Abstract
Introduction
Major depressive disorder (MDD) is a globally prevalent chronic psychiatric illness with a significant disease impact. As many as 30% of patients with MDD do not adequately respond to two therapies and are considered to be treatment resistant. This study aimed to quantify healthcare costs associated with treatment resistant depression (TRD) in the UK.
Methods
A retrospective chart review of patients with TRD was conducted in primary and secondary care settings over a 2 year period. Data abstracted from medical records of patients included demographics, clinical characteristics and healthcare resource utilization (HCRU; number of consultations, use of Crisis Resolution and Home Treatment Teams [CRHTTs], non-drug and drug interventions, and hospitalizations). HCRU per patient per month (28 days) was calculated for three health states: major depressive episode (MDE), remission and recovery. Unit costs were from the British National Formulary (BNF) and the Personal Social Services Research Unit (PSSRU).
Results
A total of 295 patients with TRD were recruited between January 2016 and May 2018. The mean age of the total sample was 43.3 years; 60.3% were female. Costs per patient, per 28 days, were highest in the MDE state, with the average cost (£992) mainly driven by consultations, non-drug treatment, hospitalizations and CRHTT, with a considerable fall in costs as patients moved into remission and subsequent recovery.
Conclusion
The results suggest that antidepressant treatments for TRD that are more effective in reducing the time spent in an MDE health state, and helping patients achieve remission and recovery, are essential for reducing the overall HCRU and costs in patients with TRD.
This observational study of TRD is the first to assess the HCRU impact associated with different predefined health states.
Using retrospective data from both primary and secondary care physicians from regions across the UK ensures a representative real-world patient population.
One limitation is that the selection of patients is based on criteria that define TRD that rely on physician judgement.
Although the study captures direct HCRU costs, the indirect costs of lost productivity and care are not included in the overall burden.
This study has defined the current clinical management of patients with TRD in the UK and provides an estimate of the associated HCRU and associated costs.
Cost of TRD in the UK Strengths and limitations of this study
Introduction
Major depressive disorder (MDD) is a highly prevalent relapsing and remitting psychiatric disorder characterized by recurrent depressive episodes. It is expected to be the second leading contributor to the global disease burden by 2020Citation1. The prevalence of MDD in people in England aged 16 years and older was estimated to be 3.3% in 2014Citation2 with a female:male ratio of approximately 2:1Citation3. Current treatment guidelines in the UK recommend that patients with mild to moderate depression should first be offered low-intensity psychosocial interventions such as cognitive behavioural therapy or a physical activity programmeCitation4,Citation5. For patients with moderate or severe depression, depressive symptoms that have been present for at least 2 years, or mild depression that does not respond to other interventions, a combination of high intensity psychological intervention or antidepressant medication is recommendedCitation4,Citation5.
Where pharmacological intervention is required, the guidelines recommend that selective serotonin reuptake inhibitors (SSRIs) be prescribed first-line for an episode of MDDCitation4,Citation5. In cases where there is a lack of efficacy (or patients experience intolerable side effects), switching to another antidepressant (an alternative SSRI, or a serotonin noradrenaline reuptake inhibitor [SNRI]) is recommended.
There is also evidence that some augmentation strategies (e.g. brexipiprazole, lithium) would be beneficial for individuals with TRDCitation6,Citation7. However, although a network meta-analysis of 522 double-blind, placebo-controlled and head-to-head trials involving 21 antidepressants found all active drugs to be more efficacious than placebo in treating adults with MDD, there were only small, nonsignificant differences between them in head-to-head trialsCitation8. A similar comprehensive meta-analysis of 234 studies to assess the comparative safety and efficacy of second-generation antidepressants in MDD had also previously reported no clinically relevant differences in efficacy or effectiveness between the active treatmentsCitation9.
While the precise definition of treatment resistant depression (TRD) is inconsistent in the literature, patients have been classified as having TRD when “consecutive treatments with at least two different antidepressants, used for a sufficient length of time (4–8 weeks), at an adequate dose, and with adequate affirmation of treatment adherence, fail to induce a clinically meaningful improvement in the disease symptoms”Citation10,Citation11. The STAR*D study reported that patients with MDD who experienced multiple treatment steps had poorer outcomes in terms of lower remission rates and higher relapse rates, confirming the benefit of early, effective treatment interventionCitation12.
The impact of TRD is substantial, and the highest costs are reported to be for inpatient care, outpatient visits, day hospital attendances and GP visits. The mean societal cost to the NHS has been estimated at £22,124 per year for patients with TRDCitation13. Informal care from family/friends and lost employment was responsible for a higher proportion of the costs than health and social care and accounted for 26% and 54%, respectively, of the total annual costCitation13. On the assumption that 12% of patients with MDD have TRD, the overall cost of TRD in the UK was estimated to be in the region of £3.9 billionCitation13. With the literature suggesting that around 10–30% of patients with MDD develop TRDCitation14, it is clear that this condition represents a considerable impact on patients, the National Health Service (NHS) and society as a whole.
Compared with those patients with MDD who respond to treatment, patients with TRD contribute a disproportionately high impact, including a higher number of depressive episodes, more frequent relapses or recurrence of their depression, increased suicidality, a higher risk of comorbidities, and a decrease in daily functioning and health-related quality of lifeCitation12,Citation15–18. Accordingly, the treatment of patients with TRD is associated with an increase in healthcare resource utilization (HCRU) and economic cost, due to a higher number of visits to both primary and secondary care practitioners, a higher rate of hospitalizations, and more psychiatric prescriptions including anxiolytics, hypnotics and antipsychoticsCitation17,Citation19,Citation20.
Despite a recent systematic literature review highlighting the association between treatment resistance within a major depressive episode (MDE), and reduced patient quality of life and health status, methodological and population differences limit the ability to meaningfully compare or synthesize results across the studiesCitation21. The direct costs associated with different TRD-related health states have rarely been quantified from the perspective of a single healthcare system. Although the overall cost of TRD for patients in the UK has been estimated previously, it is unclear how associated costs differ due to the varying responses to specific antidepressants, or health states of patients with TRDCitation13.
Objectives
This study aimed to demonstrate the economic impact in patients with TRD from a single healthcare system perspective (UK NHS) in a real-world setting, and to explore the levels of HCRU and associated direct costs in different health states (i.e. MDE, remission and recovery), in order to identify where a higher need for intervention may be required.
Methods
Study design
This was a retrospective chart review of patients with TRD to assess the HCRU associated with the management of these patients in both primary and secondary care settings. Patient data were abstracted by both general practitioners (GPs) and psychiatrists across all regions of the UK to ensure that the whole healthcare system landscape for TRD was obtained. In order to ensure that both physicians and patients remained anonymous throughout the study, physicians were recruited through a customized recruitment approach, utilizing a network of recruiters and managed by a third party agency.
Patient selection and data collection
The medical charts of adult patients classified as TRD by their treating physicians in the index window 1 January 2016–31 May 2016 were reviewed retrospectively. Data were recorded for a period of 2 years up until 31 May 2018. Each physician selected medical records of patients with TRD defined by an MDE. Data were abstracted into electronic case report forms (eCRFs). Eligible patients had to be initially diagnosed with MDD according to Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), and thereafter fulfil a diagnosis of TRD, defined as “Major Depressive Disorder in adults who have not responded to at least two separate regimens of different antidepressants of adequate dose and duration to treat the current depressive episode”. Adequate dose was considered the maximal dose recommended by the British National FormularyCitation22, while an appropriate duration was a minimum of 4 weeksCitation23.
The MDE resulting in the classification of TRD was defined as the “index MDE”. In order to provide a sufficiently large sample, and to ensure that the patient sample was not overly represented by a single respondent, each psychiatrist or GP was asked to identify up to ten eligible patients with TRD. Data assessed in this study included patient demographics, health state and outcomes over the 2 year follow up period since initiation of index antidepressant (i.e. the third or later antidepressant prescribed following failure of at least two consecutive antidepressants for treating the same MDE).
Definition of health states
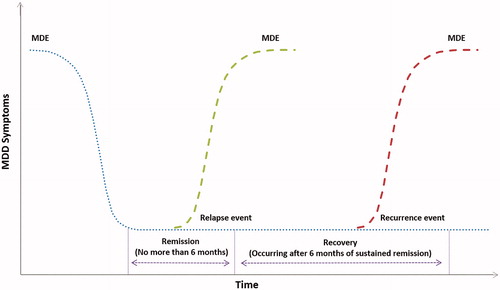
During an initial feasibility assessment to define outcomes within real-world clinical practice with practicing GPs and psychiatrists in the UK, it was recognized that “response” is not consistently assessed because most physicians do not use any depression scales, and the evaluation of depression severity is frequently based upon an informal assessment. As a result, since the magnitude of improvement deemed sufficient for response would be inconsistent or missing if response was not perceived by physicians as clinically relevant compared to remission, response was not included as an outcome in this study. Physicians were asked to provide dates for health state changes using pre-specified definitions (). This allowed healthcare resources to be assigned to specific health states.
Table 1. Definition of health states.
Healthcare resource utilization data collection
The following healthcare resource utilization (HCRU) data were extracted for each patient with health states assigned to each resource by the treating physician: number of primary and secondary care consultations; use of Crisis Resolution and Home Treatment Teams (CRHTTs); number of admissions through the emergency department (ED); hospitalizations (general admissions and psychiatric, length of stay); antidepressant treatment including dosing, duration, line of therapy; other psychiatric medications (anxiolytics, hypnotics and antipsychotics); non-drug treatments (e.g. electroconvulsive therapy [ECT], transcranial magnetic stimulation [TMS], psychotherapy).
Data analysis
Data from eligible patient medical records were captured on an eCRF system to avoid data transfer errors. Data analyses were conducted using STATA statistical software version 15.0. Descriptive statistics were performed on qualitative data to summarize all outcome variables including basic demographics, comorbidities and treatments. Categorical variables were summarized as the number and proportion of the total study population and by subgroups where appropriate. Continuous variables were reported as mean and standard deviationCitation24. No formal hypothesis testing was performed.
As it was not expected that physicians had comprehensive knowledge of all healthcare resources used by a patient, and data may be missing from the medical record, the eCRF had an option of “don’t know” for resources, potentially resulting in missing data. For the resource use totals, in order to preserve the sample sizes where possible, patients with missing data were included. Should patients with any missing data on any question have been removed, the base sizes would have fallen substantially, and the data may not have been representative of all patients with TRD. Accordingly, if a patient had any reported use of resources within a total, the total was calculated to include patients with some missing data, with missing HCRU data items set to zero. It is acknowledged that, whilst the proportion of missing data was low, the reported averages might be an underestimate of actual resource utilization.
Calculating resource costs per month (28 days)
For primary and secondary care consultations, hospitalizations and visit-based therapies, a price per visit was applied. The individual resource use per patient was multiplied by its cost and the daily costs used to calculate a cost per patient per 28 day month (PPPM) for each resource used. For each instance of resource use per patient, the health state was determined (as per data abstracted in the eCRF) and collective costs applied from all patients in that health state. This resulted in an overall cost for each of the following health state groups: MDE, remission and recovery. Each group includes data collected for patients who were in health states multiple times during the follow-up period.
Unit costs
All resources collected in the eCRF were assigned a unit cost derived from either the British National Formulary (BNF) or the Personal Social Service Research Unit (PSSRU). The costs applied to each resource are displayed in the Appendix. The cost of all pharmacological treatments prescribed was calculated on a daily basis. Where available, the BNF drug tariff cost was usedCitation22. Where these costs were not publicly available, the NHS reference cost was applied. The average cost was calculated on a monthly basis to account for the varying follow-up periods and resulted in an overall costs per health state per resource. Within each individual HCRU analysis, the resource is reported for all patients with data for that specific resource. As such, sample sizes differ between health states and resources, and the total HCRU cost for patients can therefore not be derived by totalling the cost of individual resources.
Results
Study population
A total of 30 psychiatrists and nine GPs were recruited, each to provide data on up to ten patients with TRD, resulting in a final sample of 295 patients with TRD. On average, physicians estimated that 37% of all patients with MDD that they treated typically failed at least two different antidepressant therapies and could be categorized as treatment resistant. Within the 295 patients in this study, there were 350 recorded counts of MDE, 227 counts of remission and 144 counts of recovery ().
Overall, the mean (±SD) age of the patient sample was 43.3 ± 13.6 years with a mean BMI of 25.7 ± 4.3; 60.3% were female (). Demographics and lifestyle characteristics of the eligible patients are shown by specific health state in . The average duration of follow up was 802 days; five of the 295 patients died during the follow up period, while 3% of patients entered a recurrent MDE. Common comorbid conditions at study entry included anxiety disorder (36.3%), stress (27.8%), alcoholism (18.0%) and sleep disorder (16.3%). There was little variation in demographics across the health states, although baseline comorbidities were more prevalent in patients who then progressed to second remission.
Table 2. Demographic details of patients with MDD during the management of TRD.
The mean (±SD) time from a diagnosis of MDD to enrolment (index date) was 62.1 ± 89.2 months, and just over half (51.3%) had experienced an MDE prior to enrolment. Patients who ultimately reached the recovery phase had the shortest gap between MDD diagnosis and index antidepressant therapy. Only a quarter (26.1%) of patients were employed at the index date, with 42.0% of patients unemployed or retired (). However, patients who ultimately achieved recovery during the follow-up period were more likely to be employed than at index (34.5%).
Healthcare resource utilization
An average of 0.29 ± 0.48 primary care visits per month (28 days) was recorded during the initial MDE (), consisting mainly of visits for depression (0.25 ± 0.45), primarily to a GP or nurse (0.31 ± 0.52 and 0.03 ± 0.18 visits, respectively). Patients made an average of 0.05 ± 0.13 suicide attempts during the initial MDE. More specialist visits were recorded for patients during the MDE state (0.36 ± 0.23) than either remission or recovery states (0.21 ± 0.22 and 0.21 ± 0.34, respectively) ().
Table 3. Mean (SD) number of events encountered in each health state per month (28 days) during the management of TRD.
An average of 0.13 ± 0.43 CRHTT monthly events was recorded in the MDE state, resulting in 1.70 ± 3.54 days of therapy (), substantially higher than in the remission or recovery states (0.09 ± 0.41 and 0.10 ± 0.65 days, respectively. The greatest number of hospitalizations was reported in the MDE state with an average of 1.40 ± 4.15 nights.
Pharmacological treatment
The most frequently prescribed pharmaceutical throughout the MDE state was mirtazapine (23.1%), while venlafaxine, quetiapine, aripiprazole and duloxetine were each prescribed in over 10% of patients (). A similar pattern was recorded in patients across all health states with generally greater use of pharmaceuticals in the MDE and remission states.
Table 4. Drugs prescribed to >5% of patients with TRD over a 2 year follow up period (%).
Non-pharmacological treatment
The use of non-pharmacological treatments overall was low, with occupational therapy, cognitive behavioural therapy (CBT) and counselling being the most frequently used, and the majority used during the initial MDE prior to remission ().
Table 5. Mean (SD) number of non-drug treatment sessions per month (28 days) in each health state during the management of TRD.
Monthly cost of healthcare resource utilization
When considering all combined HCRU per 28 days, the mean cost of patients in the MDE state was considerably higher (£992), compared with remission (£254) and recovery (£99) states (). Although this was driven by the higher frequency of resources used, several resources were particularly key in elevating the costs for patients in the MDE state (). The use of CRHTT was particularly high in patients in the MDE state ( and ) which, given the high unit cost of this resource, had a considerable impact on the PPPM cost for this group (£326 versus £18 in both remission and recovery). Further, the majority of hospitalizations occurred when patients were in the MDE health state, leading to an average PPPM cost of £380 versus £42 (remission) and £12 (recovery). Although the use of additional hospitalization resources was generally low, the cost associated with admission to the psychiatric ward for the MDE state was also higher (£17 versus £2 and £1 for the remission and recovery groups, respectively). Similarly, while admission to the intensive care unit (ICU) was rare, the only instances occurred in the MDE health state (£23 per 28 days).
Figure 2. Mean HCRU costs by health state (£) per patient per month (28 days) during the management of TRD.
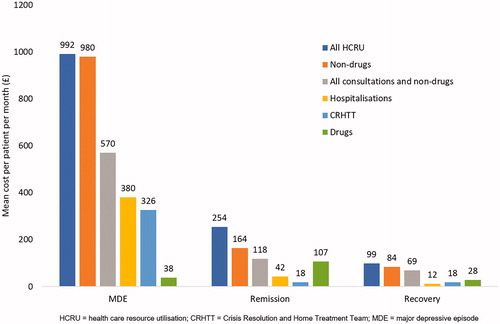
Table 6. Mean (SD) cost per patient per month (28 days) of non-drug treatment sessions in each health state during the management of TRD.
Similar to the increased CRHTT and hospitalization costs for patients in the MDE state, the frequency of consultation resulted in elevated costs for primary care and specialist visits (£52 and £11 per 28 days, respectively, exceeding the £31 and £7, respectively, for remission and £7 and £3 for recovery) highlighting the increased dependence on healthcare professionals for the more unstable patients in the MDE state.
Compared with other HCRU, the cost of drug treatment was relatively low () with the cost PPPM being highest for patients in remission (mean £107) and notably lower for patients in the MDE (£38) and recovery (£28) states. Overall, the total non-drug HCRU per 28 days is consistent in magnitude with the MDE state (£980) being considerably greater than the remission (£164) and recovery (£84) states ().
Discussion
Our study aimed to assess the HCRU and associated direct costs with patients with TRD from a single healthcare system perspective (UK NHS) in the real-world setting, not including the indirect costs of lost productivity. A systematic literature review of the economic impact of TRD found that TRD is defined inconsistently, which poses methodological challenges for between-study comparisonsCitation21. Despite this, results were relatively consistent across seven studies conducted in the US, with each study reporting a clear and consistent trend between increasing level of treatment resistance and increasing total medical costs. Overall, the annual costs for patients with TRD ranged from US$12,000 to US$19,000 (2019 GBP9400 to GBP14,900), exceeding those reported for non-TRD patientsCitation21.
In all studies, there were notable increases in total direct costs with increasing level of treatment resistance. Indeed, in a review of administrative claims data in the US, using a clinical staging algorithm to measure the cost impact of mild to complex TRD, the relationship between medical and drug costs and increasing treatment resistance was shown to be nearly linearCitation25.
A further study of commercially insured patients from a claims database in the US reported that the significant drivers of excess economic burden associated with TRD include healthcare payments related to ED visits, outpatient visits and number of prescriptionsCitation11. Among private payers, the mean annual direct healthcare costs per patient for the management of TRD (US$13,196; 2013 GBP8437) were higher than for management of treatment-responsive depression (US$7715; 2013 GBP4933)Citation14, while Feldman et al. reported that medical costs per patient-year for TRD ($16,786; 2013 GBP10,733) were markedly higher than general Medicare beneficiaries with managed depression ($11,044; 2013 GBP7061)Citation26.
The results of our study were consistent with these reports from other economies with an annual PPPM cost for patients with TRD of approximately GBP11,900. However, our study was novel in design in that it evaluated how the medical costs associated with TRD differ for three health states: MDE, remission and recovery. To allow for an accurate comparison across the health states, an analysis of the HCRU per 28 days was used to account for the varying follow-up period for all patients. The high costs associated with CRHTT and hospitalization for patients in MDE highlights the elevated resource utilization of patients in these more unstable health states, for which the more expensive resources, such as CRHTT and hospitalization, are required to manage the patient. The considerably lower HCRU costs for patients in remission and recovery demonstrate the reduction in resources and costs from stabilizing patients as rapidly as possible.
Similarly, across the majority of non-drug treatments for TRD, particularly occupational therapy and CBT, the costs reported for patients in MDE was greater. It should be noted, however, that the non-drug treatments were generally used infrequently across all patient groups, which may have been due to poor local availability. Thus, the average cost PPPM of all HCRU of patients in the MDE state (£991.82) was mainly driven by the consultations, non-drug treatment, hospitalizations and CRHTT, indicating the much higher HCRU impact in these patients, with a considerable reduction as patients moved into remission and subsequent recovery ().
It is perhaps unsurprising that the cost of drug interventions is low in patients with TRD. Patients in the MDE and recovery state of the disease (average £38 and £28 per 28 days, respectively) have a lower overall resource cost compared to stabilized patients in remission (£107). This difference in cost of drug interventions is relatively minimal and is far exceeded by the cost of all other resources for patients in MDE. This is an indication of the beneficial investment in effective treatment to quickly transition patients out of the MDE state into remission or recovery.
Strengths and limitations
The strength of conducting a primary data study to quantify HCRU is the ability to define accurately the health states of all patients, meaning that all resources captured during the follow-up window could be assigned to the health state that patients were experiencing at that point in time. This, therefore, allowed the study to be the first that assessed how the HCRU associated with TRD varies across different health states. Furthermore, primary data collection allows the collection of data specific to the research question and designed in a way that ensures that the outcomes of interest are captured.
Despite the strength of this design, one limitation is that it relies on the accuracy of physicians when completing each eCRF. To minimize the risk of collecting inaccurate data, the eCRFs were relatively short and user-friendly with electronic routing and logic applied to ensure no contradictions in responses and, where appropriate, physicians were provided the opportunity of entering “don’t know” if the information was not available. It should also be noted, due to the observational nature of this research, that the patients were not frequently monitored as they would be in a clinical trial, and their health states during the study period were retrospectively determined by their treating physicians based on the records of routine care, potentially limiting the precision of health state related analyses. However, this does in turn reflect the real-world practice of managing patients with TRD and the possible delays associated with detecting changes in health state.
Selection bias is another limitation with a primary data capture design, whereby the physicians surveyed represent a sample and may not be representative of the overall population of physicians treating patients with TRD. The current study, nevertheless, did involve a high number of physicians, working in different settings, across different geographical regions of the UK, thereby ensuring that the sample is likely to be representative of the overall population of physicians treating patients with TRD. Further, eligible patients were screened and selected by physicians, based on the consultation visits occurring retrospectively from the end of the patient selection date. It is acknowledged that patients who were visiting physicians more often are more likely to have been included in the study. To minimize this, physicians were recruited from a diverse geographical spread and mixed private/public practice where possible. The demographic data of the patient sample is similar to that found in other TRD studies, with the recent SUSTAIN-1 and TRANSFORM-2 studies both conducted in patients with a mean age of 46.3 years, although a slightly lower proportion of the sample in this study were female (60.3%) in comparison with the SUSTAIN-1 and TRANSFORM-2 trials, in which 66% and 62% (respectively) of patients were femaleCitation27–29.
Finally, it is acknowledged that the patient sample in this study is heterogeneous in nature and, as such, the distribution of the results is relatively wide. Moreover, whilst three quarters of the patients were unemployed or retired, it was considered beyond the scope of this study to capture the indirect costs of lost productivity or informal care. Despite these limitations, the key strength of this study is that it has captured the current clinical management of patients with TRD in the UK, and therefore represents the real-world HCRU impact associated with the condition.
Conclusion
While there is undoubtedly an HCRU cost associated with all patients with TRD, this study has demonstrated the substantial variation in cost between patients in different health states, with patients in remission and recovery, the more stable states of the disease, having a much lower cost associated with their disease management compared with patients in an MDE state. The high costs of certain resources, such as CRHTT and hospitalization, associated with MDE illustrate the need for a more rapid transition for patients to the more stable health states. Overall, across almost all HCRU measures, the frequency of usage and, accordingly, the total cost, was higher for patients in the MDE state, compared with the remission and recovery states. An antidepressant treatment that is more effective in reducing the duration in MDE health states and helping patients achieve remission and recovery more rapidly would be valuable in reducing the overall HCRU and costs in patients with TRD.
Transparency
Declaration of funding
This research was funded by Janssen, Titusville, NJ, USA.
Declaration of financial/other relationships
T.D. and T.M. have disclosed that they are current employees of Janssen, High Wycombe, UK. H.H.L. and Q.Z. have disclosed that they are current employees of Janssen, Titusville, NJ, USA and may own stock and/or stock options. J.W., T.B., O.R.-H. and C.M.-D. have disclosed that they are employees of Adelphi Real World, Bollington, UK and received funding from Janssen to conduct the study. No potential conflict of interest was reported by P.M. D.T. has disclosed that he has received research funding and speaking honoraria from Janssen, Angelini, Lundbeck and Sunovion.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Medical writing assistance was provided by K Ian Johnson, Harrogate House, Macclesfield, UK. This assistance was funded by Janssen, Titusville, NJ, USA.
References
- Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(1):3–7.
- McManus SBP, Jenkins R, Brugha T, editors. Mental health and wellbeing in England: adult psychiatric morbidity survey 2014. Leeds (UK): NHS Digital; 2016.
- Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90.
- NICE. National Institute for Health and Care Excellence: Clinical Guidelines. Depression in adults: recognition and management. London: National Institute for Health and Care Excellence (UK); 2009.
- Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459–525.
- Fornaro M, Fusco A, Anastasia A, et al. Brexpiprazole for treatment-resistant major depressive disorder. Expert Opin Pharmacother. 2019;20(16):1925–1933.
- Ruberto VL, Jha MK, Murrough JW. Pharmacological treatments for patients with treatment-resistant depression. Pharmaceuticals (Basel). 2020;13(6):116.
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366.
- Gartlehner G, Hansen RA, Morgan LC, et al. AHRQ comparative effectiveness reviews. second-generation antidepressants in the pharmacologic treatment of adult depression: an update of the 2007 comparative effectiveness review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011.
- EMA. Guideline on clinical investigation of medicinal products in the treatment of depression. European Medicines Agency; 2013.
- Sussman M, O’sullivan AK, Shah A, et al. Economic burden of treatment-resistant depression on the U.S. Health Care System. JMCP. 2019;25(7):823–835.
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. AJP. 2006;163(11):1905–1917.
- McCrone P, Rost F, Koeser L, et al. The economic cost of treatment-resistant depression in patients referred to a specialist service. J Ment Health. 2018;27(6):567–573.
- Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv. 2014;65(8):977–987.
- Claxton AJ, Li Z, McKendrick J. Selective serotonin reuptake inhibitor treatment in the UK: risk of relapse or recurrence of depression. Br J Psychiatry. 2000;177:163–168.
- Garcia-Toro M, Medina E, Galan JL, et al. Treatment patterns in major depressive disorder after an inadequate response to first-line antidepressant treatment. BMC Psychiatry. 2012;12:143.
- Sicras-Mainar A, Maurino J, Cordero L, et al. Assessment of pharmacological strategies for management of major depressive disorder and their costs after an inadequate response to first-line antidepressant treatment in primary care. Ann Gen Psychiatry. 2012;11(1):22.
- Jaffe DH, Rive B, Denee TR. The humanistic and economic burden of treatment-resistant depression in Europe: a cross-sectional study. BMC Psychiatry. 2019;19(1):247.
- Schultz J, Joish V. Costs associated with changes in antidepressant treatment in a managed care population with major depressive disorder. Psychiatr Serv. 2009;60(12):1604–1611.
- Sheffield RE, Lo Sasso AT, Young CH, et al. Selective serotonin reuptake inhibitor usage patterns as risk factors for hospitalization. Adm Policy Ment Health. 2002;30(2):121–139.
- Johnston KM, Powell LC, Anderson IM, et al. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195–210.
- NICE. British national formulary. London (UK): BMJ Group; 2019.
- Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52(1):46–54.
- Marsden J, White M, Annand F, et al. Medicines associated with dependence or withdrawal: a mixed-methods public health review and national database study in England. Lancet Psychiatry. 2019;6(11):935–950.
- Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370–377.
- Feldman RL, Dunner DL, Muller JS, et al. Medicare patient experience with vagus nerve stimulation for treatment-resistant depression. J Med Econ. 2013;16(1):62–74.
- Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893.
- Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22(10):616–630.
- Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–438.
- Curtis L, Burns A. Unit costs of health and social care 2017. Canterbury (UK): Personal Social Services Research Unit, University of Kent; 2017.
- McLoughlin D, Mogg A, Eranti S, et al. The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis. Health Technol Assess. 2007;11(24):1–54.
- Nightingale Hospital. Outpatient therapy fees. 2017. [cited 2018 Feb 20]. Available from: https://www.nightingalehospital.co.uk/wp-content/uploads/Nightingale-Hospital-Outpatient-Patients-Fees-2018.pdf.
- Curtis L. Unit costs of health and social care 2010. Canterbury (UK): Personal Social Services Research Unit, University of Kent; 2010.
- Kibble S, Gray D, Prat-Sala M, et al. Recovery coaching in an acute older people rehabilitation ward. BMJ Qual Improv Rep. 2014;3(1):u205646.w2316.
- Rayner L, Hotopf M, Petkova H, et al. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. Pain. 2016;157(7):1472–1479.
- Department of Health & Social Care. NHS reference costs 2016–2017. [cited 2018 Feb 20]. Available from: https://improvement.nhs.uk/documents/6467/201617_ReferenceCostData.zip.