Abstract
Background
Relapsing–remitting multiple sclerosis (RRMS) patients with high disease activity (HDA) experience more severe disease than those without HDA. This analysis describes the efficacy of cladribine tablets 3.5 mg/kg in HDA patient subgroups that were either treated with disease-modifying drugs (DMDs) prior to study entry or were treatment naïve.
Methods
Post hoc analysis of the 96 week Cladribine Tablets Treating Multiple Sclerosis Orally (CLARITY) study compared cladribine tablets 3.5 mg/kg to placebo in subgroups of patients meeting the high relapse activity plus disease activity on treatment definition of HDA. Patients were categorized into either prior DMD treatment or DMD treatment-naïve subgroups. Endpoints included annualized relapse rate (ARR), time to first relapse, time to disability progression and magnetic resonance imaging (MRI) outcomes. No inferential statistical analyses were conducted between subgroups.
Results
The DMD-naïve cohort (n = 187) was larger than the prior-DMD cohort (n = 102). In both the DMD-naïve and prior-DMD cohorts, cladribine tablets were associated with a reduction in ARR (rate ratio [RR]: 0.26; 95% confidence interval [CI]: 0.16–0.42; p < .0001 and RR: 0.55; 95% CI: 0.32–0.95; p = .0324, respectively). In both subgroups, cladribine tablets increased the time to relapse versus placebo (hazard ratio [HR]: 0.36; 95% CI: 0.21–0.62; p = .0002 for DMD-naïve cohort and HR: 0.50; 95% CI: 0.24–1.02; p = .0557 for prior-DMD cohort). Significant differences were observed for all assessed disability and MRI outcomes independently of previous treatment.
Conclusion
Post hoc evidence suggests consistent treatment benefits of cladribine tablets 3.5 mg/kg during the 96 week CLARITY study among HDA-RRMS patients who were either previously treated with DMDs or were treatment naïve.
Introduction
Relapsing–remitting multiple sclerosis (RRMS) patients with high disease activity (HDA) experience a more severe disease course marked by rapidly developing lesion load, more frequent relapses and significant increases in physical disabilityCitation1–3. A recent real-world study from Germany suggests that the incidence of HDA is quite high overall (8.5% of all RRMS patients), particularly in the youngest individuals who will have to live with the disease the longest (e.g. 30.3% of those patients aged 19 years or younger)Citation4. While there are a number of currently licensed disease-modifying drugs (DMDs) available that have been shown to help control RRMS and delay disability progression, there are fewer DMDs for patients with HDA-RRMSCitation2,Citation5–7. However, early treatment of HDA-RRMS patients with highly efficacious DMDs is of particular importance given that these patients are at significantly greater risk for accumulating permanent neurologic damageCitation8.
One therapeutic option is cladribine tablets 10 mg (3.5 mg/kg cumulative dose over two years, henceforth referred to as cladribine tablets 3.5 mg/kg; Mavencladi), approved in 2017 by the European Medicines Agency (EMA) for the treatment of adults with highly active relapsing multiple sclerosis (RMS) as defined by clinical or imaging featuresCitation9. The efficacy and safety of cladribine tablets in patients with RRMS were originally established in the pivotal phase III CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) studyCitation10,Citation11. Compared with placebo, patients receiving cladribine tablets 3.5 mg/kg experienced significant and clinically meaningful improvements in annualized relapse rate (ARR) and disability progressionCitation10. Furthermore, in a CLARITY study subgroup analysis, HDA-RRMS patients who received cladribine tablets experienced clinical and magnetic resonance imaging (MRI) responses comparable to or greater than the overall CLARITY study populationCitation12. This subgroup of HDA-RRMS patients included those previously treated with DMDs as well as treatment-naïve patients. In addition, a network meta-analysis suggested that cladribine tablets are a comparatively effective and safe alternative to other highly efficacious DMDs in patients with active RRMS as well as a HDA subpopulationCitation13.
Given the irreversible nature of RRMS in general and HDA in particular, a greater understanding of the early switching of patients to another highly effective DMD should be of paramount importance. In fact, recent real-world data reported that HDA patients identified by relapse switched to another DMD much later (over a year) and experienced significantly more disease progression than HDA patients identified by the presence of new lesionsCitation1. Additional analyses of HDA cohorts are of interest to fully characterize these patients’ response to cladribine tablets. Consequently, the objective of the CLARITY study post hoc analysis presented in this paper is to describe the efficacy of cladribine tablets 3.5 mg/kg in HDA patient subgroups that were either treated with DMDs prior to study entry or were treatment naïve. This study expands upon previous post hoc work by Giovannoni and colleagues that described the efficacy of cladribine tablets 3.5 mg/kg in two subgroups of HDA collapsed across patients that were previous treated with a DMD or notCitation12.
Methods
The CLARITY study compared low-dose cladribine (3.5 mg/kg), high-dose cladribine (5.25 mg/kg) and placebo over a 96 week periodCitation10. Each course of cladribine tablets consisted of two treatment weeks per 48 week period, administered during the beginning of the first month and the beginning of the second month of the respective treatment year. Eligible patients aged 18–65 years had a diagnosis of RRMS according to the 2005 McDonald criteriaCitation14, including at least one relapse in the last 12 months before study entry (but no relapses in the 28 days before entry), neurological lesions detectable by MRI consistent with MS, and an Expanded Disability Status Scale (EDSS) score between 0 and 5.5. A total of 1326 patients were randomized (1:1:1) with 437 patients receiving placebo, 433 patients receiving low-dose cladribine tablets and 456 patients receiving high-dose cladribine tabletsCitation10. A full description of the CLARITY study methodology, including outcomes, has been published previouslyCitation10. CLARITY was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines in accordance with the International Conference of HarmonizationCitation15.
Previous analyses of CLARITY study data have examined patient outcomes associated with cladribine tablets that were based on alternative HDA definitions using relapse activity and MRI lesion criteriaCitation12. The current analysis focused on patients randomized to cladribine tablets 3.5 mg/kg (the approved dose) or placebo using the high relapse activity plus disease activity on treatment (HRA + DAT) definition of HDACitation12. This definition includes patients with ≥2 relapses during the year prior to study entry, whether on DMD treatment or not, plus patients with ≥1 relapse during the year prior to study entry while on therapy with other DMDs and ≥1 T1 Gd + or ≥9 T2 lesions. Patients meeting HRA + DAT criteria were retrospectively categorized into either a prior DMD treatment subgroup (that had used DMDs at any time prior to study entry) or a DMD-treatment-naïve subgroup.
Cladribine tablets 3.5 mg/kg were compared to placebo within each of the HRA + DAT subgroups – DMD-naïve or prior-DMD. Outcomes of interest included ARR, time to first qualifying relapse, and time to 3 month and 6 month confirmed EDSS progression (3mCDP; 6mCDP). Data on MRI outcomes were also analyzed.
All analyses were based on the intention-to-treat (ITT) population and were post hoc in nature. As such, no multiplicity adjustments were done to the resulting p values; comparisons between the placebo and cladribine tablets 3.5 mg/kg arms for which the p value was less than .05 were considered nominally significant. The statistical models used in the analyses of the efficacy endpoints depended on the endpoint type. ARR was analyzed by Poisson regression models with the log of time on study as an offset variable. Time to first qualifying relapse and time to EDSS progression were analyzed by Cox proportional hazards models and Kaplan–Meier estimates of the proportions of patients with events at 96 weeks. MRI lesion counts (new T1 Gd + lesions, active T2 lesions, combined unique lesions and new T1 hypointense lesions) were analyzed by negative binomial regression models fitted to the cumulative numbers of lesions. Each model was adjusted for the respective baseline number of lesions and had the log number of MRI scans as an offset variable. No inferential statistical analyses were conducted between subgroups; results are presented as descriptive only.
Results
Demographics
Baseline demographics for those patients who met the HRA + DAT criteria are shown in . Additional information about the study population can be found in Giovanni et al.Citation10. The DMD-naïve cohort (n = 187) was larger than the prior-DMD cohort (n = 102). Patient demographics at baseline were generally similar between placebo and cladribine tablets 3.5 mg/kg within the two subgroups, although some numeric differences were apparent (e.g. in the DMD-naïve cohort, there was a higher proportion of placebo-treated males compared to the prior-DMD cohort).
Table 1. Baseline demographics of HRA + DAT patients by DMD-naïve and prior-DMD subgroups.
Relapse outcomes
The estimated mean number of qualifying relapses over the 96 week period was lower for patients who received cladribine tablets 3.5 mg/kg compared to placebo-treated patients in both HRA + DAT subgroups (see ). In the DMD-naïve cohort, cladribine tablets were favoured with a 74% reduction in ARR (rate ratio [RR]: 0.26; 95% confidence interval [CI]: 0.16–0.42; p < .0001). In the prior-DMD cohort, cladribine tablets were also favoured with a 45% reduction in ARR (RR: 0.55; 95% CI: 0.32–0.95; p = .0324).
Table 2. Relapse outcomes over the 96 week period for HRA + DAT patients by DMD-naïve and prior-DMD subgroups.
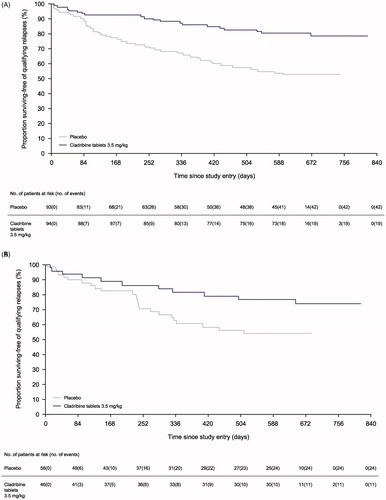
Time to first qualifying relapse in the HRA + DAT patient subgroups are shown in and . In both subgroups, cladribine tablets 3.5 mg/kg had a lower percentile of relapse at 96 weeks compared to placebo (hazard ratio [HR]: 0.36; 95% CI: 0.21–0.62; p = .0002 for the DMD-naïve cohort and HR: 0.50; 95% CI: 0.24–1.02; p = .0557 for the prior-DMD cohort).
Disability progression outcomes
Compared to placebo, cladribine tablets 3.5 mg/kg reduced the risk of 3mCDP by 71% in the DMD-naïve cohort (HR: 0.29; 95% CI: 0.14–0.63; p = .0016) and by 75% in the prior-DMD cohort (HR: 0.25; 95% CI: 0.07–0.89; p = .0322) (see ). A similar risk reduction was observed in time to 6mCDP for both HRA + DAT subgroups: cladribine tablets reduced the risk of 6mCDP by 83% in the DMD-naïve cohort (HR: 0.17; 95% CI: 0.06–0.51; p = .0015) and by 80% in the prior-DMD cohort (HR: 0.20; 95% CI: 0.04–0.91; p = .0367).
Table 3. Disability progression outcomes for HRA + DAT patients by DMD-naïve and prior-DMD subgroups.
MRI outcomes
contains a summary of MRI outcomes over the 96 week period, including number of new T1 Gd + lesions, active T2 lesions, combined unique lesions and new T1 hypointense lesions, by HRA + DAT patient subgroup. Additional safety information is reported by Leist and colleaguesCitation16. The estimated mean number of new T1 Gd + lesions per scan for patients treated with cladribine tablets 3.5 mg/kg was low in both HRA + DAT subgroups. In the DMD-naïve cohort, cladribine tablets were favoured with an 89% reduction in new T1 Gd + lesions (RR: 0.11; 95% CI: 0.06–0.20; p < .0001); in the prior-DMD cohort, cladribine tablets were also favoured with a 93% reduction in new T1 Gd + lesions (RR: 0.07; 95% CI: 0.03–0.16; p < .0001). Similar results were reported for all other MRI outcomes by DMD-naïve and prior-DMD cohorts: estimated mean number of active T2 lesions per scan (RR: 0.22; 95% CI: 0.14–0.34; p < .0001 and RR: 0.24; 95% CI: 0.12–0.48; p < .0001, respectively), estimated mean number of combined unique lesions per scan (RR: 0.19; 95% CI: 0.12–0.31; p < .0001 and RR: 0.22; 95% CI: 0.13–0.39; p < .0001, respectively) and estimated mean number of new T1 hypointense lesions per scan (RR: 0.21; 95% CI: 0.12–0.35; p < .0001 and RR: 0.13; 95% CI: 0.05–0.32; p < .0001, respectively).
Table 4. MRI outcomes over the 96 week period for HRA + DAT patients by DMD-naïve and prior-DMD subgroups.
Discussion
Although there is no cure for RRMS, treatment with approved DMDs can alter the course of the disease by reducing the rate of relapses and delaying disease progression. Current disease activity, in both previously DMD treated and DMD treatment-naïve patients, can be predictive of future disease worsening and poor long-term clinical outcomes; however, there are few available therapeutic options for patients with highly active diseaseCitation17–19. Cladribine tablets 3.5 mg/kg received a marketing authorization from the EU in 2017 for use in adults with highly active RMS as defined by clinical or imaging features. Cladribine tablets are orally administered and require only two courses 12 months apart, offering an advantage to patients in terms of adherence and complianceCitation20.
Previous post hoc analyses of CLARITY study data based on alternative HDA definitions have examined efficacy and safety associated with cladribine tablets 3.5 mg/kgCitation12. Across relapse and disability outcomes, the effect of cladribine tablets compared to placebo was larger across each HDA cohort compared to the respective non-HDA cohort, supporting a trend for a greater benefit of cladribine in HDA patientsCitation12. The new post hoc analysis of CLARITY study data presented in this paper focused on the HRA + DAT definition of HDA, with these patients then categorized into either prior DMD treatment or DMD treatment-naïve subgroups. The HRA + DAT criterion is based on both relapse rate, which can identify patients with higher clinical disease activity, and a combination of relapse rate and poor response to treatment as assessed by MRI activity; this is consistent with the EMA’s definition of patients with HDACitation21. Making a choice about next treatment in the case of inadequate response to prior DMD treatment is of particular interest as the efficacy of subsequent treatment is often suboptimal, yet delaying treatment may result in accumulated permanent disabilityCitation22.
The results of the current analysis demonstrated the consistent efficacy of cladribine tablets 3.5 mg/kg within each of the HRA + DAT subgroups. The DMD-naïve cohort was the larger subgroup of patients; nevertheless, baseline patient demographics were generally similar between placebo and cladribine tablets within the two subgroups. This is parallel with the overall CLARITY ITT population, which also reported a greater proportion of patients that were naïve to DMD treatmentCitation12. As expected, there were fluctuations in the point estimates for the efficacy results between the subgroups; however, the results for each endpoint are consistent with the greater efficacy of cladribine tablets 3.5 mg/kg as compared to placebo. It is especially relevant to patients that, for both HRA + DAT subgroups, cladribine tablets significantly reduced the risk of disability progression compared to placebo (by 71% and 83% in the DMD-naïve cohort and by 75% and 80% in the prior-DMD cohort for 3mCDP and 6mCDP, respectively). Previous comparisons between HDA and non-HDA patients showed no major difference in the adverse event profile of cladribine tablets administered as 3.5 mg/kgCitation12.
Conclusion
Post hoc evidence suggests that there is a consistent treatment benefit of cladribine tablets 3.5 mg/kg during the 96 week CLARITY study follow-up period among patients with highly active disease who were either previously treated with DMDs or were treatment naïve. Treatment with cladribine tablets 3.5 mg/kg versus placebo led to comparable results for the assessed outcomes, including ARR, time to 3 month and 6 month confirmed EDSS progression, and MRI endpoints, for both subgroups of patients with HDA.
Transparency
Declaration of funding
Financial support for this study was provided entirely by a contract with EMD Serono Inc. (an affiliate of Merck KGaA, Darmstadt, Germany). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the report.
Declaration of financial/other relationships
P.V. has disclosed that he has received honoraria or consulting fees from Biogen, Sanofi-Genzyme, Novartis, Teva, Merck KGaA (Darmstadt, Germany), Roche and Celgene; and research support from Novartis, Sanofi-Genzyme and Roche. A.G. has disclosed that he is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. F.D., D.D. and S.L.W. have disclosed that they are employees of EMD Serono Research & Development Institute Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany. G.H. and D.J. have disclosed that they are employees of Merck KGaA, Darmstadt, Germany.
Author contributions
All authors were involved in the design, analysis and interpretation of the data. All authors revised the manuscript critically for intellectual content and provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Acknowledgements
The authors wish to thank Jason Allaire PhD of Generativity Solutions Group for his assistance with editing the paper.
Data availability statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck KGaA’s Data Sharing Policy. All requests should be submitted in writing to Merck KGaA’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavour to gain agreement to share data in response to requests.
Notes
i. Mavenclad is a registered trademark of Merck KGaA, Darmstadt, Germany.
References
- Spelman T, Freilich J, Anell B, et al. Patients with high-disease-activity relapsing–remitting multiple sclerosis in real-world clinical practice: a population-based study in Sweden. Clin Ther. 2020;42(2):240–250.
- Huisman E, Papadimitropoulou K, Jarrett J, et al. Systematic literature review and network meta-analysis in highly active relapsing–remitting multiple sclerosis and rapidly evolving severe multiple sclerosis. BMJ Open. 2017;7(3):e013430.
- Enzinger C, Fuchs S, Pichler A, et al. Predicting the severity of relapsing–remitting MS: the contribution of cross-sectional and short-term follow-up MRI data. Mult Scler. 2011;17(6):695–701.
- Ohlmeier C, Gothe H, Haas J, et al. Epidemiology, characteristics and treatment of patients with relapsing remitting multiple sclerosis and incidence of high disease activity: real world evidence based on German claims data. PLoS One. 2020;15(5):e0231846.
- Farber RS, Sand IK. Optimizing the initial choice and timing of therapy in relapsing–remitting multiple sclerosis. Ther Adv Neurol Disord. 2015;8(5):212–232.
- Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9:S5–S48.
- Johnson KM, Zhou H, Lin F, et al. Real-world adherence and persistence to oral disease-modifying therapies in multiple sclerosis patients over 1 year. JMCP. 2017;23(8):844–852.
- Fernandez O. Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS? Mult Scler Relat Disord. 2017;17:75–83.
- eMC. Mavenclad 10mg tablets [Internet]. London (UK): EMC; 2017 [cited 2017 Dec 18]. Available from: https://www.medicines.org.uk/emc/medicine/34044
- Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426.
- Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing–remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10(4):329–337.
- Giovannoni G, Soelberg Sorensen P, Cook S, et al. Efficacy of cladribine tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: a post hoc analysis of the CLARITY study. Mult Scler. 2019;25(6):819–827.
- Siddiqui MK, Khurana IS, Budhia S, et al. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing–remitting multiple sclerosis. Curr Med Res Opin. 2018;34(8):1361–1371.
- Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–846.
- Dixon JR Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65–74.
- Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord. 2020;46:102572.
- Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon β. Ann Neurol. 2013;73(1):95–103.
- Rudick RA, Polman CH. Current approaches to the identification and management of breakthrough disease in patients with multiple sclerosis. Lancet Neurol. 2009;8(6):545–559.
- Mowry EM. Natural history of multiple sclerosis: early prognostic factors. Neurol Clin. 2011;29(2):279–292.
- Alroughani R, Inshasi JS, Deleu D, et al. An overview of high-efficacy drugs for multiple sclerosis: Gulf region expert opinion. Neurol Ther. 2019;8(1):13–23.
- Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. Amsterdam (The Netherlands): European Medicine Agency; 2015.
- Giovannoni G, Comi G, Cook S, et al. Clinical efficacy of cladribine tablets in patients with relapsing–remitting multiple sclerosis (RRMS): final results from the 120-week phase IIIb extension trial to the CLARITY study. Neurology. 2016;86(16 Supplement):P3.028.