Abstract
Objectives
Previous network meta-analysis (NMA) demonstrated advantageous or similar efficacy of baloxavir marboxil (baloxavir) over neuraminidase inhibitors in otherwise healthy (OwH) influenza patients. This analysis assessed the efficacy and safety of baloxavir in the subgroup of high-risk (HR) patients and in the population of uncomplicated influenza consisting of OwH and HR patients with influenza.
Methods
A systematic literature review (SLR) was performed in Medline, Embase, CENTRAL and ICHUSHI up to August 8th, 2018. A Bayesian NMA was conducted to compare baloxavir with oseltamivir, zanamivir, laninamivir and peramivir in HR patients and all uncomplicated patients.
Results
Based on the SLR, a total of 32 studies were identified as pertinent for the analysis, including 7 studies on HR patients, 13 trials on OwH patients and 14 studies on OwH + HR population. NMA of 10 trials assessing HR patients demonstrated comparable time to alleviation of symptoms for all treatments. Mean decline in virus titer from baseline at 24 h after treatment was significantly greater for baloxavir compared with oseltamivir and peramivir. The risks of total complications and drug-related adverse events were comparable between baloxavir and zanamivir, oseltamivir and laninamivir. These findings were highly consistent with results of the NMA using pooled evidence on the uncomplicated population of OwH and HR patients.
Conclusions: Baloxavir was significantly more effective than placebo regarding all outcomes except for the risk of pneumonia. Besides, baloxavir was associated with similar clinical efficacy and safety, and superior antiviral activity compared to other antivirals in HR patients, as well as in the entire population of uncomplicated patients with influenza.
Introduction
Influenza is an acute viral infection of the respiratory tract, which occurs seasonally. It is a frequent cause of mild to severe illness, but it can also lead to death. The symptoms of influenza are often similar to those caused by other respiratory viruses circulating in temperate climates, including some or all of the following: fever or feeling feverish/chills, cough, sore throat, runny or stuffy nose, muscle or body aches, headaches, and fatigue (tiredness)Citation1. Therefore, epidemiologists often use the term “influenza-like illness” (ILI) to refer to themCitation2. The World Health Organization (WHO) defines ILI as an acute respiratory infection with a measured fever of ≥38 °C and cough; with an onset within the last 10 daysCitation3; however, other definitions are also used in the literature.
Symptoms of influenza are most often mild, and patients recover within 2 weeks without the need for medical care. In some patients, however, influenza leads to the development of a variety of complications, including life-threatening conditions, which require inpatient care. For this reason, influenza does not only impose a huge economic burden due to sick leave among patients but is responsible for humanistic burden in terms of mortality and lost quality of life. Molinari et al. predicted that the total economic burden of influenza would reach $87 billion in the USCitation4. In Japan, the number of influenza patients in the 2018/19 season reached a near epidemic level of around 12 million infectedCitation5. Also in the 2018/19 season, the hospitalized influenza surveillance reported a total of 20,389 hospitalized influenza patients, similarly as in the preceding season (20,584 in 2017/18) but much higher compared to previous seasons (15,405 in 2016/17; 12,275 in 2015/16)Citation5–8.
The US Centers for Disease Control and Prevention (CDC) defines patients who are at particularly high risk of developing complications following ILICitation9. This group consists of people with a variety of underlying health complications, including asthma, diabetes, cancer, cardiovascular diseases, AIDS/HIV or children with neurologic conditions and others. Older age (>64 years old), children <2 years old, pregnancy and gestation have also been recognized by CDC as independent risk factors for developing ILI-associated complicationsCitation9. At an individual level, high-risk (HR) patients, such as young children, elderly, pregnant women and patients with comorbidities, can experience much more severe symptoms than otherwise healthy patients. The population at risk infected with influenza occasionally develop complications including pneumonia, otitis media and dehydration or encephalopathy with or without liver failureCitation1.
Antiviral therapies have been developed to shorten the disease duration, improve recovery and reduce the risk of ILI-associated complications. The recent clinical practice guidelines issued by the Infectious Diseases Society of America recommend immediate initiation of antiviral treatment in hospitalized patients, people with progressing diseases and subjects with a high underlying risk of ILI complicationsCitation10. A study conducted by Havers et al., however, showed that antiviral drugs are under-utilized in the group of HR patients with an acute respiratory illnessCitation11.
The most commonly used antivirals include neuraminidase inhibitors. They interfere with the release of progeny influenza virus from infected host cells via blocking of neuraminidase activity; therefore, they prevent infection of new host cells and stop the spread of infectionCitation12. Nevertheless, there is a need to create new, more effective drugs since the development of resistance to already existing drugs has been identified during treatment of seasonal influenzaCitation4. A cap-dependent endonuclease inhibitor baloxavir marboxil (further referred as baloxavir) was developed as a single-dose, oral drug, with the purpose to target viral replication of influenza A and B. In the CAPSTONE-1 trial, baloxavir demonstrated superior efficacy to the placebo in alleviating influenza symptoms, and superior to both oseltamivir and the placebo in virologic outcomesCitation13. A recently revealed CAPSTONE-2 trial demonstrated that baloxavir administered in HR patients was associated with a significantly shorter time to improvement of influenza symptoms compared with the placebo (median 73.2 h vs. 102.3 h, p<.0001) and numerically shorter than oseltamivir (81.0 h, p=.8347). The median time to cessation of viral shedding in baloxavir patients was 48 h– significantly less than 96 h in both the placebo and oseltamivir patients. Baloxavir compared with the placebo reduced the frequency of influenza-related complications (2.8 and 10.4%) and the need for systemic antibiotic use (3.4 vs. 7.5%)Citation14. A recent network meta-analysis by Taieb et al. demonstrated that in the otherwise healthy (OwH) population, baloxavir was associated with a reduced time to alleviation of all symptoms compared to zanamivir, whereas time to cessation of viral shedding was significantly shorter for baloxavir than zanamivir and oseltamivirCitation15. The mean decline in virus titer from the baseline at 24 h after treatment was significantly greater for baloxavir than for other drugsCitation15. Baloxavir demonstrated a comparable safety profile to other antivirals, except for total drug-related adverse events (DRAE) where it demonstrated a decrease compared to oseltamivir and laninamivirCitation15.
This study was conducted to assess the comparative efficacy and safety profile of baloxavir in the subset of high-risk patients, as well as in the entire population of uncomplicated patients with influenza.
Methods
Systematic literature review (SLR)
This analysis was preceded by a systematic literature review (SLR) of randomized controlled trials (RCT) assessing the efficacy and safety of antiviral medications administered in patients with influenza symptoms and ILI. The identification of studies was based on SLR conducted on the 14November 2016 and further updated on the 8August 2018. The systematic search was conducted in the following electronic databases: MEDLINE, MEDLINE In-Process, EMBASE, CENTRAL, and ICHUSHI. Websites of conferences/congresses related to infectious diseases were screened for relevant clinical data. Additional sources were consulted in order to capture relevant documents for the review (e.g. ClinicalTrials.gov, Shionogi proprietary unpublished data). No geographical restrictions were applied. The search strategies are reported in the Supplementary Appendix. Clinical trials were selected by two reviewers working independently and the discrepancies were resolved by a third reviewer. Extracted data included publication characteristics, study details, patient characteristics, results and study limitations. All extracted data were quality-checked by a second reviewer. The eligibility criteria are summarized in .
Table 1. Inclusion and exclusion criteria of SLR and NMA.
Statistical analysis
The CAPSTONE-2 trial was designed to compare between baloxavir and the placebo or oseltamivir regarding the time to the improvement of influenza symptoms as the primary endpoint, while the time to alleviation of all symptoms (TTAS) was assessed as the secondary efficacy measure. Since all identified trials for comparators assessed only TTAS, it was chosen as the primary efficacy outcome of this analysis. A sensitivity analysis was also carried out, in which the primary outcome from the CAPSTONE-2 trial was pooled together with TTAS reported in the remaining trials.
Network meta-analysis (NMA) was performed to compare baloxavir with antivirals [oseltamivir 75 mg twice a day (BID) for 5 days, zanamivir 10 mg BID for 5 days, laninamivir 40 mg single administration, peramivir 600 mg single or repeated administration] and the placebo in terms of efficacy and safety in HR patients.
The analyses were conducted in Bayesian framework, using the Markov Chain Monte Carlo (MCMC) method, as outlined by the National Institute for Health and Care Excellence Decision Support Unit (NICE DSU) guidelinesCitation16. The analyses of efficacy outcomes were conducted in the influenza-infected population and the analyses of safety outcomes were conducted in the total population. For continuous outcomes, the mean change from the baseline for each treatment and associated standard errors (SE) were used as inputs. For binary outcomes, the number of patients experiencing the outcomes and the total numbers of patients by study arm were used. For time to event outcomes, the analysis was conducted assuming that the survival function for time to recovery outcomes followed an exponential distribution, the input of the analysis was the logarithm of the hazard rate [log(λ)] and associated SE.
Vague prior distributions were used for the model parameters. The between-treatment differences regarding estimates of virus titer and time to event endpoints were considered statistically significant when the associated 95% credibility intervals (Crl) did not include zero. An odds ratio was considered statistically significant if the associated 95% Crl did not include 1. All data regarding time to alleviation of symptoms and time to resolution of fever were converted to hours for the analysis.
Fixed-effects (FE) and random-effects (RE) models were fitted for the NMA. The final model was selected based on the deviance information criterion (DICs). The RE model was chosen if DIC was reduced by >5 compared to the FE modelCitation17.
Analyses were conducted using R 3.5.0 statistical software and WinBUGS 1.4.3.
Results
SLR results
The SLR yielded a total of 2342 references, of which 365 were duplicates, and the remaining 1977 were included in the title and abstract screening. Of those, 1743 records were excluded based on pre-defined selection criteria and 234 were considered for full-text analysis. Finally, 90 records related to 69 studies were identified, of which 32 studies (36 records) reported data pertinent for the NMA, including:
5 studies (7 records) recruiting HR patients only;
14 studies (14 records) recruiting a general uncomplicated population (HR + OwH);
13 studies (15 records) studies recruiting OwH only population.
The study selection process is presented in . An overview of the studies included in the analysis is reported in (HR population), (OwH population) and (uncomplicated population).
Figure 1. PRISMA flowchart of selected studies. Abbreviations. CT, clinical trials; EMA, European Medicines Agency; FDA, Food and Drug Administration; NMA, network meta-analysis; PMDA, Pharmaceuticals and Medical Devices Agency; SLR, systematic literature review.
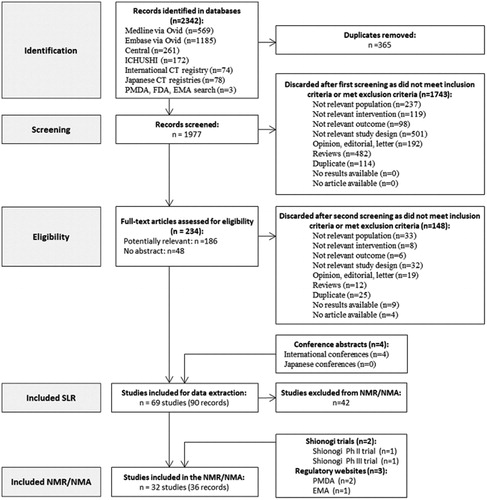
Table 2. List of studies included in the analysis – HR population.
Table 3. List of studies included in the analysis – OwH population.
Table 4. List of studies included in the analysis – uncomplicated population.
NMA results
The network of evidence for the HR population is reported in , and a summary of available evidence for each outcome of interest is provided in . The network of evidence for the entire evidence set (HR + OwH) is presented in , and a summary of the available evidence is provided in .
Figure 2. Network of evidence – HR population. Abbreviations. BXM, Baloxavir; HR, high risk; PMDA, Pharmaceuticals and Medical Devices Agency.
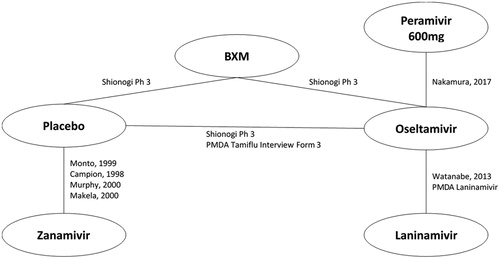
Figure 3. Network of evidence – entire evidence set (HR + OwH). Abbreviations. BXM, Baloxavir; HR, high risk; OwH, otherwise healthy.
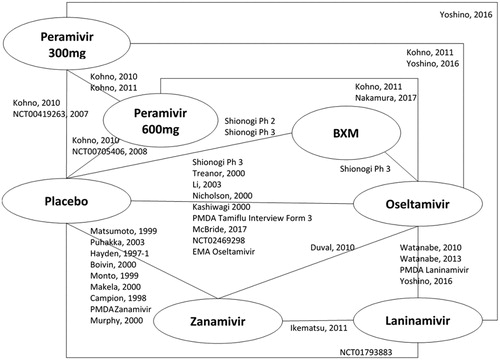
Table 5. Summary of evidence for efficacy and safety outcome measures – high-risk population.
Table 6. Summary of evidence for efficacy and safety outcome measures – entire evidence set (HR + OwH).
Table 7. Results summary in HR population – Efficacy and safety outcomes versus baloxavir.
Table 8. Values of DIC for NMA on HR + OwH population.
Table 9. Results summary in HR + OwH population – Efficacy and safety outcomes versus baloxavir.
Efficacy outcomes
Time to alleviation of symptoms (TTAS)
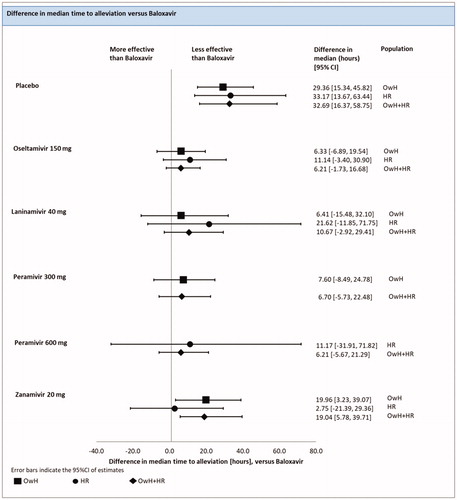
There were 6 studies (6 treatments; 1931patients) included in the analysis of the median TTAS in HR population.
A fixed-effect model NMA revealed that baloxavir was associated with a significantly shorter TTAS compared with the placebo [difference in median of 33.17 h (13.67; 63.44)]. The effect of baloxavir did not differ significantly from zanamivir 20 mg [difference in median of 2.75 h (−21.39; 29.36)], laninamivir [difference in median of 21.62 h (−11.85; 71.75)], oseltamivir [difference in median of 11.14 h (−3.40; 30.90)] and peramivir 600 mg [difference in median of 11.17 h (−31.91; 71.82)] (; ).
Figure 4. Time to alleviation of symptoms – NMAs on OwH/HR/OwH + HR. Abbreviations. CI, credibility interval; HR, high risk; NMA, network meta-analysis; OwH, otherwise healthy.
A total of 23 RCTs (8265 patients) were included in the analysis pooling all evidence regardless of underlying risk, of which 7 studies were conducted on HR patients, 7 on OwH patients and the remaining 9 on a mixed population of OwH and HR patients.
The results of the FE model NMA pooling all available evidence were consistent with the outcomes of the NMA, including HR patients, except that baloxavir was associated with significantly shortened TTAS compared with zanamivir 20 mg [19.04 h (5.78, 39,71)] (; ).
Time to resolution of fever (TTRF)
There were 3 studies (5 treatments; 1438 patients) included in the analysis of the median TTRF in HR population.
An FE model NMA revealed that baloxavir was associated with a significantly lower TTRF compared to the placebo [difference in median of 21.18 h (13.77; 30.25)]. The effect of baloxavir did not differ significantly from laninamivir [difference in median of 8.49 h (−2.96; 22.25)], oseltamivir [difference in median of 3.63 h (−1.24; 8.31)]) and peramivir 600 mg [difference in median of 6.55 h (−8.34; 26.36)] (; ).
Figure 5. Time to resolution of fever – NMAs on OwH/HR/OwH + HR. Abbreviations. CI, credibility interval; HR, high risk; NMA, network meta-analysis; OwH, otherwise healthy.
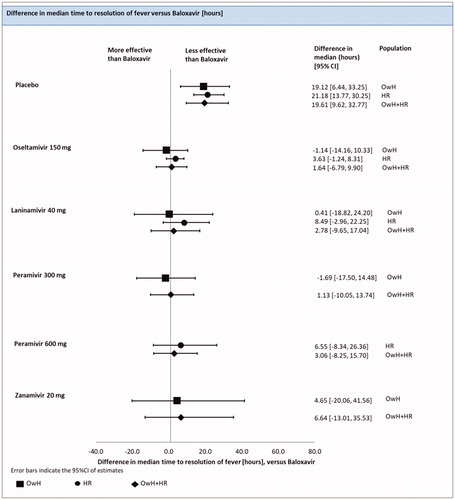
There were 16 studies (7 treatments; 5931 patients) included in the pooled analysis of all RCTs for the median TTRF, among which 4 studies were conducted on HR patients, 5 on OwH patients and remaining 7 trials on a mixed population of HR and OwH patients ().
The results of the RE model NMA pooling all available evidence were consistent with the outcomes of the NMA, including HR patients (; ).
Time to improvement of influenza symptoms
There were 6 studies (6 treatments; 1931 patients) included in the analysis of the median time to the improvement of influenza symptoms in the HR population. In the CAPSTONE-2 study, the endpoint was defined as time to the improvement of influenza symptoms, while in the remaining trials, TTAS of influenza was reported.
An FE model NMA revealed no significant differences between baloxavir and any other treatments. Baloxavir was found significantly more effective only when compared to the placebo, with a difference of median time to improvement equal to 37.57 h (16.59; 70.53). The analysis of the entire evidence set (HR + OwH) was not feasible due to the lack of data for the OwH population ().
Time to cessation of viral shedding
The analysis of this outcome on the HR population was not feasible due to the lack of evidence for this population.
Change in virus titer from baseline at 24 h after treatment
There were 2 studies (4 treatments; 1115 patients) included in the analysis of change in virus titer from the baseline at 24 h after treatment in the HR population.
An FE model NMA revealed that baloxavir showed a significantly greater change in virus titer from the baseline at 24 h after treatment when compared with all treatments: oseltamivir [difference in mean of 1.60 (1.27; 1.93) log10TCID50/mL], peramivir 600 mg [difference in mean of 1.46 (0.65; 2.26) log10TCID50/mL) and the placebo [difference in mean of 2.11 (1.77; 2.45) log10TCID50/mL] (; ).
Figure 6. Change in virus titer from baseline at 24 h after treatment – NMAs on OwH/HR/OwH + HR. Abbreviations. CI, credibility interval; HR, high risk; NMA, network meta-analysis; OwH, otherwise healthy.Comparison with laninamivir 40 mg was infeasible due to lack of data on change in virus titer from baseline to 24 h.
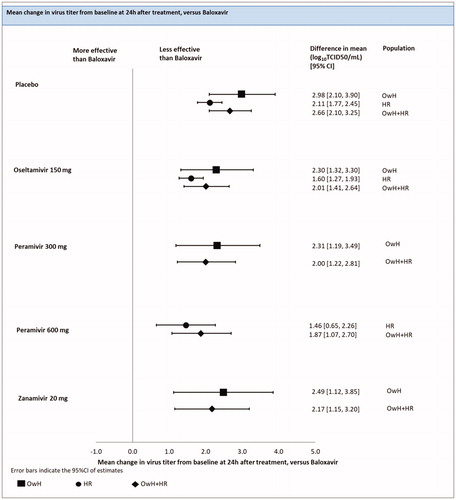
There were 11 studies (6 treatments; 4755 patients) included in the pooled analysis of all RCTs for the change in virus titer from the baseline at 24 h after treatment, among which 2 studies were conducted on HR patients, 5 on OwH patients and remaining 4 on a mixed population of HR and OwH patients.
The results of the RE model NMA pooling all available evidence were consistent with the outcomes of the NMA, including HR patients (; ).
Change in virus titer from the baseline at 48 h after treatment
There were 2 studies (4 treatments; 1208 patients) included in the analysis of change in virus titer from the baseline at 48 h after treatment in the HR population.
An FE model NMA revealed that the effect of baloxavir in a change in virus titer from the baseline at 48 h after treatment was significantly better compared to all other treatments: oseltamivir [difference in mean of 0.66 (0.31; 1.02) log10TCID50/mL], laninamivir [difference in mean 0.91 (0.14; 1.68) log10TCID50/mL] and the placebo [difference in mean of 0.93 (0.57; 1.29) log10TCID50/mL] (; ).
Figure 7. Change in virus titer from baseline at 48 h after treatment - NMAs on OwH/HR/OwH + HR. Abbreviations. CI, credibility interval; HR, high risk; NMA, network meta-analysis; OwH, otherwise healthy.
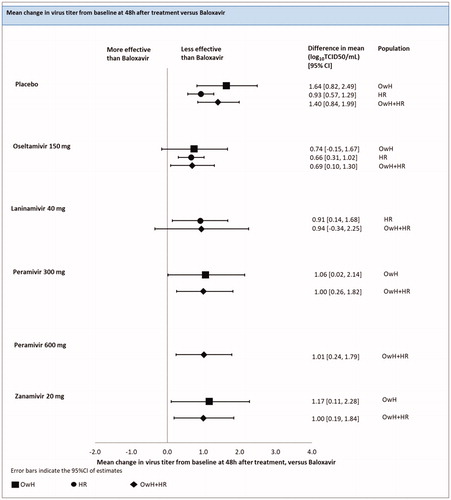
There were 13 studies (7 treatments; 4,974 patients) included in the pooled analysis of all RCTs for the change in virus titer from the baseline at 48 h after treatment, among which, 3 studies were conducted on HR patients, 3 on OwH patients and remaining 7 trials on a mixed population consisted of HR and OwH patients.
The results of RE model NMA pooling all available evidence were consistent with the outcomes of the NMA, including HR patients, except that baloxavir did not differ significantly from laninamivir [0.94 (−0.34; 2.25)] (; ).
Total complications
There were 4 studies (6 treatments; 1486 patients) which reported the number of patients with complications.
An FE model NMA revealed that in terms of the risk of total complications, baloxavir did not differ significantly from zanamivir [odds ratio in median of 1.20 (0.20; 6.59)], oseltamivir [odds ratio in median of 1.68 (0.79; 3.72)], peramivir [odds ratio in median of 1.03 (0.20; 4.94)] and laninamivir [odds ratio in median of 1.41 (0.35; 5.74)]. A significant difference was recorded when baloxavir was compared with the placebo [odds ratio in the median of 4.04 (2.10; 8.41)] ().
The analysis of the entire evidence set (HR + OwH) was not feasible due to the lack of evidence for the OwH population.
Incidence of pneumonia
There were 4 studies (5 treatments; 1740 patients) that reported the number of patients with pneumonia in the HR population. However, one study (Watanabe 2013) was excluded from the analysis due to the lack of events in both study arms. As a result, 3 studies (4 treatments; 1539 patients) were included in the NMA.
An FE model revealed no significant differences between baloxavir and oseltamivir [odds ratio in median of 9.56 (0.62; 4275.03)], peramivir [odds ratio in median of 4.15 (0.05; 2625.25)] and the placebo [odds ratio in median of 14.20 (0.99; 6170.20)] in incidence of pneumonia. Since the number of events was very low in all treatments arms, NMA results were associated with a high credible interval and subjected to instability (; ).
Figure 8. Incidence of pneumonia – NMAs on OwH/HR/OwH + HR. Abbreviations. CI, credibility interval; HR, high risk; NMA, network meta-analysis; OwH, otherwise healthy. Comparison with laninamivir 40 mg was infeasible due to lack of data on pneumonia. Zanamivir 20 mg was assessed in one trial (Duval, 2010) recruiting patients without respiratory complications, including recent exacerbations of chronic obstructive pulmonary disease, asthma or severe chronic disease. The proportion of HR patients was therefore estimated solely based on age distribution.
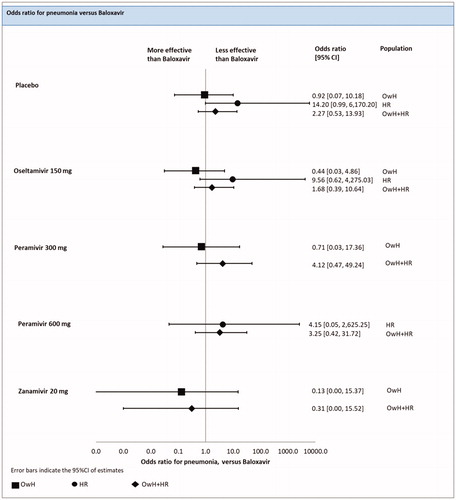
There were 10 studies (6 treatments; 4409 patients) included in the pooled analysis of all RCTs for the risk of pneumonia, among which 4 studies were conducted on HR patients, 3 on OwH patients and remaining 3 studies on a mixed population consisted of HR and OwH patients.
The results of the FE model NMA pooling all available evidence were consistent with the outcomes of the NMA including HR patients (; ).
Incidence of bronchitis
There were 3 studies (5 treatments; 1456 patients) reported the number of patients with bronchitis in the HR population.
An FE model NMA revealed no significant differences between baloxavir and oseltamivir [odds ratio in the median of 1.31 (0.47; 3.76)], peramivir [odds ratio in the median of 0.25 (0.001; 8.65)]. A significant difference between baloxavir and the placebo was recorded [odds ratio in the median of 3.56 (1.57; 9.23)] ().
The analysis of the entire evidence set (HR + OwH) was not feasible due to the lack of evidence for the OwH population.
Safety outcomes
Adverse events (AEs)
There were 4 studies (5 treatments; 2870 patients) that reported the number of patients with adverse events in the HR population.
An FE model NMA revealed that the risk of total adverse events (AE) for baloxavir was significantly lower than for the placebo [odds ratio in median of 1.26 (1.002; 1.59)], but did not differ significantly when baloxavir was compared with zanamivir [odds ratio in median of 0.98 (0.66; 1.46)], oseltamivir [odds ratio in median of 1.16 (0.92; 1.47)] and peramivir [odds ratio in median of 0.34 (0.10; 1.06)] (; ).
Figure 9. Total adverse events – NMAs on OwH/HR/OwH + HR. Abbreviations. CI, credibility interval; HR, high risk; NMA, network meta-analysis; OwH, otherwise healthy.
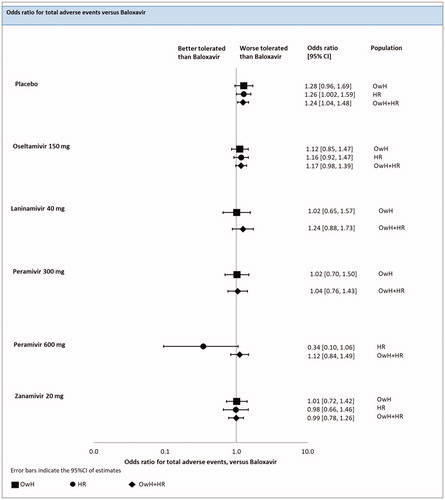
There were 18 studies (7 treatments; 9,806 patients) included in the pooled analysis of all RCTs for the risk of AEs, among which 4 studies were conducted on HR patients, 6 on OwH patients and remaining 8 trials on a mixed population consisted of HR and OwH patients.
The results of the FE model NMA pooling all available evidence were consistent with the outcomes of the NMA, including HR patients (; ).
Drug-related adverse events (DRAEs)
There were 4 studies (6 treatments; 2820 patients) reported the number of patients with drug-related adverse events in the HR population.
An FE model NMA revealed that the risk of DRAEs was significantly lower for baloxavir compared with the placebo [median odds ratio of 1.52 (1.002; 2.30)]. Baloxavir did not differ significantly from zanamivir [median odds ratio of 1.53 (0.74; 3.22)], peramivir [median odds ratio of 0.10 (0.0002; 1.69)] and laninamivir [median odds ratio of 5.20 (0.51; 170.90)] (; ).
Figure 10. Drug-related adverse events – NMAs on OwH/HR/OwH + HR. Abbreviations. CI, credibility interval; HR, high risk; NMA, network meta-analysis; OwH, otherwise healthy.
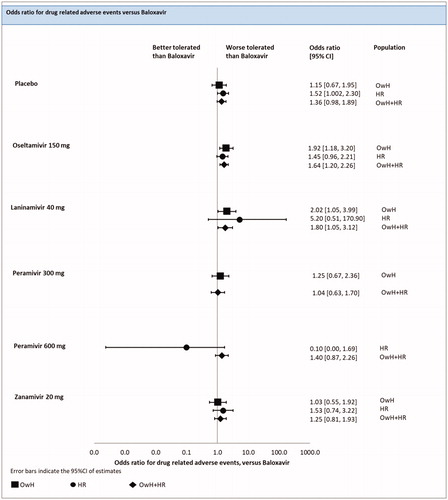
There were 13 studies (7 treatments; 7,754 patients) included in the pooled analysis of all RCTs for the risk of DRAEs, among which 5 studies were conducted on HR patients, 3 on OwH patients and the remaining 5 on a mixed population that consisted of HR and OwH patients.
The results of FE model NMA pooling all available evidence were consistent with the outcomes of the NMA including HR patients, except that baloxavir was significantly better than oseltamivir [odds ratio in the median of 1.64 (1.20; 2.26)] and laninamivir [1.80 (1.05; 3.12)] (; ).
Discussion
This study provides a comprehensive comparison of treatment outcomes between baloxavir and neuraminidase inhibitors in terms of safety conducted in patients with ILI and efficacy conducted in the influenza-infected population. In the primary analysis, the relative between-treatment differences were assessed in the subset of patients with the underlying high risk of complications. According to the up to date clinical practice guidelines, these patients should be treated with antivirals following diagnosis without any delays to prevent severe and life-threatening conditions.
The results of this NMA conducted among HR patients suggest that baloxavir was significantly more efficacious than the placebo for all efficacy outcomes, except pneumonia. Baloxavir was also associated with a significantly greater reduction of virus titer at 24 h since treatment initiation compared with oseltamivir 150 mg and peramivir 600 mg, and better reduction of viral titer at 48 h after treatment compared with oseltamivir 150 mg and laninamivir 40 mg. These results are consistent with previous findings reported in the NMA on OwH patients conducted by Taieb et al. showing that baloxavir was more effective than other antivirals regarding the reduction of viral titer within 24 h. Interestingly in the OwH population, baloxavir was more effective than zanamivir in the alleviation of influenza symptoms, which was not confirmed among the HR populationCitation15.
A traditional NMA has also been conducted to pool all identified studies on uncomplicated population, including HR patients and OwH. The results of all conducted analyses (NMA on HR, NMA on the uncomplicated population) were highly consistent suggesting the superiority of baloxavir over the placebo for all efficacy outcomes, except pneumonia. The analysis pooling all evidence regardless of underlying risk was consistent with the results presented by Taieb et al. showing that baloxavir was significantly better than zanamivir 20 mg for TTAS and significantly better than zanamivir 20 mg, oseltamivir 150 mg and both peramivir 300 mg and 600 mg in change in a virus titer from the baseline at 24 h and at 48 h after treatmentCitation15. Baloxavir was more efficacious in control of the virus load (change in virus titer from baseline at 24 h and 48 h after treatment) than all other comparators for which data were available, except laninamivir 40 mg in the change in virus titer from the baseline at 48 h after treatment. The safety profile of baloxavir was significantly better than the placebo regarding total AEs and significantly better than laninamivir 40 mg and oseltamivir 150 mg regarding DRAEs.
The emergence of drug-resistant strains of the influenza virus imposes a potential threat, in particular for immunocompromised patients and other seriously ill subjects. Although, the overall resistance to neuraminidase inhibitors is considered as low with around 3.5 and 1% of circulating viruses resistant to oseltamivir and zanamivir, respectivelyCitation18. The survey of susceptibility patterns conducted in the 2018–2019 season indicated that 1% of A(H1N1)pdm09 strains were resistant to both oseltamivir and peramivir but sensitive to zanamivirCitation5,Citation19. Baloxavir, with its high antiviral activity, serves a promising option in the treatment of patients with influenza., however human-to-human transmission was detected in 5 patients in the antiviral resistance surveillance in Japan in 2018/2019 and it needs to be continuously monitored through the surveillanceCitation19. Substitutions of the 38th amino acid position in polymerase acidic protein conferring reduced baloxavir susceptibility emerged in 2.2 and 9.7% of patients in phase 2 and phase 3 of CAPSTONE-1 trial respectively, as well as in 5.2% of patients in phase 3 CAPSTONE-2 trialCitation13,Citation14.
There are some potential limitations caused by considering studies that enrolled patients with a wide range of risk factors, as well as studies with single risk factors that may result in potential heterogeneity and inconsistency within the network of evidence in this analysis. The comparability of these populations was confirmed by the clinical expert. Another limiting factor is that the differences regarding the definition of the outcomes across the included studies cannot be excluded. Although the majority of studies assessed resolution of ILI symptoms, some papers did not specify which symptoms contributed to this endpoint. No evidence was collected that any of these differences would affect the relative treatment effect; therefore, the analysis was conducted despite heterogeneity in outcome definitions. The comparability between studies may be questioned due to the fact that the response to treatment could potentially vary between seasons and countries depending on circulating strains of the virusCitation20,Citation21. Finally, the pivotal studies assessing baloxavir were designed and powered to demonstrate clinical superiority versus placebo. Moreover, the number of eligible trials seems to be limited, given the complexity of the evidence networks. Therefore, this NMA is likely underpowered to demonstrate the difference between baloxavir and other antivirals regarding the time to alleviation of disease symptoms and other clinically relevant outcomes.
Transparency
Declaration of funding
This study (design and conduct, data collection, management, analysis and interpretation of the data, review and approval of the manuscript) was funded by Shionogi & Co.
Declaration of financial/other relationships
VT, PW, KJ and SA report grant funding from Shionogi & Co., Ltd during the conduct of the study and outside of the submitted work. MH is an employee of Shionogi Limited. HI is an employee of Shionogi & Co., Ltd. NH reports personal fees and other from Shionogi & Co., Ltd., outside the submitted work.
Author contributions
Conception and design: HI, MH, VT, SA
Acquisition of data: VT, SA, PW, KJ
Analysis and interpretation of the data: HI, MH, VT, SA, PW, KJ, NH
Drafting of the manuscript: VT, SA, PW, KJ, HI, MH
Critical revision of the manuscript for important intellectual content: VT, SA, PW, KJ, HI, MH, NH
Obtaining funding: HI, MH
Acknowledgements
Creativ-Ceutical Ltd performed the NMA in HR patients, funded by Shionogi & Co., Ltd. The authors wish to thank Yuki Yoshida (Shionogi) for biostatistics analysis support. The authors also thank Yoshie Onishi (Creativ-Ceutical) for collaboration and valuable technical inputs, and Malgorzata Biernikiewicz (Creativ-Ceutical) for medical writing support.
References
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2012;1:CD008965. DOI:10.1002/14651858.CD008965.pub3
- Kelly H, Birch C. The causes and diagnosis of influenza-like illness. Aust Fam Physician. 2004;33(5):305–309.
- World Health Organization. WHO surveillance case definitions for ILI and SARI; 2014 [cited 2017]. Available from: http://www.who.int/influenza/surveillance_monitoring/ili_sari_surveillance_case_definition/en/.
- Molinari N-AM, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096.
- National Institute of Infectious Diseases. Influenza 2018/19 season, Japan; 2019. Available from: https://www.niid.go.jp/niid/en/iasr-vol33-e/865-iasr/9288-477te.html.
- National Institute of Infectious Diseases. Influenza 2015/16 season, Japan; 2016. Available from: https://www.niid.go.jp/niid/en/iasr-vol33-e/865-iasr/6885-441te.html.
- National Institute of Infectious Diseases. Influenza 2016/17 season, Japan; 2017. Available from: https://www.niid.go.jp/niid/en/iasr-vol33-e/865-iasr/7674-453te.html.
- National Institute of Infectious Diseases. Influenza 2017/18 season, Japan; 2018. Available from: https://www.niid.go.jp/niid/en/iasr-vol33-e/865-iasr/8438-465te.html.
- CDC. People at high risk for flu complications; 2018. Available from: https://www.cdc.gov/flu/highrisk/index.htm.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1–e47.
- Havers F, Flannery B, Clippard JR, et al. Use of influenza antiviral medications among outpatients at high risk for influenza-associated complications during the 2013-2014 influenza season. Clin Infect Dis. 2015;60(11):1677–1680.
- Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353(13):1363–1373.
- Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913–923.
- Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204–1214.
- Taieb V, Ikeoka H, Ma FF, et al. A network meta-analysis of the efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors for the treatment of influenza in otherwise healthy patients. Curr Med Res Opin. 2019;35(8):1355–1364.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011.
- Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. J Royal Statistical Soc B. 2002;64(4):583–639.
- Lee N, Hurt AC. Neuraminidase inhibitor resistance in influenza: a clinical perspective. Curr Opin Infect Dis. 2018;31(6):520–526.
- National Institute of Infectious Diseases. The antiviral resistance surveillance in Japan in the 2018/2019; 2019. Available from: https://www.niid.go.jp/niid/images/flu/resistance/20191227/dr18-19e20191227-1.pdf.
- Jefferson T, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;2014(4):CD008965. DOI:10.1002/14651858.CD008965.pub4
- Burch J, Paulden M, Conti S, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technol Assess. 2009;13(58):1–265.
