT, Foley RN, et al. Clinical practice guidelines for assessment and management of iron deficiency. Kidney Int Suppl. 2008;74(110):S7–S11.
- White CT, Barrett BJ, Madore F, et al. Clinical practice guidelines for evaluation of anemia. Kidney Int Suppl. 2008;74(110):S4–S6.
- Ministry of Health British Colombia. Iron deficiency – diagnosis and management. 2019. [cited 2019 November 6]. Available from: https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/iron-deficiency.pdf
- Ezekowitz JA, O’Meara E, McDonald MA, et al. Comprehensive update of the Canadian Cardiovascular Society Guidelines for the management of heart failure. Can J Cardiol. 2017;33(11):1342–1433.
- Canadian Paediatric Surveillance Program. Iron deficiency anemia in children. 2011. [cited 2019 Nov 7]. Available from: https://www.cpsp.cps.ca/uploads/publications/RA-iron-deficiency-anemia.pdf.
- National Institute for Health and Care Excellence. Chronic kidney disease: managing anemia. 2015. [cited 2019 Nov 6]. Available from: https://www.nice.org.uk/guidance/ng8.
- Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309–1316.
- Pavord S, Daru J, Prasannan N, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2020;188(6):819–830.
- Thomas DW, Hinchliffe RF, Briggs C, et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161(5):639–648.
- Ministry of Health and Family Welfare. Guidelines for control of iron deficiency anaemia. 2013. [cited 2019 Nov 6]. Available from: https://www.nhm.gov.in/images/pdf/programmes/child-health/guidelines/Control-of-Iron-Deficiency-Anaemia.pdf
- Federation of Obstetric and Gynaecological Societies of India (FOGSI). Management of Iron Deficiency Anemia in adolescent girls. 2016 [cited 2019 November 7]. Available from: https://www.fogsi.org/wp-content/uploads/2016/05/recommendation-adolescence-ida-stride-17-May-2016.pdf
- Gastroenteroligcal Society of Australia. Iron Deficiency. 2015. [cited 2019 November 6]. Available from: https://www.gesa.org.au/resources/clinical-guidelines-and-updates/iron-deficiency/
- Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese Society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3(1):36.
- Cappellini MD, Comin-Colet J, de Francisco A, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol. 2017;92(10):1068–1078.
- Shander A, Van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012;109(1):55–68.
- Franchini M, Marano G, Veropalumbo E, et al. Patient Blood Management: a revolutionary approach to transfusion medicine. Blood Transfus. 2019;17(3):191–195.
- Summary of recommendation statements. Kidney Int Suppl. 2011;2(5):341–342.
- Munoz M, Acheson AG, Bisbe E, et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia. 2018;73(11):1418–1431.
- World Health Organization. Global forum for blood safety: patient blood management. 2011. [cited 2020 July 9]. Available from: https://www.who.int/bloodsafety/events/gfbs_01_pbm_concept_paper.pdf
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200.
- Gauthier H, Thery L, Le Maignan C, et al. Functional iron deficiency during the first-line chemotherapy in cancer patients: results of a prospective study. Eur J Cancer. 2015;51:S235.
- Okam MM, Koch TA, Tran MH. Iron supplementation, response in iron-deficiency anemia: analysis of five trials. Am J Med. 2017;130(8):991 e1–991 e8.
- Butcher A, Richards T. Bringing women into the spotlight: the impact of preoperative anemia in gynecological surgery. Eur J Obstet Gynecol Reprod Biol. 2017;211:212.
- Beverina I, Macellaro P, Parola L, et al. Extreme anemia (Hb 33 g/L) in a 13-year-old girl: is the transfusion always mandatory? Transfus Apher Sci. 2018;57(4):512–514.
- Mantadakis E. Advances in pediatric intravenous iron therapy. Pediatr Blood Cancer. 2016;63(1):11–16.
- Powers JM, Shamoun M, McCavit TL, et al. Intravenous ferric carboxymaltose in children with iron deficiency anemia who respond poorly to oral iron. J Pediatr. 2017;180:212–216.
- Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: a new paradigm. Hematology Am Soc Hematol Educ Program. 2016;2016(1):57–66.
- DeLoughery TG. Safety of oral and intravenous iron. Acta Haematol. 2019;142(1):8–12.
- Ingolf S, Aksan A, Wehkamp J, et al. Expert consensus on managing iron deficiency anaemia in patients with gastrointestinal bleeding - a European E-Delphi Survey. Gastroenterology. 2019;156(6):S-469.
- Aksan A, Işık H, Farrag K, et al. The comparative safety of different intravenous iron preparations in inflammatory bowel disease: a systematic review and network meta-analysis. J Crohns Colitis. 2019;13(Supplement_1):S471–S472.
- Hofmarcher T, Cabrales Alin D, Linde C. Cost effectiveness of implementing ESC guidelines for treatment of iron deficiency in heart failure in the Nordic countries. Scand Cardiovasc J. 2018;52(6):348–355.