Abstract
Objective
Depressive episodes and symptoms of bipolar I disorder are commonly misdiagnosed as major depressive disorder (MDD) in primary care. The novel and pragmatic Rapid Mood Screener (RMS) was developed to screen for manic symptoms and bipolar I disorder features (e.g. age of depression onset) to address this unmet clinical need.
Methods
A targeted literature search was conducted to select concepts thought to differentiate bipolar I from MDD and screener tool items were drafted. Items were tested and refined in cognitive debriefing interviews with individuals with self-reported bipolar I or MDD (n = 12). An observational study was conducted to evaluate predictive validity. Participants with clinical interview-confirmed bipolar I or MDD diagnoses (n = 139) completed a draft 10-item screening tool and other questionnaires. Data were analyzed to identify the smallest possible subset of items with optimized sensitivity and specificity.
Results
Adults with confirmed bipolar I (n = 67) or MDD (n = 72) participated in the observational study. Ten draft screening tool items were reduced to 6 final RMS items based on the item-level analysis. When 4 or more items of the RMS were endorsed (“yes”), sensitivity was 0.88 and specificity was 0.80; positive and negative predictive values were 0.80 and 0.88, respectively. These properties were an improvement over the Mood Disorder Questionnaire in the same analysis sample while using 60% fewer items.
Conclusion
The pragmatic 6-item RMS differentiates bipolar I disorder from MDD in patients with depressive symptoms, providing real-world guidance to primary care practitioners on whether a more comprehensive assessment for bipolar I disorder is warranted.
Introduction
Bipolar I disorder is a chronic and debilitating mental illness that is characterized by a mixture of manic, depressive, and subsyndromal symptoms [Citation1]. Although the presence or history of at least one fully syndromal manic episode is required for a diagnosis of bipolar I disorder [Citation2], depressive symptoms are the more common presentation. Delayed or missed diagnosis of bipolar disorder is exceedingly common; the delay between the onset of illness and diagnosis of bipolar disorder was reported to be 6–13 years [Citation3,Citation4]. In a large survey of individuals with bipolar disorder, 69% of respondents reported being initially misdiagnosed, with patients receiving an average of 3.5 misdiagnoses before the correct bipolar disorder diagnosis was made [Citation4]. Not surprisingly, unipolar depression was cited as the most common misdiagnosis (60%). As a matter of fact, 1 in 4 patients treated for major depressive disorder (MDD) may actually have bipolar disorder, which is a clinical concern given the importance of early intervention and appropriate treatment [Citation5,Citation6]. As timely and accurate diagnoses are precursors of good clinical outcomes, the high rate of missed bipolar disorder diagnosis is a critical unmet need, which provides an incentive for routine and systematic bipolar disorder screening of all patients who present with depressive symptoms [Citation7].
It has been reported that up to 40% of patients with bipolar disorder are treated exclusively in primary care [Citation8], suggesting that primary care providers, including nurse practitioners, are uniquely positioned to detect and diagnose the bipolar disorder early in the illness trajectory [Citation9]. In a survey of primary care providers, respondents reported that almost one-third of their patients were being treated for a mental health issue, with depression the most common psychiatric condition treated [Citation10]. Further, up to 10% of all visits to primary care are related to depression and as many as 64% of all clinical encounters for depression occur in primary care [Citation11]. In a study that reported on the consulting patterns of adults with a positive bipolar disorder screening result, the most frequently consulted specialty for bipolar disorder symptoms was primary care (41%); of note, primary care providers made an incorrect diagnosis or did not identify bipolar disorder 78% of the time [Citation12].
The importance of timely and accurate diagnosis of bipolar I disorder cannot be overstated. Untreated bipolar disorder may result in poor outcomes such as persistent symptoms, impaired functioning, recurrences, cognitive impairment, comorbidities, neurobiological changes, and increased suicidality, while early identification and prompt treatment have been associated with improved outcomes across symptomatic and functional domains [Citation13–15]. Although mania is the hallmark of a bipolar I diagnosis, there are challenges to quickly identifying prior manic symptoms. As such, screening for other clinical features that are more likely to be associated with bipolar I disorder than with MDD may help clinicians differentiate these diagnoses. For example, not only are clinical characteristics such as early age of depression onset, prior negative response to antidepressant treatment, and positive bipolar family history prognostic for bipolar I disorder, they are also likely to be collected in routine patient evaluation [Citation16]. Differentiating bipolar I disorder and MDD is a clinical priority as appropriate pharmacologic management differs depending on the disorder. Most importantly, if patients with bipolar I disorder are treated with a conventional antidepressant as monotherapy, they may be at risk for mood destabilization or treatment-emergent mania [Citation17].
Evidence of frequent misdiagnosis and diagnostic delay suggests that the use of a brief, clinically validated, patient-administered, and easy-to-use screening tool for bipolar I disorder would be a welcome addition to clinical practice. Although various bipolar disorder screening tools are available, there are impediments to their use in primary care settings, including length and reliance on screening for manic symptoms only. Given that office visits in primary care are usually very short, a bipolar I disorder screening tool should ideally have only a small number of easily understood items that can be completed and scored quickly so that results are immediately informative to the clinician and the patient. While screening for MDD appears to be commonplace, with 82% of healthcare providers reporting that they currently use an MDD screening tool, only 32% reported screening for bipolar disorder (data on file, AbbVie) even though historical and contemporary screening is recommended to rule out bipolar disorder before a diagnosis of MDD is made. For example, although most primary care nurse practitioners see patients with depressive symptoms, only half reported using a screening tool to assess for bipolar disorder; cited barriers to screening included uncertainty about when to screen, insufficient knowledge of bipolar disorder symptoms, lack of screening tools, and lack of time to screen during a visit [Citation18].
Using a bipolar I disorder screening tool should help clinicians triage those patients for whom further workup for bipolar I disorder may be required, thus providing better patient care more efficiently. The Rapid Mood Screener (RMS) for bipolar I disorder is a novel and pragmatic patient-reported screening tool that utilizes easily understood terminology to screen for manic symptoms and bipolar depression risk factors in less than 2 min during or outside of a clinical visit (e.g. online, via electronic medical record system, waiting room). Designed to help clinicians identify patients with bipolar I disorder, the RMS may be an efficient and accurate bipolar I disorder screening option that could easily be integrated into a busy primary care setting.
Methods
Qualitative evaluation
Concept selection and item development
The RMS was developed in a multistep process beginning with a targeted literature search to identify concepts that differentiate bipolar I disorder from MDD. A search of the literature was conducted to identify relevant articles cited in PubMed (1 March 2019); results were limited to English language articles published in the previous 10 years. Multiple combinations of terms related to bipolar disorder and MDD indications (e.g. bipolar, unipolar, depression, depressive) and diagnosis (e.g. differential diagnosis, diagnostic conversion) were searched. Reference lists from retrieved articles were manually searched for additional articles of interest. Existing bipolar disorder tools and diagnostic criteria were also reviewed.
A multidisciplinary group of 6 mental health and psychometric experts then participated in two modified Delphi consensus panels. During Panel 1 (pre-panel online activity and web conference [30 April 2019]), participants reviewed the identified concepts and ranked them in order of the ones that would best identify patients with a diagnosis of MDD who may instead have bipolar I disorder. During Panel 2 (pre-panel online activity and in-person or web conference [20 May 2019]) participants further refined the reduced concept list developed during Panel 1 proceedings and again ranked them by their potential to differentiate patients with bipolar I disorder from those with MDD. Patient-friendly item wording, and specific thresholds (e.g. age of depression onset, number of episodes) and variations (e.g. comorbidities, family history) were explored and determined for draft screening tool items. All decisions related to item reduction and selection were made based on a consensus from the majority of participants (i.e. at least 4 of 6). A pool of bipolar I disorder screening tool items were drafted for subsequent qualitative evaluation.
Cognitive interviews
Two rounds of in-depth qualitative cognitive debriefing interviews were conducted with adults who were identified from databases belonging to qualitative research firms. Participants had a self-reported clinician-provided diagnosis of bipolar I disorder or MDD; individuals with bipolar II disorder were excluded. Participant feedback was used to test and refine wording as needed to improve draft item clarity and interpretability. The qualitative interviews also provided further support for the content validity of the bipolar I disorder screening tool draft items.
Quantitative evaluation
To test the final set of draft screening tool items, a multisite, cross-sectional, observational study was conducted in adults with a clinically confirmed diagnosis of bipolar I disorder or MDD. The study protocol was approved by the institutional review board (IRB; RTI International IRB) and written informed consent was obtained from participants. Data collected from study participants were used to identify screening tool items that had the greatest potential to optimize the sensitivity and specificity of the bipolar I disorder screening tool being developed. Based on the totality of item permutations and numeric thresholds that were evaluated, the best combination of items was identified, with a goal of high accuracy while using the fewest possible number of items and maintaining high sensitivity and specificity.
Participants
Using a standardized recruitment form, medical recruiters selected potential study participants from databases belonging to qualitative research firms located in the United States (North Carolina, Texas, Colorado, Florida, and California); social network initiatives were also used to recruit participants as needed. In-person interviews were scheduled for people who were eligible and interested.
At the screening, participants had to be at least 18 years of age, with a self-reported clinician-provided diagnosis of bipolar I disorder or MDD, current or past depressive symptoms, and current use of an antidepressant, atypical antipsychotic, or mood-stabilizing medication. The diagnosis of bipolar disorder had to be specified as bipolar I disorder or a past manic episode of at least 7 days’ duration had to be reported. Participants were excluded if they had been hospitalized or admitted to the emergency room due to mental health issues in the past 30 days, or if they were currently experiencing a manic episode.
Study measures
For participants who met inclusion criteria at screening, the self-reported diagnosis was confirmed by a Mini-International Neuropsychiatric Interview (MINI) structured clinical interview [Citation19]. Two MINI modules were administered for diagnostic purposes (depressive disorder [Module A] and bipolar disorder [Module C]), and 2 modules were administered to rule out other diagnoses (any psychotic disorder [Module K] and medical, organic, or drug causes [Module O]). A MINI question related to repeated thoughts about death and suicide was not administered to minimize emotional risk to study participants and preempt discussion of the topic given the nonclinical study setting. The items of the draft bipolar I disorder screening tool were administered to participants; more clinical information was elicited via self-reported responses to additional questions that provided numeric variations (e.g. number of episodes, age of onset, prior number of antidepressants) and content permutations (e.g. comorbidities, family mental health history) for some draft items. Additional study measures included the 15-item Mood Disorder Questionnaire (MDQ) to screen for bipolar disorder [Citation20] and the 20-item Center for Epidemiologic Studies Depression Scale (CES-D), used to assess the severity of depressive symptoms over the prior week [Citation21].
MINI diagnostic interviews were conducted by 4 Ph.D. level clinicians, including a licensed clinical psychologist, who had participated in training activities to ensure rater accuracy and consensus; interviewers collaborated throughout the interview process to monitor rating agreement and address discrepancies. All other study measures were participant reported.
Statistical analysis
Planned evaluations were conducted using standard psychometric evaluation techniques. A sample size of 120 participants was selected to provide adequate precision. To obtain an analysis sample with approximately equal numbers of individuals with MDD and bipolar I disorder (∼60 each), recruitment quotas were established and adjusted based on clinical interview confirmation for each diagnosis throughout the recruitment process.
Descriptive statistics were tabulated and summarized by diagnostic subgroup for demographic and clinical characteristics, responses to the bipolar I disorder screening tool items, responses elicited from the additional questions, bipolar disorder classification based on the MDQ, and CES-D total score. Combinations of potential bipolar I disorder screening tool items (and numeric thresholds or item permutations where applicable) were reviewed collaboratively by the screening tool development team to optimize scoring and test characteristics for the final item set while using the fewest possible number of items. Prediction criteria for multiple sets of bipolar I disorder screening tool items with various thresholds elicited from the additional questions (e.g. number of depressive episodes, age of depression onset) were examined.
Item responses were summed (Yes = 1; No = 0); logistic regression was conducted for each set of items tested to provide the summed score that best separated bipolar I disorder from MDD. The score that maximized predictive ability was selected as the total number of items requiring endorsement. Because thresholds below and above those in the original items were tested, responses to the additional questions were used to inform comparisons. Analyses were performed using SAS version 9.3 or higher for Windows statistical software (SAS Institute Inc.; Cary, NC; 2012) in a nonvalidated personal computer environment.
Prediction criteria included sensitivity (the proportion of participants who were identified as having bipolar I disorder based on the structured clinical interview who screened positive for bipolar I disorder); specificity (proportion of participants who were identified as having MDD based on the structured clinical interview who screened negative for bipolar I disorder); positive predictive value (PPV; the proportion of participants in this sample who screened positive for bipolar I disorder who were identified as bipolar I disorder based on the structured clinical interview); and negative predictive value (NPV; the proportion of participants in this sample who screened negative for bipolar I disorder who were identified as MDD based on the structured clinical interview). The concordance index (C-index; equivalent to the area under the receiver operating characteristic curve) was calculated to assess overall screening tool performance and accuracy (the proportion of participants correctly identified as bipolar I disorder or MDD) was determined.
Results
From 85 articles identified via targeted literature search, 54 concepts that could differentiate bipolar I disorder from MDD were extracted. Clinical experts who participated in modified Delphi panel activities ranked and refined the concepts based on their capacity to discriminate between bipolar I disorder and MDD; 18 additional panelist-suggested concepts were included in the ranking process. During multiple rounds of iterative discussions and concept ranking, panelists reduced the initial 72-item list to 6 concepts that were considered to be the most discriminative: number of prior depressive episodes, comorbidities, age of onset, family history, treatment response, and manic symptoms. Mindful of using patient-friendly terminology, the screening tool development team drafted a pool of 11 screener items encompassing the 6 identified concepts for qualitative evaluation in cognitive debriefing interviews.
The initial draft items were tested and refined for clarity and comprehension across 2 rounds of debriefing interviews in participants with a self-reported diagnosis of bipolar I disorder or MDD (n = 6 for each round). Based on participant feedback, minor modifications were made to the draft items after each round of interviews. After round 2, the pool of 11 draft items was reduced to 10 items by combining 2 items that assessed 3 manic symptoms (unusually energetic, unusually happy, unusually outgoing) into 1 item (Item 8) to avoid redundancy; 10 draft screener items were retained for quantitative evaluation. Participants reported that all items were relevant, not redundant, and easy to interpret and understand.
Quantitative evaluation
Observational study participants
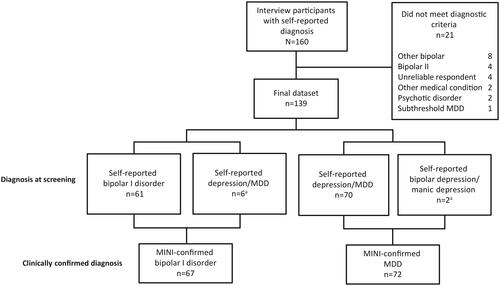
A total of 187 participants were recruited and scheduled for interview sessions; 27 (14.4%) people who were recruited missed their scheduled interview appointments and 160 interviews were conducted. Among the participants who were interviewed, 21 (13.1%) did not meet diagnostic criteria for either bipolar I disorder or MDD based on the MINI clinical interview and were not included in the analysis sample (). The final dataset included 139 participants with a confirmed diagnosis of bipolar I disorder or MDD based on the MINI; most MINI-confirmed diagnoses (n = 131) were consistent with the diagnosis reported by the participant at the screening.
Figure 1. Observational study participants. a6 patients with self-reported depression/MDD had a clinically confirmed diagnosis of bipolar I disorder; 2 patients with self-reported bipolar depression/manic depression had a clinically confirmed diagnosis of MDD. Abbreviations. MDD, major depressive disorder; MINI, Mini International Neuropsychiatric Interview.
The mean (SD) age of study participants was 46 (13) years; most were female and white (). Slightly more than one-quarter of the overall study population were experiencing a current depressive episode; all participants had current depressive symptoms, with mean CES-D scores suggesting mild-to-moderate severity in both diagnostic subgroups.
Table 1. Participant demographic and mood disorder characteristics.
Bipolar I disorder draft screener tool items
The 10 draft bipolar I disorder screening tool items and additional questions designed to evaluate various item permutations are presented in ; responses to the additional questions were used to inform comparisons among potential solutions. Numeric thresholds both above and below the corresponding threshold referenced in the draft screening tool were evaluated for 3 items (Items 1, 2, and 3). Multiple thresholds for each of these items were retained for consideration of potential item sets for the final screener tool.
Table 2. Final draft screener items with additional threshold values and concept variations for testing.
Final rapid mood screener items
After considering various permutations of item combinations, including different individual item thresholds where applicable, the screener development team selected the combination of items that would collectively provide an optimal balance of specificity and sensitivity for the final RMS while using the smallest possible number of items. Six final RMS items, 3 that screen for bipolar I disorder risk factors and 3 that screen for manic symptoms, were chosen (; the full version with instructions to healthcare providers is provided in English in Supplemental Material; for certain other languages (Chinese [Mandarin], Chinese [Cantonese], French, Spanish [Spain, USA, Mexico], Tagalog [Philippines], Vietnamese) please contact [email protected]). Based on responses to the additional question for draft Item 1, improved test characteristics were achieved when the numeric threshold was revised from “at least 3” to “at least 6” different periods of time feeling deeply depressed, and the higher threshold was selected for the final RMS item.
Table 3. Rapid Mood Screener final 6-item set.
The 6 final items of the RMS were summed to yield predictive criteria (). Since Items 1 and 2 were tested with varying thresholds, responses to additional questions were used to estimate final RMS test characteristics; test performance results were calculated in a sample of 122 participants with complete RMS information (17 participants had missing responses to an additional question). The C-index was 0.87 and a score of at least 4 maximized the predictive ability of the summary score. The highest estimated accuracy was observed with 4 or more “yes” responses; in this case, the sensitivity of the RMS for identifying participants with bipolar I disorder was 0.88 and the specificity was 0.80. Three or more “yes” responses also signaled a higher likelihood for bipolar I disorder than for MDD ().
Table 4. Rapid Mood Screener Test performance.
Rapid mood screener and mood disorder questionnaire: results in the same analysis population
Test characteristics for participants classified as bipolar disorder by the RMS and the MDQ are presented in . Estimated test characteristics for the RMS were better than those estimated for the MDQ in the study population comprising 122 participants with complete RMS information (). Predictive criteria for the MDQ in this modified participant sample were almost identical to criteria estimated in the full analysis sample (sensitivity = 0.87; specificity = 0.78).
Table 5. RMS and MDQ classification results.
Table 6. RMS and MDQ test characteristics.
Discussion
Screening for bipolar I disorder in patients with depression is an appropriate and judicious strategy to help primary care practitioners identify when a more comprehensive assessment for bipolar I disorder is warranted. The RMS was developed with the goal of creating a pragmatic and highly accurate tool that screens for bipolar I disorder using carefully constructed items with clear and precise wording. The 6 items of the RMS were selected based on multiple considerations, including clinical validity, optimized sensitivity and specificity, and pragmatism. Item permutations and numeric thresholds tested via the draft screener tool were evaluated to identify the combination of items that provided an optimal balance of test characteristics using the fewest possible number of items. To screen for characteristics that are associated with bipolar I disorder, the RMS asks about the number of prior depressive episodes (Item 1), the onset of depression before the age of 18 years (Item 2), and discontinuation of an antidepressant because it caused the respondent to feel highly irritable or hyper (Item 3). To screen for past manic symptoms, the RMS asks if there has ever been a week in which the respondent was more talkative than normal with racing thoughts (Item 4), felt unusually happy, outgoing, or energetic (Item 5), or needed much less sleep than usual (Item 6). Based on qualitative feedback, all items were precisely worded to provide easy interpretation and clarity so respondents could readily identify events that may indicate past manic characteristics. Since symptoms of bipolar I disorder may first present early in life, no timeframe restrictions were used, with the exception of the question designed to establish the early onset of depression (i.e. before 18 years), which is characteristic of bipolar I disorder. While an early life (childhood) mood episode might generate an affirmative RMS response that is not related to bipolar I disorder, RMS questions are designed to create an opportunity for comprehensive dialogue. Although endorsing 4 or more items yielded the best test characteristics signaling a very high likelihood of bipolar disorder, it should be noted that 3 “yes” answers also signaled a higher likelihood for bipolar disorder than MDD. A positive screen would be followed up with a more comprehensive diagnostic evaluation. Based on the small number of items and easily interpreted wording, the RMS is estimated to take less than 2 minutes to complete by a patient.
As many patients with bipolar disorder are treated in primary care settings [Citation8] and upwards of half of these patients present with depressive symptoms [Citation16], it is imperative that clinicians have the knowledge and tools to differentiate bipolar I depression from MDD. Misdiagnosis of bipolar I disorders as MDD is especially concerning given the potential for inappropriate treatment with antidepressant monotherapy and the long attendant delay in receiving appropriate treatment. A longer duration of untreated bipolar illness has been associated with worse outcomes, including a greater frequency of episodes and increased risk of hospitalization and suicidality, further supporting the clinical relevance of timely diagnosis and treatment with an approved pharmacological agent [Citation13–15]. The urgent need for timely and accurate diagnosis is further highlighted by findings that multiple mood episodes and longer duration illness are associated with less effective neuroprotective mechanisms and more apparent harmful biological changes [Citation14]; for example, it has been shown that the incidence of manic episodes was related to volume decrease in frontal brain regions [Citation22]. Since identifying prior manic symptoms is challenging and may require robust dialogue to help patients identify past mood elevation, using the RMS, a screening tool with well-crafted and precisely worded manic symptom items, as well as items that characterize other bipolar I disorder features, may be a distinct clinical advantage for identifying bipolar I disorder in patients with depressive symptoms.
Although other self-reported bipolar disorder screening tools are available, reliance on screening for manic symptoms only or length may be obstacles to use in the clinic. For example, the MDQ, which was also administered in the RMS observational study, consists of 15 total items, and screens for manic symptoms, manic symptoms clusters, and level of impairment to identify bipolar I or II disorder. For a positive screen, 7 of 13 MDQ manic symptom items must be endorsed, and in 2 additional items, patients must affirm that several of these symptoms have occurred during the same time period and caused at least moderate impairment. In contrast, the RMS consists of 6 items and requires an endorsement of 4 or more of the items to identify patients who may potentially have bipolar I disorder. Other available self-report bipolar screening tools of note include the 48-item Hypomanic Personality Scale (HPS) [Citation23], the 32-item Mania/Hypomanic Checklist (HCL-32) [Citation24], the Bipolar Spectrum Diagnostic Scale (BSDS) [Citation25], and the Mood Spectrum Self-Report (MOODS-SR) [Citation26]. Obvious disadvantages that may limit the usefulness of the HPS and the HCL-32 in clinical practice include length and reliance on hypomanic/manic symptoms. Further, although the BSDS (19 items in paragraph form) and MOODS-SR (161 items) include depressive symptoms, they too are long and potentially more complicated than a simple checklist because of their scoring and formats. While the General Behavior Inventory (GBI) also screens for both depressive and manic symptoms [Citation27–29] and its psychometric properties have been estimated in clinical and non-clinical samples [Citation27,Citation30,Citation31], its utility is also restricted by length (52–73 items) and multiple versions that provide inconsistent cut-off scores for positive screening, which limits its generalizability.
Since few studies provide direct comparisons of screener tools administered in the same analysis population, RMS and MDQ test properties estimated in the observational study are of particular interest. When 4 or more RMS items were endorsed, indicating a positive screen for bipolar I disorder, the C-index was 0.87, sensitivity was 0.88, and specificity was 0.80. By comparison, when the MDQ screened positive for bipolar disorder in the same study sample, the C-index was 0.82, sensitivity was 0.86, and specificity was 0.78. In addition to these test characteristics, the RMS may also be more convenient than the MDQ in a clinical practice setting. Namely, the RMS has less than half the number of items than the MDQ, it screens for both bipolar I disorder features and manic symptoms, it uses a simpler scoring algorithm, and it is estimated to take less time to complete than the commonly cited 5 minute completion time for the MDQ [Citation32–34]. Further, the RMS is precisely worded to better differentiate manic symptoms, which is an important consideration since it may be difficult to elicit confirmation of a prior manic episode from a patient with bipolar I disorder.
The ultimate value of a bipolar I disorder screening tool depends on a clinician’s willingness to employ it in their practice. In a survey of 200 HCPs who were asked about bipolar disorder screening tools (data on file, AbbVie), 85% of respondents indicated that although they were familiar with the MDQ, only 29% currently use it. Most HCPs (81%) reported that they would be more likely to use the RMS than the MDQ, with the RMS significantly outperforming the MDQ across the most valued bipolar disorder screening tool attributes (e.g. easily answered questions, practical to use, easy scoring, the minimal number of questions). Further, over two-thirds of respondents thought that the RMS was better than other screening tools and 84% believed that the new RMS would have a positive impact on their practice. Of particular clinical relevance, almost half of primary care providers indicated that they would screen a greater percentage of their patients because of the RMS and 76% stated that they would be likely to use it to screen new patients with depressive symptoms. Overall, survey responses indicated that the RMS would be a valuable addition to clinical practice settings, with a majority of primary care providers indicating that this pragmatic and patient-friendly tool would encourage them to screen more patients with depressive symptoms.
Limitations of this study include its reliance on participants recruited from qualitative research facilities, which may limit generalizability to real-world samples. Additionally, since the RMS was designed to identify bipolar I disorder among patients who may have been misdiagnosed or initially thought to have MDD, participants with bipolar II disorder were not included in the study; the RMS is not validated in bipolar II/hypomania. As is the case with any new screening tool, additional evaluation of RMS test characteristics is warranted. Of particular relevance, sensitivity and specificity for Item 1 (the number of previous episodes of depression) and Item 2 (age of depression onset) were estimated using responses to additional questions, with these data used to inform the selection of the final item set. Further, since Item 1 was modified from the version administered to participants during the quantitative evaluation, it has not been administered in its final form. Although the MINI item related to suicidal ideation or thoughts about death was omitted during the quantitative evaluation, participants with a current or past depressive episode met the required criteria for MDD (depressed mood or anhedonia) and had a sufficient number of other criteria (appetite/weight change, sleep change, psychomotor agitation/retardation, fatigue, guilt/worthlessness, cognitive impairment) so that no diagnoses were reliant on the excluded criteria. Additionally, all participants reported that they were on medication to treat MDD or bipolar disorder, which may limit the ability to generalize the results to nonmedicated patients. Future validation studies in larger clinical samples, racial/ethnic groups, and other settings (e.g. inpatient, primary care offices, community mental health centers, phone/telemedicine administration) are warranted.
The RMS is a practical new bipolar I disorder screening tool that was developed to help clinicians rapidly identify patients with depressive symptoms who may have bipolar I disorder instead of MDD. Designed with the objective of combining pragmatism and accuracy, the RMS uses a small number of succinctly worded patient-friendly items to minimize respondent burden and maximize the potential for use in a busy clinical setting. When compared with the MDQ in the same analysis sample, the RMS had better test characteristics while using 60% fewer items. To the authors’ knowledge, no prior study evaluating a bipolar disorder screening tool has yielded better collective specificity and sensitivity than the values estimated for the RMS in this study. Screening with the RMS is a practical and efficient new way to help address the all too common problem of misdiagnosing bipolar I disorder as MDD in patients with depressive symptoms. Although a screening tool does not provide a diagnosis, patients who score 4 or more on the brief self-report RMS questionnaire have a strong probability of bipolar I, thus alerting primary care physicians that more thorough evaluation is needed.
Transparency
Declaration of funding
This manuscript was supported by funding from Allergan (prior to its acquisition by AbbVie). The authors had full control of the content and approved the final version.
Declaration of financial/other relationships
R.S. McIntyre has received research grant support from Lundbeck, Shire, Otsuka, National Institute of Mental Health, Canadian Institutes for Health Research, The Brain and Behavior Research Foundation, and the Chinese National Natural Research Foundation. He has also received speaker/consultant fees from Lundbeck, Pfizer, Janssen, Sunovion, Bausch Health, Intra-Cellular, Takeda, Otsuka, Allergan (now AbbVie), Purdue, Minerva, and Neurocrine. Dr. McIntyre is also CEO and shareholder of Champignon Brands.
P.S. Masand has received research grant support from Allergan (now AbbVie). He has received speaker/consultant fees from Acadia, Allergan (now AbbVie), Intra-Cellular Therapies, Lundbeck, Sunovion, and Takeda. He is also a shareholder of Centers of Psychiatric Excellence (COPE), and Global Medical Education.
M.D. Patel, A. Harrington, and P. Gillard are employees and shareholders of AbbVie.
S.L. McElroy has been a consultant to or member of the scientific advisory board of Allergan (now AbbVie), Avanir, Bracket (now Signant Health), F. Hoffmann-La Roche Ltd., Idorsia, Mitsubishi Tanabe Pharma Corporation, Myriad, Naurex, Novo Nordisk, Otsuka, Shire, Sunovion, and Takeda Pharmaceutical Company Limited (Shire). She has also been a principal or co-investigator on studies sponsored and/or funded by Alkermes, Allergan (now AbbVie), Avanir, Azevan Pharmaceuticals, Forest, Jazz, Marriott Foundation, Medibio, Myriad, National Institute of Mental Health, Naurex, Neurocrine, Novo Nordisk, Otsuka, Shire, Sunovion, and Takeda Pharmaceutical Company Limited (Shire). She is also an inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and along with the patent’s assignee, the University of Cincinnati, Cincinnati, Ohio, has received payments from Johnson & Johnson, which has exclusive rights under the patent.
K. Sullivan has received consultant and speaker honoraria from Allergan (now AbbVie).
C.B. Montano has received research funding from Arbor, Avanir, Axsome, Sanofi, Sunovion, and Tonix. Additionally, he has received consultant and speaker honoraria from Allergan (now AbbVie), Arbor, Neos, Rhodes, Shire, and Takeda.
T.M. Brown and L. Nelson are employees of RTI-HS.
R. Jain has served as a consultant to Addrenex, Allergan (now AbbVie), Avanir, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shionogi, Shire, Sunovion, Supernus, Takeda, and Teva; paid speaker for Addrenex, Alkermes, Allergan (now AbbVie), Lilly, Lundbeck, Merck, Neos Therapeutics, Otsuka, Pamlab, Pfizer, Rhodes, Shionogi, Shire, Sunovion, Takeda, and Tris Pharmaceuticals; received research support from Allergan (now AbbVie), AstraZeneca, Lilly, Lundbeck, Otsuka, Pfizer, Shire, and Takeda; and served on advisory board for Addrenex, Alkermes, Avanir, Forum, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shionogi, Shire, Sunovion, Supernus, Takeda, and Teva.
Author contributions
All authors participated in the writing, editing, and critical revision for intellectual content, and approval of the final version of this manuscript. All authors met ICMJE authorship criteria and agree to be accountable for all aspects of the work. Neither honoraria nor payments were made for authorship.
Reviewer disclosure
A reviewer on this manuscript has disclosed that they have received manuscript or speaker’s fees from Astellas, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Kyowa Yakuhin, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Novartis, Otsuka Pharmaceutical, Shionogi, Shire, Tsumura, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Eisai, Mochida Pharmaceutical, Meiji Seika Pharma and Shionogi. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download PDF (87.2 KB)Acknowledgements
Writing and editorial assistance was provided to the authors by Carol Brown, MS, of Prescott Medical Communications Group (Chicago, IL), a contractor of AbbVie. Project development assistance was provided by Sheri Fehnel, Ph.D., of RTI-HS. Statistical assistance was provided by Nicole Williams of RTI-HS.
Data availability statement
AbbVie is committed to responsible data sharing. This includes access to anonymized, individual and/or study-level data (analysis data sets), as well as other information (e.g. protocols and Study Reports), as long as the studies are not part of an ongoing or planned regulatory submission and consistent with the informed consent conditions for the study. This includes requests for clinical trial data for unlicensed products and indications. Study data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). For more information on the process, or to submit a request, contact the authors.
References
- McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993–2005.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th Ed. Arlington (VA): American Psychiatric Association; 2013.
- Dagani J, Signorini G, Nielssen O, et al. Meta-analysis of the interval between the onset and management of bipolar disorder. Can J Psychiatry. 2017;62(4):247–258.
- Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64(2):161–174.
- Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord. 2014;169(1):S12–S16.
- Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233–239.
- McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorder: clinical challenges, new research findings, and treatment opportunities. Lancet. 2020;396(10265):1841–1856.
- Kilbourne AM, Goodrich DE, O’Donnell AN, et al. Integrating bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14(6):687–695.
- Rolin D, Whelan J, Montano CB. Is it depression or is it bipolar depression? J Am Assoc Nurse Pract in Press. 2020;32(10):703–713.
- Abed Faghri NM, Boisvert CM, Faghri S. Understanding the expanding role of primary care physicians (PCPs) to primary psychiatric care physicians (PPCPs): enhancing the assessment and treatment of psychiatric conditions. Ment Health Fam Med. 2010;7(1):17–25.
- Unutzer J, Park M. Strategies to improve the management of depression in primary care. Prim Care. 2012;39(2):415–431.
- Frye MA, Calabrese JR, Reed ML, et al. Use of health care services among persons who screen positive for bipolar disorder. Psychiatr Serv. 2005;56(12):1529–1533.
- Joyce K, Thompson A, Marwaha S. Is treatment for bipolar disorder more effective earlier in illness course? A comprehensive literature review. Int J Bipolar Disord. 2016;4(1):19.
- Vieta E, Reinares M, Rosa AR. Staging bipolar disorder. Neurotox Res. 2011;19(2):279–285.
- Altamura AC, Dell’Osso B, Berlin HA, et al. Duration of untreated illness and suicide in bipolar disorder: a naturalistic study. Eur Arch Psychiatry Clin Neurosci. 2010;260(5):385–391.
- Mitchell PB, Goodwin GM, Johnson GF, et al. Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord. 2008;10(1):144–152.
- Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant-emergent mania in bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2018;20(3):195–227.
- Kriebel-Gasparro AM. Advanced practice registered nurses: gateway to screening for bipolar disorder in primary care. Open Nurs J. 2016;10:59–72.
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33.
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873–1875.
- Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Applied Psychol Meas. 1977;1(3):385–401.
- Abe C, Ekman CJ, Sellgren C, et al. Manic episodes are related to changes in frontal cortex: a longitudinal neuroimaging study of bipolar disorder 1. Brain. 2015;138(11):3440–3448.
- Eckblad M, Chapman LJ. Development and validation of a scale for hypomanic personality. J Abnorm Psychol. 1986;95(3):214–222.
- Angst J, Adolfsson R, Benazzi F, et al. The HCL-32: towards a self-assessment tool for hypomanic symptoms in outpatients. J Affect Disord. 2005;88(2):217–233.
- Ghaemi NS, Miller CJ, Berv DA, et al. Sensitivity and specificity of a new bipolar spectrum diagnostic scale. J Affect Disord. 2005;84(2–3):273–277.
- Dell’Osso L, Armani A, Rucci P, et al. Measuring mood spectrum: comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Compr Psychiatry. 2002;43(1):69–73.
- Depue RA, Klein DN. Relatives at risk for mental disorder. New York (NY): Raven Press.
- Depue RA, Slater JF, Wolfstetter-Kausch H, et al. A behavioral paradigm for identifying persons at risk for bipolar depressive disorder: a conceptual framework and five validation studies. J Abnorm Psychol. 1981;90(5):381–437.
- Mallon JC, Klein DN, Bornstein RF, et al. Discriminant validity of the General Behavior Inventory: an outpatient study. J Pers Assess. 1986;50(4):568–577.
- Depue RA, Krauss S, Spoont MR, Arbisi P. General behavior inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. J Abnorm Psychol. 1989;98(2):117–126.
- Klein DN, Dickstein S, Taylor EB, et al. Identifying chronic affective disorders in outpatients: validation of the General Behavior Inventory. J Consult Clin Psychol. 1989;57(1):106–111.
- CEDC. The California Evidence-Based Clearinghouse for Child Welfare website [Internet]. The Mood Disorder Questionnaire (MDQ). 2015 [Cited 2020 July 8]. Available from: https://www.cebc4cw.org/assessment-tool/the-mood-disorder-questionnaire/
- Psych Tools. Psych Tools website [Internet]. Mood Disorder Questionnaire (MDQ). 2018 [cited 2020 July 8]. Available from: https://www.psychtools.info/mdq/
- Psychiatry & Behavioral Health Learning Network. Psychiatry & Behavioral Health Learning Network website [Internet]. Mood Disorder Questionnaire (MDQ). 2020 [cited 2020 July 8]. Available from: https://www.psychcongress.com/saundras-corner/scales-screeners/bipolar-disorder/mood-disorder-questionnaire-mdq
