Abstract
Objective
The aim of this study was to review clinical evidence supporting the use of fixed-dose combination of tiotropium and olodaterol, a long-acting muscarinic antagonist (LAMA) and a long-acting β2-agonist (LABA), respectively, as the initial and follow-up treatment choice in patients with chronic obstructive pulmonary disease (COPD) as per Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020 recommendations—the impact of this treatment strategy on the reduction in the risk of exacerbations—and the importance of early therapeutic interventions.
Methods
For this narrative review, the available literature was searched to identify studies including patients with COPD receiving tiotropium and olodaterol as either monotherapy or combination therapy and studies including patients with COPD receiving inhaled corticosteroids (ICS) in addition to long-acting bronchodilators. Relevant studies were included in the review.
Results
Patients with COPD are often prescribed ICS therapy, which, when used over a long term, can be associated with local and systemic adverse effects. The GOLD 2020 report recommends dual bronchodilator therapy as both an initial and follow-up treatment option. A LABA + LAMA combination is mechanistically synergistic, and cumulative evidence surrounding the efficacy and safety of fixed-dose combination of tiotropium and olodaterol supports therapeutic advantages over monotherapy in most patients with COPD.
Conclusions
The early stages of COPD may represent a “window of therapeutic opportunity” during which initiation of tiotropium and olodaterol dual bronchodilator therapy may improve lung function and quality of life and reduce exacerbations in patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality, and is expected to rise from the fifth to the third leading cause of death globally by 2030Citation 1 . Furthermore, the management of COPD is a considerable health care liability—the total economic burden (direct and indirect costs) associated with COPD is projected to be $49 billion in the United States by 2020Citation 2 .
Our understanding of COPD and the development of treatment strategies have progressed substantially, and the appreciation of differences in patient and disease characteristics has facilitated individualization of treatmentCitation 3 . Therapeutic options for COPD are varied and include mono-bronchodilation or dual bronchodilation (short- and long-acting β2-agonists [SABAs and LABAs, respectively] and long-acting muscarinic antagonists [LAMAs]), inhaled corticosteroids (ICS), phosphodiesterase-4 inhibitors, and antibioticsCitation 3–8 . Per the Global Initiative for Chronic Obstructive Lung Disease (GOLD), patients are categorized into one of four stages (stage 1 [mild] to stage 4 [very severe]), based on the degree of airway obstruction, and to one of the four groups (group A to group D), based on individualized assessment of symptoms and exacerbation riskCitation 3 (). According to GOLD 2020 recommendations, initial treatment in patients with COPD should include a bronchodilator (SABA, LABA, or LAMA)Citation 3 . Thereafter, patient response to treatment initiation should be reviewed and pharmacologic therapy adjusted regardless of the patient’s GOLD group at diagnosisCitation 3 .
Figure 1. Classification of COPD based on spirometric determination of the severity of airway obstruction and the combination of symptomatic severity and history of exacerbationsCitation 3 . Abbreviations. CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified British Medical Research Council. Note: Reused from Global Initiative for Chronic Obstructive Lung Disease 2020 report (Copyright © 2020 Global Initiative for Chronic Obstructive Lung Disease, Inc.).
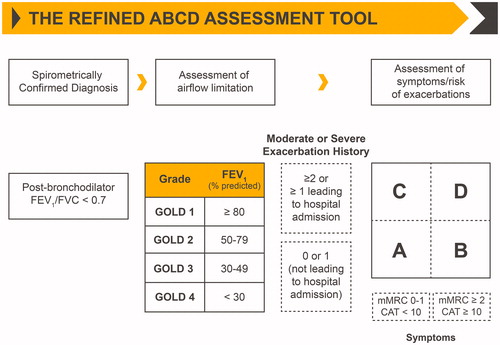
COPD exacerbations are defined as episodic worsening of symptoms that require a modification of treatment strategy or hospitalization, and reduction in the risk of exacerbations is a salient goal of COPD managementCitation 3 . Exacerbations severely impact the natural progression of COPD in many patients, and frequency of exacerbations correlates with a decline in lung function, an increase in mortality, and a reduction in quality of life (QoL)Citation 9–12 . An analysis of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) trial data revealed that increased risk of exacerbations, including those leading to hospitalization, was associated with high St. George’s Respiratory Questionnaire (SGRQ) scores (poor health status) in patients with COPDCitation 13 . Additionally, evidence clearly indicates that exacerbations lead to increased health care resource utilization and are major contributors to costs associated with COPD managementCitation 14 , Citation 15 . Conventionally, exacerbation severity has been classified as mild, moderate, or severe based on the treatment needed to manage or control symptomsCitation 3 . However, causes of exacerbations vary. In many patients, exacerbations may be inflammatory in nature and associated with high levels of T-helper cellsCitation 16 . Common treatment interventions, therefore, may not be effective in reducing exacerbations. Thus, identifying and carefully characterizing patients based on the underlying cause of exacerbations is crucial.
Here, we review clinical evidence supporting the use of LAMA + LABA combinations. Specifically, we focus on the fixed-dose combination (FDC) of tiotropium and olodaterol (T + O), a LAMA and a LABA, respectively, as the initial and follow-up treatment choice in patients with COPD as per GOLD 2020 recommendationsCitation 3 . We also review the impact of this treatment strategy on reducing exacerbations.
Methods
A PubMed literature search was conducted from inception until July 31, 2018, using the following search string: (tiotropium AND olodaterol) AND (COPD OR chronic obstructive pulmonary disease). Additional searches were conducted for studies on tiotropium monotherapy and ICS. English-language articles reporting the efficacy and safety of the FDC of T + O in patients with COPD were included. The reference list of the articles was also scanned for relevant articles. Review articles, preclinical studies, congress reports, letters, commentaries, and other articles not meeting the inclusion criteria were excluded.
Tiotropium monotherapy in the treatment of COPD
Tiotropium is a United States Food and Drug Administration–approved LAMA for long-term, once-daily maintenance treatment of bronchospasms associated with COPD and for the reduction of COPD exacerbationsCitation 17 , Citation 18 . Tiotropium is available as a dry powder formulation, delivered via HandiHaler (Spiriva HandiHalerFootnote i ; 18 µg once daily)Citation 17 , and as an aqueous solution, delivered via Respimat Soft Mist Inhaler (Spiriva RespimatFootnote ii ; 5 µg once daily)Citation 18 . Tiotropium delivered via HandiHaler or Respimat improved lung functionCitation 19 , Citation 20 and QoLCitation 19–21 and reduced exacerbationsCitation 19–22 compared with placebo or other active comparators in patients with COPD.
The effect of tiotropium on exacerbations was evaluated in a number of studiesCitation 19 , Citation 21 , Citation 23–28 . Findings from two separate reviews (a Cochrane review of 22 studies [N = 23,309] of tiotropium HandiHaler 18 µg or tiotropium Respimat 5 or 10 µgCitation 20 and a pooled analysis of nine studies with tiotropium HandiHaler 18 µg [N = 6171])Citation 29 showed that tiotropium significantly reduced the risk of exacerbations and exacerbation-associated hospitalizations. For example, tiotropium HandiHaler 18 µg significantly delayed the median time to first exacerbation (16.7 vs 12.5 months vs placebo [14% risk reduction]Citation 19 ; 187 vs 145 days vs salmeterol [17% risk reduction])Citation 23 and reduced the mean number of exacerbations vs placebo (by 14%)Citation 19 , annual rate of exacerbations (by 11% [moderate and severe by 7% and 27%, respectively])Citation 23 , and risk of moderate and severe exacerbations vs salmeterol (by 14% and 28%, respectively)Citation 23 in the Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT; N = 5993)Citation 19 and 1-year Prevention Of Exacerbations with Tiotropium in COPD (POET-COPD; N = 7376)Citation 23 trials. Of note, in UPLIFT, patients in the placebo group received usual treatment for COPD, which included ICS and/or LABA in up to 62% of patients at baseline and up to 74% of patients at any time during follow-up. Further, in several 1-year trials, tiotropium Respimat 5 µgCitation 21 , Citation 24 significantly delayed the time to first exacerbation (160 vs 86 daysCitation 21 ; 169 vs 119 days)Citation 24 and reduced the proportion of patients with ≥1 exacerbation (37.2% vs 44.1% [25% risk reduction]Citation 21 ; 35.3% vs 43.1% [31% risk reduction])Citation 24 , exacerbation rates (per patient-year: 0.93 vs 1.91Citation 21 ; 0.69 vs 0.87)Citation 24 , and exacerbations requiring hospitalization (0.12 vs 0.15 [19% risk reduction])Citation 24 compared with placebo.
Olodaterol monotherapy in the treatment of COPD
β2-agonists have been used for COPD treatment for over 50 years; however, initial formulations had short durations of actionCitation 30 . Over the years, twice-daily (e.g. salmeterol and formoterol) and eventually once-daily (e.g. indacaterol, vilanterol, and olodaterol) β2-agonists became available. Olodaterol is a highly selective LABA that provides bronchodilation for more than 24 hCitation 31 , Citation 32 . The short- and long-term efficacy (improvements in lung function, QoL, and exercise tolerance) and safety of olodaterol were demonstrated in patients with moderate-to-very severe COPDCitation 33–35 . In two replicate, placebo-controlled, phase 3 trials, both olodaterol and formoterol monotherapies were significantly superior to placebo in improving lung function, but only olodaterol significantly improved QoLCitation 34 . In an indirect treatment comparison, the impact of olodaterol on lung function, QoL, and exacerbations was similar to that of indacaterolCitation 36 . In a study by Maltais et al.Citation 37 , fewer patients experienced exacerbations with olodaterol 10 µg (2.7%–4.9%) compared with olodaterol 5 µg (6.0%–7.5%) and placebo (6.7%–7.0%).
LAMA + LABA combinations in the treatment of symptomatic COPD
LAMA and LABA monotherapies have been compared in several studies, and, in general, LABAs are more efficacious in improving lung function and health statusCitation 38–40 , whereas LAMAs are more efficacious in reducing the risk of exacerbationsCitation 23 , Citation 41–43 . Differences in efficacies coupled with nonoverlapping, synergistic mechanisms of action provided a compelling rationale for combination treatment with a LABA and a LAMACitation 44 . In fact, LAMA + LABA combinations have become the mainstay of COPD treatment, offering beneficial effects on lung function, health status, and exacerbations compared with respective monotherapies or LABA + ICS combinationsCitation 45 , Citation 46 . In addition, LAMA + LABA improved cardiac function by increasing left-ventricular end-diastolic volumeCitation 47 . The GOLD 2020 report provides different recommendations for initial treatment (based on symptom and exacerbation risk) and a follow-up algorithm (based on most treatable trait and current medications), and LAMA + LABA combination therapy is recommended as a part of both algorithmsCitation 3 .
Impact of T + O on lung function, QoL, and physical activity in patients with COPD
Tiotropium was considered a promising LAMA for dual bronchodilator (LAMA + LABA) therapy because of its favorable efficacy. Further, tiotropium may also improve left ventricular function in patients with COPD and chronic heart failure and may have anti-inflammatory effectsCitation 48 , Citation 49 . Additionally, with a similar duration of action and complementary mechanism of action, olodaterol was considered a viable LABA to pair with tiotropium in an FDC. Results of the ANHELTO and ToViTO trials demonstrated the clinical efficacy of this combination in managing COPD symptomsCitation 50 , Citation 51 ().
Figure 2. Clinical trials assessing the efficacy of T + O in patients with COPDCitation 51–53 , Citation 55 , Citation 59 , Citation 60 , Citation 63 , Citation 76 , Citation 77 . Abbreviations. CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; CWRCE, constant work-rate cycle ergometry; EET, exercise endurance time; ESWT, endurance shuttle walk test; ExT, exercise training; FDC, fixed-dose combination; FEV1, forced expiratory volume in 1 second; FEV1AUC0-3, area under the curve for FEV1 within 3 hours of dosing; FEV1AUC0-12, area under the curve for FEV1 within 12 hours of dosing; FEV1AUC0-24, area under the curve for FEV1 within 24 hours of dosing; IC, inspiratory capacity; O, olodaterol; SGRQ, St. George's Respiratory Questionnaire; SMBM, self-management behavior-modification; T, tiotropium.
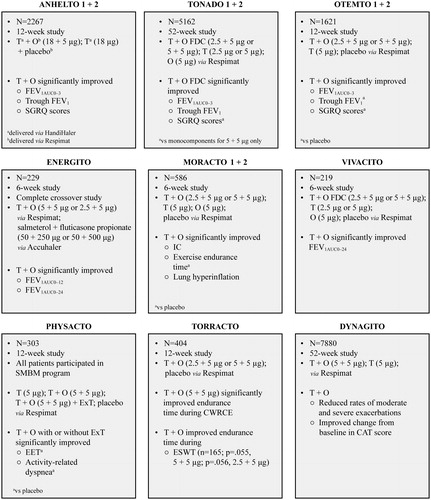
Results of two short-term phase 3 ANHELTO trials showed that once-daily tiotropium 18 µg (delivered via HandiHaler) and olodaterol 5 µg (delivered via Respimat) significantly improved lung function and QoL vs tiotropium 18 µg aloneCitation 51 . In the two replicate, 52-week, phase 3 Tiotropium + Olodaterol Fixed Dose Combination (FDC) Versus Tiotropium and Olodaterol in COPD (TONADO) trials, the once-daily FDC T + O 2.5 + 5 µg or T + O 5 + 5 µg delivered via Respimat led to significant improvements in lung function vs tiotropium (2.5 and 5 µg, and 5 µg, respectively) or olodaterol (5 µg) monotherapy. However, significant improvement in QoL was observed only with T + O (5 + 5 µg)Citation 52 . In the two replicate, 12-week, phase 3 Tiotropium + Olodaterol Fixed Dose Combination (FDC) in Chronic Obstructive Pulmonary Disease (OTEMTO) trials, FDC T + O (5 + 5 µg and 2.5 + 5 µg) delivered via Respimat resulted in improved lung function and QoL vs placebo and tiotropium monotherapyCitation 53 . Further, pooled analyses of TONADO and OTEMTO data showed that FDC T + O significantly improved QoL and breathlessness vs placebo or either monocomponentCitation 54 . Finally, once-daily FDC T + O (5 + 5 µg and 2.5 + 5 µg) was superior to twice-daily salmeterol + fluticasone (50 + 500 µg and 50 + 250 µg) in improving lung function in patients with moderate-to-severe COPD in the 6-week ENERGITO studyCitation 55 .
Regular physical activity is associated with reduction in lung function decline and in the risk of COPD among smokersCitation 56 and of readmission to hospital with COPDCitation 57 . Therefore, improvement in exercise tolerance is another goal of COPD managementCitation 3 . Improved physical activity is an indicator of better QoLCitation 58 , and FDC T + O Respimat had favorable effects on physical activity in multiple trials. In the 12-week TORRACTO trial, in which exercise endurance was evaluated, FDC T + O 5 + 5 µg, but not 2.5 + 5 µg, significantly improved endurance time during constant work-rate cycle ergometryCitation 59 . A strong tendency toward improved walking endurance time was also demonstrated with both doses. FDC T + O (5 + 5 µg) improved exercise endurance time and reduced physical activity–related dyspnea with or without exercise training in patients with COPD participating in a self-management behavior-modification program in the PHYSACTO trialCitation 60 .
Of note, the benefits of the T + O combination are not at the expense of an increased risk of adverse events. Robust evidence from about 10,000 patients suggests that the T + O combination is a suitable treatment option for patients with COPD, including those with a history of cardiovascular risk factorsCitation 61 , Citation 62 .
Impact of T + O on exacerbation control in patients with COPD
As described previously, exacerbations are substantial determinants of disease prognosis in COPD. Of interest, studies evaluating the impact of FDC T + O Respimat on exacerbation control have yielded encouraging results ( and Video 1).
Table 1. Summary of clinical trials assessing the impact of FDC T + O on exacerbations in patients with COPDCitation 52 , Citation 63 , Citation 64 .
In the TONADO trials, FDC T + O significantly reduced exacerbations in patients with moderate or very severe COPD compared with olodaterol aloneCitation 52 . The effect of FDC T + O and tiotropium monotherapy on the annual rate of exacerbations was also evaluated in the large-scale, 52-week, phase 3 DYNAGITO trialCitation 63 . FDC T + O reduced the rate of moderate and severe exacerbations by 7% compared with tiotropium alone; however, the predefined significance level of 0.01 was not met. Moreover, a post hoc analysis using multiple covariate models similar to those used in the SPARK and FLAME trials showed a 11% reduction in moderate-to-severe exacerbations. Furthermore, FDC T + O significantly reduced exacerbations treated with corticosteroids and those treated with corticosteroids + antibiotics vs tiotropium monotherapy ().
Figure 3. Benefits of FDC T + O treatment in exacerbation management compared with tiotropium monotherapyCitation 63 . Abbreviations. CI, confidence interval; FDC, fixed-dose combination; O, olodaterol; RR, rate ratio; T, tiotropium.
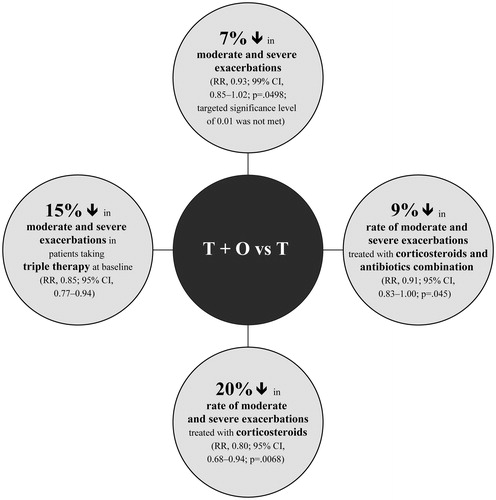
Interestingly, in a prespecified subgroup analysis of Japanese patients in the DYNAGITO trial, FDC T + O reduced moderate-to-severe COPD exacerbations by 29% vs tiotropium monotherapyCitation 64 .
Appropriate use of ICS in COPD
The therapeutic benefits of ICS in asthma prompted the investigation of the efficacy of ICS, such as fluticasone and budesonide, in the management of symptomatic COPD; monotherapy with ICS is indeed associated with modest improvements in COPD. In the Inhaled Steroids in Obstructive Lung Disease in Europe (ISOLDE) trial, fluticasone twice daily produced an increase in the forced expiratory volume in 1 s (FEV1) levels after bronchodilator use and resulted in fewer exacerbations and a slower decline in health status compared with placeboCitation 65 . In the TOwards a Revolution in COPD Health (TORCH) trial, the combination of salmeterol and fluticasone improved lung function and health status and reduced exacerbationsCitation 66 .
The use of ICS is associated with adverse events, including a slight increase in risk of pneumonia, reduced bone mineral density, and cataractCitation 67 . Although the GOLD 2020 report recommends considering ICS use in the patient population at risk for exacerbations with elevated eosinophil countsCitation 3 , LABA + ICS continues to be more widely prescribedCitation 3 , Citation 68 . Thus, it is important to ascertain the feasibility and impact of switching patients already on LABA + ICS to LAMA + LABA treatment regimens. In an effort to highlight the efficacy and safety of treatment de-escalation, the CRYSTAL trial showed that a switch from LABA or LAMA monotherapy or LABA + ICS to LAMA + LABA was associated with superior improvements in lung function with comparable safetyCitation 69 . Such findings necessitate careful selection and identification of patient populations in whom the use of ICS can be limited to prevent adverse effects, and recommendations on effective ways to wean off ICS in patients with COPD have been proposedCitation 70 . Emerging data, although only suggestive, indicate that patients with COPD in whom the eosinophil count is 300 cells/µL or higher are more likely to benefit from continued treatment with ICSCitation 71 . Hence, the GOLD 2020 report recommends that the use of ICS should be guided by a biomarker assessmentCitation 3 . For example, LABA + ICS could be considered for initial treatment in subpopulations of Group D patients with eosinophil counts ≥300 cells/µL3. LABA + ICS can also be considered as a follow-up pharmacological option for patients with exacerbations despite LAMA or LABA therapy if eosinophil counts are ≥300 cells/µL or are ≥100 cells/µL with ≥2 moderate or ≥1 severe (requiring hospitalization) exacerbation in the previous yearCitation 3 . Similarly, use of triple therapy is recommended only as a follow-up pharmacological option if patients who have eosinophil counts ≥100 cells/µL do not respond to LAMA + LABA therapy or in patients who fail to respond to LABA + ICS3. Importantly, patients can be switched from LABA + ICS to LAMA + LABA when they fail to respond to ICS, the safety associated with ICS use is a concern, or resolution of symptoms requires less therapyCitation 3 .
Early intervention in COPD
The progressive nature of COPD is well appreciated, and lung function in patients with COPD deteriorates over timeCitation 72 . In this regard, underdiagnosed, early-stage COPD is likely to progress to more severe disease and lead to a greater impact on exacerbations and QoL. However, the conventional opinion is that early therapeutic interventions are futile and smoking cessation is the only intervention capable of improving disease progression in early stages of COPDCitation 73 .
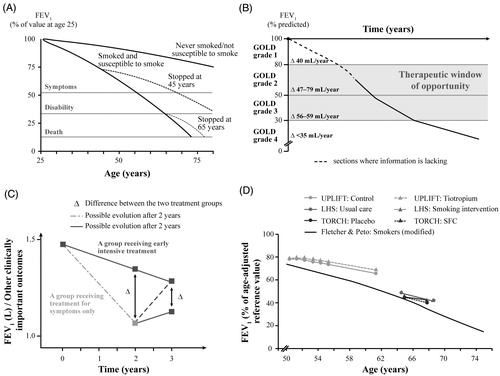
Initial research indicated that the rate of decline in lung function increased with disease progressionCitation 72 (). However, findings from other investigations suggested that decline in lung function, as assessed by the decline in FEV1, is most rapid in the early stages of COPD and slows down in the advanced stages of COPDCitation 74 (). Further, as shown in , intensive treatment could be more beneficial at an early stage rather than at a later stage in patients receiving symptomatic treatmentCitation 73 . In , data from published studies (Lung Health Study, TORCH, and UPLIFT) have been compared with the overall clinical course of COPDCitation 73 . These findings suggest that a “window of therapeutic opportunity” might exist in the early stages of COPD, during which appropriate treatment interventions may be most effective in arresting disease progression and considerably improving prognosis. Of note, subgroup analyses of data from UPLIFT showed that, compared with placebo, tiotropium reduced the rate of decline in mean postbronchodilator FEV1 in patients with GOLD stage 2 COPD. Further, both median time to first exacerbation and median time to first exacerbation resulting in hospitalization were longer with tiotropium. Tiotropium also significantly reduced the number of exacerbations in patients in the early stages of COPDCitation 75 . The potential symptomatic and exacerbation benefits associated with FDC T + O suggest that this dual bronchodilator therapy might be a promising intervention to prevent disease progression in the early stages of COPD. The enhanced efficacy of the combination and comparable safety with monotherapies provide physicians a steroid-free treatment option which is suitable for early initiation.
Figure 4. Therapeutic benefits of early intervention in the management of patients with COPDCitation 72–74 . Abbreviations. COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LHS, Lung Health Study; SFC, salmeterol-fluticasone combination; TORCH, TOwards a Revolution in COPD Health trial; UPLIFT, Understanding Potential Long-Term Impacts on Function with Tiotropium trial. Note: Panel A has been adapted with permission from Fletcher and Peto, British Medical Journal, 1977 (Copyright © 2020 BMJ Publishing Group Ltd); panel B has been adapted with permission from Tantucci and Modina, International Journal of Chronic Obstructive Pulmonary Disease, 2012 (Copyright © 2012 Tantucci and Modina, publisher and licensee Dove Medical Press Ltd.); panels C and D are adapted with permission from Decramer and Cooper, Thorax, 2010 (Copyright © 2010 Decramer and Cooper, publisher and licensee BMJ Publishing Group Ltd & British Thoracic Society).
Conclusions
COPD is the most common chronic lung disease worldwide and results in considerable morbidity, mortality, and health care burden. COPD exacerbations are critical drivers of disease prognosis in COPD—they diminish QoL and increase mortality and exacerbation management is associated with considerable costs. Much progress has been made in our understanding of the etiology of COPD, which has resulted in the development of a number of pharmacological strategies for the management of COPD throughout its natural history—from early to advanced stages of the disease. Although bronchodilators are the recommended initial treatment for COPD, evidence indicates that patients with COPD are often prescribed ICS therapy, which, when used over a long term, is associated with local and systemic adverse effects. A LABA + LAMA combination is mechanistically synergistic, and cumulative evidence supports the therapeutic advantage of such a combination over individual monotherapies in most patients with COPD. The dual bronchodilator combination of T + O improves lung function and QoL in patients with COPD and may reduce exacerbations. The early stages of COPD may represent a “window of therapeutic opportunity” during which initiation of T + O may reduce the rate of lung function decline and improve QoL and disease control.
Transparency
Declaration of funding
This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc.
Declaration of financial/other relationships
GC has received grants from NIH-NHLBI, PA DOH, GSK, Boehringer Ingelheim, Novartis, AstraZeneca, Respironics, MedImmune, Actelion, Forest, Pharmaxis, Pulmonx, Aeris, PneumRx, Pearl, Spiration, Broncus, and Genentech; has pending grants from NIH-NHLBI, GSK, AstraZeneca, Boehringer Ingelheim, Nuvaira, Genentech, Patara, and Galapagos; provides consultation for MedImmune, Bayer, GSK, Amgen, Chiesi, Respironics, Nuvaira, CSA, Boehringer Ingelheim, AstraZeneca, Novartis, Verona, Free Flow Medical, EOLO, Celerion, PneumRx, Olympus, Broncus, Patara, and Prometics; and has equity interest in HGE Health Care Solutions. SD has nothing to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Both authors, GC and SD, contributed equally towards development of this manuscript. Both authors agree to be accountable for all aspects of the work.
Impact of T+O therapy on COPD.
Download MP4 Video (24.1 MB)Acknowledgements
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance was provided by Kushal Banerjee, PhD, Saurabh Gagangras, PhD, Suchita Nath-Sain, PhD, and Maribeth Bogush, PhD, of Cactus Life Sciences (part of Cactus Communications), which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Notes
i Boehringer Ingelheim Pharmaceuticals, Inc; Ridgefield, CT, USA.
ii Boehringer Ingelheim Pharmaceuticals, Inc; Ridgefield, CT, USA.
References
- World Health Organization. Chronic obstructive pulmonary disease (COPD). [cited 2020 Apr 10]. Available from: http://www.emro.who.int/health-topics/chronic-obstructive-pulmonary-disease-copd/index.html.
- Ford ES , Murphy LB , Khavjou O , et al. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45.
- Global Strategy for Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2020 . [cited 2020 Apr 7]. Available from: https://goldcopd.org/.
- Anzueto A , Miravitlles M. The role of fixed-dose dual bronchodilator therapy in treating COPD. Am J Med. 2018;131(6):608–622.
- Singh D , D'Urzo AD , Donohue JF , et al. An evaluation of single and dual long-acting bronchodilator therapy as effective interventions in maintenance therapy-naïve patients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:2835–2848.
- Kaplan AG. Inhaled corticosteroid treatment in chronic obstructive pulmonary disease (COPD): boon or bane? J Am Board Fam Med. 2020;33(2):289–302.
- Chong J , Leung B , Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;9:CD002309.
- Miravitlles M , Anzueto A. Antibiotic prophylaxis in COPD: why, when, and for whom? Pulm Pharmacol Ther. 2015;32:119–123.
- Makris D , Moschandreas J , Damianaki A , et al. Exacerbations and lung function decline in COPD: new insights in current and ex-smokers. Respir Med. 2007;101(6):1305–1312.
- Dransfield MT , Kunisaki KM , Strand MJ , COPDGene Investigators, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330.
- Rubinsztajn R , Przybyłowski T , Maskey-Warzechowska M , et al. Exacerbations of chronic obstructive pulmonary disease and quality of life of patients. Adv Exp Med Biol. 2016;884:69–74.
- Suissa S , Dell'Aniello S , Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963.
- Wilke S , Jones PW , Müllerova H , et al. One-year change in health status and subsequent outcomes in COPD. Thorax. 2015;70(5):420–425.
- Mittmann N , Kuramoto L , Seung SJ , et al. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102(3):413–421.
- Pasquale MK , Sun SX , Song F , et al. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2012;7:757–764.
- Bhat TA , Panzica L , Kalathil SG , et al. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12 (Suppl 2):S169–S175.
- SPIRIVA® HANDIHALER® (tiotropium bromide) inhalation powder, for oral inhalation use. Highlights of prescribing information. February 2018. Boehringer Ingelheim. [cited 2020 Apr 10]. Available from: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva/Spiriva.pdf.
- SPIRIVA® RESPIMAT® (tiotropium bromide) inhalation spray, for oral inhalation use. Highlights of prescribing information. March 2019. Boehringer Ingelheim. [cited 2020 Apr 10]. Available from: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdf.
- Tashkin DP , Celli B , Senn S , et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554.
- Karner C , Chong J , Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD009285.
- Bateman E , Singh D , Smith D , et al. Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis. 2010;5:197–208.
- Halpin DM , Vogelmeier C , Pieper MP , et al. Effect of tiotropium on COPD exacerbations: a systematic review. Respir Med. 2016;114:1–8.
- Vogelmeier C , Hederer B , Glaab T , et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103.
- Bateman ED , Tashkin D , Siafakas N , et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104(10):1460–1472.
- Casaburi R , Mahler DA , Jones PW , et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224.
- Brusasco V , Hodder R , Miravitlles M , et al. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58(5):399–404.
- Dusser D , Bravo ML , Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006;27(3):547–555.
- Tonnel AB , Perez T , Grosbois JM , et al. Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(2):301–310.
- Halpin D , Menjoge S , Viel K. Patient-level pooled analysis of the effect of tiotropium on COPD exacerbations and related hospitalisations. Prim Care Respir J. 2009;18(2):106–113.
- Incorvaia C , Montagni M , Makri E , et al. Striving for optimal bronchodilation: focus on olodaterol. Int J Chron Obstruct Pulmon Dis. 2016;11:439–444.
- Casarosa P , Kollak I , Kiechle T , et al. Functional and biochemical rationales for the 24-hour-long duration of action of olodaterol. J Pharmacol Exp Ther. 2011;337(3):600–609.
- van Noord JA , Smeets JJ , Drenth BM , et al. 24-hour bronchodilation following a single dose of the novel beta(2)-agonist olodaterol in COPD. Pulm Pharmacol Ther. 2011;24(6):666–672.
- Ferguson GT , Feldman GJ , Hofbauer P , et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629–645.
- Koch A , Pizzichini E , Hamilton A , et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:697–714.
- Maleki-Yazdi MR , Beck E , Hamilton AL , et al. A randomised, placebo-controlled, Phase II, dose-ranging trial of once-daily treatment with olodaterol, a novel long-acting beta2-agonist, for 4 weeks in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(5):596–605.
- Roskell NS , Anzueto A , Hamilton A , et al. Once-daily long-acting beta-agonists for chronic obstructive pulmonary disease: an indirect comparison of olodaterol and indacaterol. Int J Chron Obstruct Pulmon Dis. 2014;9:813–824.
- Maltais F , Kirsten AM , Hamilton A , et al. Evaluation of the effects of olodaterol on exercise endurance in patients with chronic obstructive pulmonary disease: results from two 6-week crossover studies. Respir Res. 2016;17(1):77.
- Kerstjens HA , Deslée G , Dahl R , et al. The impact of treatment with indacaterol in patients with COPD: a post-hoc analysis according to GOLD 2011 categories A to D. Pulm Pharmacol Ther. 2015;32:101–108.
- Mahler DA , Kerstjens HA , Donohue JF , et al. Indacaterol vs tiotropium in COPD patients classified as GOLD A and B. Respir Med. 2015;109(8):1031–1039.
- Rodrigo GJ , Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting β-agonists for stable COPD: a systematic review. Chest. 2012;142(5):1104–1110.
- Chong J , Karner C , Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD009157.
- Decramer ML , Chapman KR , Dahl R , et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533.
- Maia IS , Pincelli MP , Leite VF , et al. Long-acting muscarinic antagonists vs. long-acting β2 agonists in COPD exacerbations: a systematic review and meta-analysis. J Bras Pneumol. 2017;43(4):302–312.
- Cazzola M , Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23(4):257–267.
- Rodrigo GJ , Price D , Anzueto A , et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922.
- Aziz MIA , Tan LE , Wu DB , et al. Comparative efficacy of inhaled medications (ICS/LABA, LAMA, LAMA/LABA and SAMA) for COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:3203–3231.
- Hohlfeld JM , Vogel-Claussen J , Biller H , et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6(5):368–378.
- Kato M , Komamura K , Kitakaze M. Tiotropium, a novel muscarinic M3 receptor antagonist, improved symptoms of chronic obstructive pulmonary disease complicated by chronic heart failure. Circ J. 2006;70(12):1658–1660.
- Trevethick M , Clarke N , Strawbridge M , et al. Inhaled muscarinic antagonists for COPD-does an anti-inflammatory mechanism really play a role? Curr Opin Pharmacol. 2009;9(3):250–255.
- Spiolto Respimat in COPD. Boehringer Ingelheim. [cited 2020 Apr 10]. Available from: https://www.boehringer-ingelheim.com/copd/copd/information-tovito-clinical-trial-program.
- ZuWallack R , Allen L , Hernandez G , et al. Efficacy and safety of combining olodaterol Respimat(®) and tiotropium HandiHaler(®) in patients with COPD: results of two randomized, double-blind, active-controlled studies. Int J Chron Obstruct Pulmon Dis. 2014;9:1133–1144.
- Buhl R , Maltais F , Abrahams R , et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979.
- Singh D , Ferguson GT , Bolitschek J , et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319.
- Ferguson GT , Karpel J , Bennett N , et al. Effect of tiotropium and olodaterol on symptoms and patient-reported outcomes in patients with COPD: results from four randomised, double-blind studies. NPJ Prim Care Respir Med. 2017;27(1):7.
- Beeh KM , Derom E , Echave-Sustaeta J , et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study). Int J Chron Obstruct Pulmon Dis. 2016;11:193–205.
- Garcia-Aymerich J , Lange P , Benet M , et al. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463.
- Garcia-Aymerich J , Farrero E , Félez MA , et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105.
- Esteban C , Quintana JM , Aburto M , et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292–300.
- Maltais F , O'Donnell D , Gáldiz Iturri JB , et al. Effect of 12 weeks of once-daily tiotropium/olodaterol on exercise endurance during constant work-rate cycling and endurance shuttle walking in chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2018;12:1753465818755091.
- Troosters T , Maltais F , Leidy N , et al. Effect of bronchodilation and exercise training with behavior modification on exercise tolerance and downstream effects on symptoms and physical activity in COPD. Am J Respir Crit Care Med. 2018;198(8):1021–1032.
- Miravitlles M , Urrutia G , Mathioudakis AG , et al. Efficacy and safety of tiotropium and olodaterol in COPD: a systematic review and meta-analysis. Respir Res. 2017;18(1):196.
- Ferguson GT , Buhl R , Bothner U , et al. Safety of tiotropium/olodaterol in chronic obstructive pulmonary disease: pooled analysis of three large, 52-week, randomized clinical trials. Respir Med. 2018;143:67–73.
- Calverley PMA , Anzueto AR , Carter K , et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344.
- Ichinose M , Nishimura M , Akimoto M , et al. Tiotropium/olodaterol versus tiotropium in Japanese patients with COPD: results from the DYNAGITO study. Int J Chron Obstruct Pulmon Dis. 2018;13:2147–2156.
- Burge PS , Calverley PM , Jones PW , et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303.
- Calverley PM , Anderson JA , Celli B , et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789.
- Price D , Yawn B , Brusselle G , et al. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22(1):92–100.
- Nishi SPE , Maslonka M , Zhang W , et al. Pattern and adherence to maintenance medication use in medicare beneficiaries with chronic obstructive pulmonary disease: 2008-2013. Chronic Obstr Pulm Dis. 2018;5(1):16–26.
- Vogelmeier CF , Gaga M , Aalamian-Mattheis M , CRYSTAL study investigators, et al. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respir Res. 2017;18(1):140.
- Miravitlles M , Cosío BG , Arnedillo A , et al. A proposal for the withdrawal of inhaled corticosteroids in the clinical practice of chronic obstructive pulmonary disease. Respir Res. 2017;18(1):198.
- Watz H , Tetzlaff K , Wouters EF , et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398.
- Fletcher C , Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648.
- Decramer M , Cooper CB. Treatment of COPD: the sooner the better? Thorax. 2010;65(9):837–841.
- Tantucci C , Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99.
- Decramer M , Celli B , Kesten S , et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–1178.
- O’Donnell DE , Casaburi R , Frith P , et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur Respir J. 2017;49(4)pii:1601348.
- Beeh KM , Westerman J , Kirsten AM , et al. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;32:53–59.
