Abstract
Objective
A chronic pain patient sample living in the United States who participated in a cross-sectional study to evaluate the validity and reproducibility of the Prescription Opioid Misuse and Abuse Questionnaire is characterized.
Methods
Patients with chronic pain identified through electronic medical records as refilling at least one opioid prescription within the prior 3 months were recruited from five United States Department of Defense Military Health System clinics. Patients completed the Prescription Opioid Misuse and Abuse Questionnaire, Brief Pain Inventory-Short Form, Medical Outcomes Study: 36-item Short Form, and sociodemographic questions online. Clinical characteristics and electronic medical records for 1 year prior to consent were collected.
Results
809 (86.2%) participants completed the Prescription Opioid Misuse and Abuse Questionnaire. Mean (± standard deviation) age was 55.4 ± 12.7 years; the majority female (55.5%) and white (74.8%). Mean duration of chronic pain was 14.7 ± 10.5 years; the most common pain conditions were lower back pain (76.6%), neck or shoulder pain (60.3%), and osteoarthritis (38.7%). The most commonly prescribed opioids were oxycodone (35.7%), tramadol (34.5%), and hydrocodone (26.9%); 54.8% took one opioid, 44.9% took 2 or more opioids.
Discussion
Participants’ health status was poor; pain severity and interference were moderate. Electronic medical record data revealed high healthcare resource utilization. This chronic pain population was severely impacted by their pain condition(s).
Introduction
Estimated to impact anywhere from 25 million to 100 million Americans, chronic pain is a significant public health problem in the United States (US)Citation1,Citation2. Chronic pain is a major contributor to national rates of morbidity, mortality, and disability and is one of the most common reasons for physician visitsCitation1. Contributing an estimated $560 billion annually in the United States to direct medical costs, lost work productivity, and disability programsCitation1 and linked to lower levels of life satisfaction, reduced health-related quality of life, and higher rates of suicideCitation3, the impacts of chronic pain extend beyond economics and underlie numerous psychological and social issues. The diverse consequences associated with chronic pain underscore the importance of appropriate and compassionate patient care.
Although there is little evidence to support long-term opioid use, the long-term use of prescription opioid pain medications in the treatment of moderate to severe chronic pain has become a therapeutic mainstay for select subgroups of patientsCitation4. Unfortunately, long-term use can result in problematic patterns of use and non-therapeutic use potentially leading to opioid use disorder (OUD)Citation5. Chronic pain is an extraordinarily complex condition that is influenced by a multitude of factors, including biologic processes, and psychological, social, and environmental influencesCitation6. Adding to this complexity are the various patient-level risk factors that are associated with the development of OUD, including the history of substance abuse and mental health disordersCitation7. Despite a decline in opioid prescriptions in recent years due to general concerns surrounding the national opioid crisis and increased awareness of OUD, opioid prescriptions remain very high in the United StatesCitation8.
Increased prescribing of opioid medications in the United States over the past 20 years has been correlated with the development of a national crisis of increasing addiction to and misuse of both prescription and non-prescription opioids, resulting in the Department of Health and Human Services (HHS) declaring opioid use disorder a public health emergency in 2017Citation9. In response to the urgent, complex, and intertwined public health priorities of effective chronic pain treatment and prevention of opioid misuse and abuse, the US Food and Drug Administration (FDA) has launched several initiatives to evaluate and improve research methods and measurement in the evaluation of potential new pain therapiesCitation1. In the absence of a “gold standard” measure of prescription opioid misuse/abuse, the FDA requested a study as part of their post-marketing requirement (PMR) of pharmaceutical makers of extended-release/long-acting prescription opioids, to develop and validate a measure of the opioid-related adverse events of misuse and abuse among patients with chronic pain prescribed long-term opioid therapyCitation10,Citation11 (see Supplemental Digital Content). While there are several widely used patient screeners for opioid riskCitation12–17, these instruments are not validated for use in a chronic pain population or are not designed to assess intentionality and/or do not distinguish between behaviors of misuse and abuse. To address the FDA’s PMR, the Prescription Opioid Misuse and Abuse Questionnaire (POMAQ) was derived from the Self-Reported Misuse, Abuse and Diversion of Prescription Opioids (SR-MAD)Citation16, with extensive modifications and additional questions included to address the specific PMR research needs. The POMAQ was designed to fill the need for assessment of misuse/abuse and intentionality of prescription opioid use.
The POMAQ underwent content validation via qualitative interviews (Protocol 3033-3Citation18), but quantitative evidence of its validity as a measure of misuse and abuse of prescription opioid medication had yet to be established. Therefore, a validation study (Protocol 3033-4), described herein, was implemented to evaluate the validity and reproducibility of the POMAQ to identify opioid abuse and misuse behaviors among patients who have chronic pain requiring long-term opioid use and to identify patterns of behaviors that commonly co-occur within these individuals. The purpose of this paper is to present the descriptive results of the POMAQ validation sample in terms of demographics and clinical characteristics, thereby depicting patients living with chronic pain in the United States and sharing insights gained from this population. Complete results from the POMAQ validation study, including evaluation of its construct validity and reproducibility, will be presented in separate publications.
Methods
Key definitions
The working definitions of misuse and abuse that provided the conceptual framework for the POMAQ were obtained from the Analgesic, Anaesthetic, and Addiction Clinical Trials, Translation, Innovations, Opportunities, and Networks (ACTTION) reviewCitation19. These terms were defined as follows:
Misuse: Intentional use of a drug for therapeutic purpose (to reduce an aversive symptom or state), inappropriately outside label directions or in a way other than prescribed or directed by a healthcare practitioner; this includes patients using a drug for a condition different from that for which the drug is prescribed, patients taking more drugs than prescribed, or at different dosing intervals.
Abuse: Intentional use of a drug for non-therapeutic purposes, repeatedly or sporadically, for the purpose of achieving a positive psychological or physical effect.
Study design
The study population was recruited from the US Department of Defense (DoD) Military Health System (MHS) which serves approximately 10 million beneficiaries (i.e. active duty military, national guard members/reservists, family members, and military retirees) and is supported by a robust electronic medical record (EMR) system. Participants were recruited from five DoD clinics located across the US including: Naval Medical Center Portsmouth (Portsmouth, VA); Naval Medical Center San Diego (San Diego, CA); Wright-Patterson Medical Center (Dayton, OH); Walter Reed National Military Medical Center (Bethesda, MD); and San Antonio Military Medical Center (San Antonio, TX). Dorrance and colleagues found the DoD population to be representative of the insured US population in terms of age, gender, occurrence of common conditions and overall healthCitation20. Various geographic locations were utilized to increase the generalizability of the sample. Patients were eligible from clinics within each of the selected centers provided they had a chronic pain diagnosis, were currently taking prescription opioids, and had refilled at least one prescription for opioid pain medication within the prior 3 months. Potentially eligible patients were identified by site staff through the DoD MHS EMR database and screened for study eligibility over the phone or in-person.
To be eligible, patients had to be ≥18 years, diagnosed with a chronic (≥3 months) pain condition requiring long-term treatment with opioids, willing to provide informed consent and a urine sample, and able to participate in an internet-based survey and telephone interview in English. Patients diagnosed with a terminal illness or who were currently active-duty service members of the military were not eligible to participate. Eligible and interested participants were scheduled for an initial clinic visit during which they provided written informed consent. Upon obtaining consent from the patient, the site coordinators assigned the participant a unique study identification (ID) and enrolled the participant into the web-based enrolment portal.
All recruitment procedures complied with the current Health Insurance Portability and Accountability Act (HIPAA) regulations. The protocol was approved by an Institutional Review Board (IRB) at each participating site, and a Certificate of Confidentiality (CoC) from the National Institutes of Health was obtained.
Procedures
Upon providing consent, participants were given a study packet that included a welcome letter, study information, and directions on how to complete a series of questionnaires via a secure website on their own time and at a location of their choice. The study packet included the participant’s unique study ID and registration number which allowed them access to the online survey and linked their responses to their de-identified EMRs. iPads were also available at clinical sites for participants to use to complete the survey onsite if desired. If completed at the site, patients were provided with a private location and unrestricted time to complete the survey. The survey battery included: the POMAQ, Brief Pain Inventory-Short Form (BPI-SF), Medical Outcomes Study: 36-item Short Form Health Survey Instrument (MOS SF-36), and sociodemographic questions. Following the clinic visit, participants were given up to 21 days to complete the series of questionnaires included in the survey. After the participant completed all the internet-based questionnaires, they received a $25 gift code via e-mail. Clinic staff also completed a clinical case report form (CRF) for each participant enrolled in the study.
Measures
Prescription Opioid Misuse and Abuse Questionnaire (POMAQ)
The POMAQ was designed as a series of questions with various response patterns about behaviors divided into 19 specific items. The questions specifically focused on behaviors related to misuse and abuse and the intention behind each behavior to identify prescription opioid misuse and abuse as well as prescription opioid diversion behaviors. A behavior or combination of behaviors were classified as opioid misuse or abuse (or both) based upon how the person responded to the intent of the specific behavior. Questions assessing behaviors over the previous one-year timeframe were used at baseline. Scoring guidelines for the POMAQ will be detailed in the algorithm validation manuscript (in development).
Medical Outcomes Study: 36-item Short Form Health Survey Instrument (MOS SF-36)
The MOS SF-36 is a self-administered, validated questionnaire that measures the following eight health aspects – physical functioning, role limitations due to physical problems, social functioning, bodily pain, mental health, role limitations due to emotional problems, vitality, and general health perceptionCitation21–24. Higher scores indicate better health-related quality of life (HRQL). The MOS SF-36 has been used extensively in numerous clinical trials and studies with published reliability and validityCitation22,Citation25 and provides benchmark generic health status data on the patient sample.
Brief Pain Inventory – Short Form (BPI-SF)
The patient-completed BPI-SF assessed pain intensity through four questions (pain at its worst, least, average and now [current pain]); these four questions were analyzed separately and averaged to obtain a composite Pain Severity score. Further, the BPI-SF’s pain interference item with seven sub-questions assessed the degree that pain interferes with various daily activitiesCitation26. All items used an 11-point numerical rating scale, with higher scores indicating greater pain intensity or interference. The BPI-SF has been used, tested, and validated extensively in various patient populationsCitation27,Citation28 and provides benchmark data regarding the level of pain the participants experience. A 24 hour recall period was used for each item except for the questions on average and current pain intensity which did not stipulate a specific timeframe for the response.
Sociodemographic form
Participants completed a brief sociodemographic questionnaire after completing the other patient-reported outcome measures. The sociodemographic form collected the participant’s age, ethnicity, living situation, employment, and education.
Clinical form
Based on EMR data and clinical notes, clinical site staff completed a clinical CRF to document basic information related to opioid use history (e.g. type of opioid pain medication, duration of opioid use, known history of misuse or abuse) for each participant who completed a study visit.
Electronic medical record (EMR) data
Participants’ available EMR data were obtained from the DoD military healthcare data repository for one year prior to the consent date and included data on diagnoses, healthcare resource utilization, and prescriptions filled through this healthcare system.
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Descriptive statistics were derived for demographic and clinical variables to describe the study population. Descriptive statistics were derived for each POMAQ item as well as the other ancillary study measures including the MOS SF-36 and BPI-SF. Scores were calculated using each instrument’s scoring manual.
Results
Screening and sample disposition
A total of 3263 patients were screened; 1588 patients (48.7%) declined to participate due to lack of interest or time, or they disagreed with the study purpose; 604 (18.5%) were ineligible due to a variety of reasons (e.g. no longer taking opioids, too ill, living too far away) while 133 (4.1%) were eligible and willing to participate but were not enrolled due to difficulties with scheduling and/or site logistics; and 938 (28.7%) were determined to be eligible, consented to participate, and were enrolled. The POMAQ survey was completed by 809 (86.2%) of those who consented to be in the study. Of those who did not complete the POMAQ (13.8%), the majority (89.9%) were non-responsive to follow-up phone calls from the site, and the remainder (10.1%) withdrew from the study.
Self-reported demographic and clinical characteristics
The mean age ± standard deviation (SD) of participants was 55.4 ± 12.7 years () with the majority being female (55.5%). Most participants were of non-Hispanic/Latino ethnicity (n = 733, 90.6%), and 74.8% (n = 605) of the sample were White/Caucasian. Participants tended to be employed full-time (n = 167, 20.6%), retired (n = 231, 28.6%), or on disability (n = 200, 24.7%). The majority either had some college education (n = 295, 36.5%) or a college degree (n = 273, 33.7%).
Table 1. Participant-reported demographic and clinical characteristics.
Arthritis was the most commonly self-reported comorbid condition (n = 541, 66.9%) followed by hypertension (n = 372, 46.0%). Only 8.8% (n = 71) reported no comorbid conditions. Many participants (n = 348, 43.0%) reported “Other” comorbid conditions. The three most commonly self-reported pain conditions (not mutually exclusive) were lower back pain (n = 620, 76.6%), neck or shoulder pain (n = 488, 60.3%), and osteoarthritis (n = 313, 38.7%). Slightly over a quarter of patients (n = 224, 27.7%) reported “Other” pain conditions.
Site-reported clinical characteristics
Based on the staff-completed clinical CRF, participants had been seen at their respective medical practices for an average of 6.2 ± 7.8 years (range: 0.0–50.0 years, median: 3.0 years) (). The vast majority (n = 806, 99.6%) of participants were currently taking opioid pain medications, with a mean duration of opioid medication use of 7.3 ± 5.6 years (range: 0.2–47.0 years, median 7.0). The remaining three (0.4%) had previously taken opioids but were no longer taking opioids at the time they completed the questionnaire. The most commonly prescribed current opioid pain medications were oxycodone alone or in combination with another medication (n = 289, 35.7%), tramadol alone or in combination with another medication (n = 279, 34.5%), and hydrocodone alone or in combination with another medication (n = 218, 26.9%); 45% were currently prescribed two or more prescription opioids.
Table 2. Site-reported clinical characteristics.
Based on their medical records, most participants (n = 757, 93.6%) had no reported prior history of misuse or abuse of opioids or other substances. However, for those with a history of abuse (n = 19 for abuse of opioids and n = 28 for abuse of other substances) or misuse (n = 11 for misuse of opioids and n = 5 for misuse of other substances), the mean duration of abuse or misuse was 13.3 ± 14.6 years and 6.4 ± 6.5 years, respectively.
Health status as reported on the MOS SF-36 and BPI-SF
MOS SF-36 domain scores ranged from 0 to 100 with higher scores indicating more favorable health status. Mean ± SD domain scores demonstrated lower scores on the physical health domains compared to the mental health domains, with scores ranging from 17.8 ± 30.9 (role limitations due to physical health) to 69.0 ± 20.6 (mental health) (), which is significantly lower than the general population norm-based scores of 50 with a standard deviation of 10.
Figure 1. Descriptive Statistics for the MOS SF-36. 1Scores have a possible range of 0–100. Higher scores indicate more favorable health status. The general population norm-based score is 50 with a standard deviation of 10. 2The scoring algorithm used to calculate the MOS SF-36 scores was developed by Hays et al. [Citation41]. This version of the scoring algorithm does not incorporate weights for calculating the mental component summary (MCS) and physical component summary (PCS). The updated weights for the MCS and PCS are copyrighted and not publicly available for use. As such, in these analyses, MCS and PCS scores were not calculated for the MOS SF-36.
![Figure 1. Descriptive Statistics for the MOS SF-36. 1Scores have a possible range of 0–100. Higher scores indicate more favorable health status. The general population norm-based score is 50 with a standard deviation of 10. 2The scoring algorithm used to calculate the MOS SF-36 scores was developed by Hays et al. [Citation41]. This version of the scoring algorithm does not incorporate weights for calculating the mental component summary (MCS) and physical component summary (PCS). The updated weights for the MCS and PCS are copyrighted and not publicly available for use. As such, in these analyses, MCS and PCS scores were not calculated for the MOS SF-36.](/cms/asset/daf5a232-9ab8-4645-95e0-c1696c31b25b/icmo_a_1865889_f0001_c.jpg)
Scores for each item of the BPI-SF range from 0 to 10, with 10 indicating more pain and/or interference with activities. Mean ± SD scores for the four individual BPI-SF severity items ranged from 3.3 ± 2.0 (least pain over past 24 hours) to 6.9 ± 2.1 (worst pain over past 24 hours) while the Pain Severity score (average of the four items) was 4.9 ().
Figure 2. Descriptive Statistics for the BPI-SF. 1BPI-SF item scores range from 0 to 10, with higher scores indicating greater pain severity or interference. Pain Severity is a composite of the four pain items (a mean severity score). Pain interference is scored as the mean of the seven interference items if more than 50%, or four of seven, of the total items have been completed on a given administration
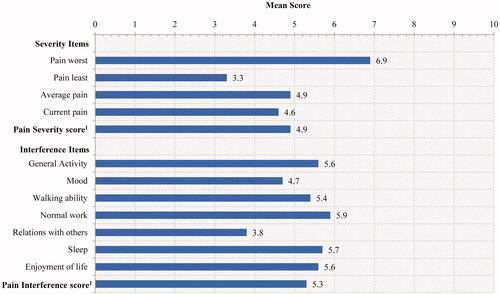
Mean ± SD scores on the BPI-SF interference items ranged from 3.8 ± 3.1 (relations with others) to 5.9 ± 3.0 (normal work). The mean pain interference summary score (a mean of the seven interference items if >50% of items were completed) was 5.3 ± 2.5.
POMAQ item characteristics
More than half the sample (54.8%) reported currently taking only one prescription opioid pain medication on item 1 of the POMAQ, while 44.9% reported taking two or more opioids. Key findings for POMAQ behaviors reported as having occurred in the past year (prior to the date of POMAQ completion) yielded a range of prevalence from 0% (purchasing or stealing a prescription pad) to 43.6% (taking less opioids than prescribed) with most POMAQ behaviors being reported by 2–20% of the sample as listed in . Most participants (n = 671, 82.7%) reported not taking any street drugs in the past year, and of the 135 participants who did, marijuana (n = 81, 10.0%) and sleeping pills (n = 40, 4.9%) were the drugs most widely reported.
Table 3. POMAQ results (items 1–8 and 10–19).
EMR results
Extracted EMR data demonstrated high healthcare resource utilization among this chronic pain patient population. Emergency room visits were reported for 40.8% of participants, while 14.3% had been hospitalized at least once in the year prior to the study (). In the year prior to study enrolment, the mean (± SD) number of visits per to a DoD Military Health System (MHS) outpatient facility was 33.4 (± 29.3) visits per participant.
Table 4. EMR data (for year prior to study start).
The EMR data revealed a high number of unique International Classification of Diseases, Ninth Revision (ICD-9) diagnoses per patient with an average of 15.9 ± 6.7 diagnoses per person (diagnoses recorded in the 1 year prior to study), indicating the presence of many comorbid conditions. Examples of common comorbid conditions were anxiety (24.0%), major depression (28.8%), orthopaedic conditions (61.9%), neurologic conditions (58.3%), and sleep disorders (38.6%) (see Supplemental Digital Content Table S1). Nearly all participants (99.8%) were prescribed medications other than opioids. The most common of those medications included: gastrointestinal medication (77.1%), non-steroidal anti-inflammatory drugs (NSAIDs) (70.1%), cardiac medication (65.6%), anti-infectives (64.8%), antidepressants (62.2%), anticonvulsants (57.1%), and muscle relaxants (48.3%) (see Supplemental Digital Content Table S2). The mean number of opioid prescriptions filled per person over the past year was 15.4 ± 8.2.
Discussion
This paper describes the characteristics of a large sample (n = 809) of patients with chronic pain currently prescribed long-term treatment with opioids recruited through DoD MHS clinics. These patients were recruited to participate in a cross-sectional study (to be presented in separate publications) intended to evaluate the validity and reproducibility of the POMAQ as a measure of opioid-related adverse events of misuse and abuse among patients with chronic pain prescribed long-term opioid therapy. The self-reported general health status of patients in this sample was poor, as assessed by the MOS SF-36, while their pain severity and interference were moderate, based on the BPI-SF. The majority had lower back pain and/or neck or shoulder pain and nearly all (>90%) reported at least one comorbid condition. EMR data revealed numerous comorbid conditions, including a high prevalence of major depression (28.8%) and anxiety (24.0%), as well as substantial healthcare resource utilization and other prescription medication usage. In short, results reveal a chronically ill patient population suffering from numerous comorbid conditions layered on top of moderate to severe chronic pain. The complex health profile of this patient population requires extensive coordinated care through various avenues of the healthcare system.
The EMR data surrounding comorbidities and healthcare resource utilization in this study complement previously published work conducted among chronic pain populationsCitation29–32. For example, Engel and colleagues examined a group of primary care patients with chronic back pain and found that having chronic pain along with other conditions, including multiple types of chronic pain, was linked to heightened use of the healthcare system and increased costsCitation29. A large retrospective analysis which examined insurance claims data of patients with chronic low back pain alongside matched controls noted that patients with chronic low back pain were characterized by greater comorbidity and economic burdens, including more than double the estimated direct medical costs compared to those without low back painCitation30.
The high prevalence of depression and anxiety in this sample was not surprising given that painful and depressive symptoms often co-occurCitation33. Depression rates in this sample were rather high, similar to those found in previous studies evaluating depression and anxiety in chronic pain populationsCitation30,Citation34. Coyne and colleagues noted in a cross-sectional study among adult patients recruited through a primary care setting, levels of anxiety, depression and impact on patients’ usual activities, sleep, and mood were all higher among patients with chronic pain compared to those without chronic painCitation34. Gore and colleagues also found higher rates of depression, anxiety, and sleep disorders in patients with chronic low back pain compared to matched controls without chronic low back painCitation30. Importantly, despite the multiple comorbid conditions experienced by this study sample, a relatively small proportion of patients endorsed POMAQ items that would indicate either abuse or misuse behaviors surrounding their prescription opioid medication.
Despite chronic pain being one of the most common reasons for seeking medical care in the USCitation1, there is a lack of systematic screening, inconsistent recording of pain, and fractured delivery of patient careCitation35–37. As supported by findings within this study population and others, patients often pursue numerous primary and specialty care services to address their pain and associated conditionsCitation1,Citation38. This is evidenced by the high number of outpatient visits (33.4 ± 29.3) per participant over the one-year study period found in our study. This approach to pain management can lead to unfavorable outcomes and significantly increased healthcare resource utilization costsCitation38,Citation39. Thus, it is imperative that the needs of chronic pain patients are met through careful, individualized and coordinated multidisciplinary care.
Several limitations of this study should be noted. First, this study population was recruited from five DoD military hospitals and their supporting clinics. Although noted by Dorrance and colleagues to be similar to the overall US population in terms of key demographics and common medical conditions, this sample is not representative of uninsured populations, which face a different set of health problems and utilize health care resources differentlyCitation20. It should also be noted that recruitment for this study was difficult with nearly half of the screened patients declining to enroll. This poor response rate can be attributed to a number of typical reasons, such as lack of time or interest and inconvenience as well as a lack of trust and/or a concern for losing access to their medication(s). There is no way to determine if the large number of patients who declined to participate in the study (48.7%) would have responded similarly or differently on the POMAQ or other measures compared to those who enrolled. Despite these limitations, the present sample was carefully and systematically recruited to provide an appropriate examination of the suitability of the POMAQ items for the population of chronic pain outpatients in the United States.
The description of this study sample revealed some important observations of chronic pain patients. Amid the growing focus on reducing exposure to opioids and enhancing rationalized prescribing patterns by the FDA and Centers for Disease Control and Prevention (CDC), patients’ fear of losing access to their necessary medication is top of mind for many of those battling chronic painCitation40. While not the primary purpose of this study, our data present a useful picture of the complexity of pain care in this population. What we cannot learn from our data is the degree to which the co-morbidities and high level of healthcare utilization are due to the illnesses that create the chronic pain versus any possible issues that might arise from the patients’ chronic use of opioids. This is an important issue that clearly requires additional study. Regardless, monitoring for misuse/abuse behaviors is critical in evaluating whether a patient takes their prescription opioid medication properly; administering the POMAQ could assist physicians in identifying problematic behaviors and facilitating communication between patients and healthcare providers as potential issues arise. What is clear is that the association of any two findings in a cohort study does not demonstrate causality, and great care must be taken in the interpretation of this type of data, including in this study. Importantly, to effectively address the complex health profiles of chronic pain patients, their care will need to be delivered through a thoughtful and highly coordinated, multidisciplinary approach.
Conclusion
The large chronic pain population recruited for this cross-sectional study was severely impacted by their pain condition(s) but reported relatively few behaviors indicative of prescription opioid misuse or abuse.
Transparency
Declaration of funding
This project was conducted as part of a Food and Drug Administration (FDA)-required post-marketing study for extended-release and long-acting opioid analgesics and was funded by the Opioid Postmarketing Consortium (OPC) consisting of the following companies: Allergan; Assertio Therapeutics, Inc.; BioDelivery Sciences, Inc.; Collegium Pharmaceutical, Inc.; Daiichi Sankyo, Inc.; Egalet Corporation; Endo Pharmaceuticals, Inc.; Hikma Pharmaceuticals USA Inc.; Janssen Pharmaceuticals, Inc.; Mallinckrodt Inc.; Pernix Therapeutics Holdings, Inc.; Pfizer, Inc.; Purdue Pharma, LP.
Declaration of financial/other relationships
K. S. Coyne and B. M. Currie are employees of Evidera, a company that received funding from the OPC to conduct this study. At the time of the study, A. I. Barsdorf was employed by Pfizer, a member company of the OPC. At the time of the study, J. L. Poon was an employee of Evidera and is now an employee of Eli Lilly and Company. At the time of the study, J. Y. Mazière was an employee of PPD, a parent company to Evidera and is now an employee of Astellas. R. F. Pierson is an employee of Janssen, a member company of the OPC. S. H. Schnoll is an employee of Pinney Associates, a company that received funding from the OPC for time spent consulting on this study. S. F. Butler received funding from the OPC for time spent consulting on this study. J. T. Farrar is an employee of the University of Pennsylvania and received funding from the OPC for time spent consulting on this study to support his salary. H. J. Fisher is an employee of Health ResearchTx, a company that received funding from the OPC to conduct this study. All aspects of the study design, interpretation, and decision to submit for publication were determined by the authors.
Source of support
Research data derived from approved Naval Medical Research Unit-Dayton, Dayton, Ohio and Naval Medical Center, Portsmouth, Virginia IRB, protocol; number NAMRUD.2015.0004. The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. CDR Michael Franks Jr. is a member of the U.S. Military. This work was prepared as part of his official duties. Title 17U.S.C. 105 provides that "Copyright protection under this title is not available for any work of the United States Government." Title 17U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person's official duties.
Supplemental Table 2
Download MS Word (30.8 KB)Supplemental Table 1
Download MS Word (39.8 KB)Supplemental Material
Download MS Word (22.4 KB)Acknowledgements
The authors thank Kawthar Nakayima, Amara Tiebout, and Michael Grossi for their production and editorial assistance.
References
- Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780.
- Medicine I. O. Pain as a public health challenge. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
- Rosenblum A, Marsch LA, Joseph H, et al. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405–416.
- Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569–576.
- Raffaeli W, Arnaudo E. Pain as a disease: an overview. J Pain Res. 2017;10:2003–2008.
- Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse: part 1. Pain Physician. 2017;20(2S):S93–S109.
- Burcher KM, Suprun A, Smith A. Risk factors for opioid use disorders in adult postsurgical patients. Cureus. 2018;10(5):e2611.
- Health and Human Services. HHS Acting Secretary Declares Public Health Emergency to Address National Opioid Crisis 2017. Available from: https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html
- Food and Drug Administration. Labeling Supplement and PMR required 2013. [cited 2018 October 4]. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM367697.pdf
- Food and Drug Administration. New PMR required 2016. [cited 2018 October 4]. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM484415.pdf
- Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7(9):671–681.
- Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the current opioid misuse measure. Pain. 2007;130(1–2):144–156.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9(4):360–372.
- Coambs R, Jarry J, Santhiapillai A, et al. The SISAP: a new screening instrument for identifying potential opioid abusers in the management of chronic nonmalignant pain within general medical practice. Pain Res Manage. 1996;1(3):155–162.
- Setnik B, Roland CL, Barsdorf AI, et al. The content validation of the Self-Reported Misuse, Abuse and Diversion of Prescription Opioids (SR-MAD) instrument for use in patients with acute or chronic pain. Curr Med Res Opin. 2017;33(6):1067–1076.
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432–442.
- Coyne KS, Barsdorf AI, Brooks A, et al. Establishing the content validity of the Prescription Opioid Misuse and Abuse Questionnaire (POMAQ) among chronic pain patients. CMRO. 2020. DOI:10.1080/03007995.2020.1865891
- Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154(11):2287–2296.
- Dorrance KA, Ramchandani S, Neil N, et al. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142–145.
- Maruish M. User’s manual for the SF-36v2 health survey. Lincoln, RI: QualityMetric Incorporated; 1993.
- McHorney CA, Ware JE Jr, Lu JF, et al. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66.
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483.
- Ware JE. Jr. SF-36 Health Survey update. The use of psychological testing for treatment planning and outcome assessment, Vol. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. p. 693–718.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129–138.
- Cleeland C. Assessment in cancer. In: Osoba D, editor. Effect of cancer on quality of life. Boca Raton, FL: CRC Press; 1991. p. 293–305.
- Cleeland C. The brief pain inventory user guide. Houston, TX: University of Texas M.D. Anderson Cancer Center; 2009.
- Engel CC, von Korff M, Katon WJ. Back pain in primary care: predictors of high health-care costs. Pain. 1996;65(2–3):197–204.
- Gore M, Sadosky A, Stacey BR, et al. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine. 2012;37(11):E668–E677.
- Mehra M, Hill K, Nicholl D, et al. The burden of chronic low back pain with and without a neuropathic component: a healthcare resource use and cost analysis. J Med Econ. 2012;15(2):245–252.
- Romanelli RJ, Shah SN, Ikeda L, et al. Patient characteristics and healthcare utilization of a chronic pain population within an integrated healthcare system. Am J Manag Care. 2017;23(2):e50–e56.
- Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445.
- Coyne KS, Currie BM, Donevan S, et al. Psychometric validation of the electronic chronic pain questions (eCPQ) in a primary care setting. Curr Med Res Opin. 2017;33(1):137–148.
- Glajchen M. Chronic pain: treatment barriers and strategies for clinical practice. J Am Board Fam Pract. 2001;14(3):211–218.
- Redmond K. Organizational barriers in opioid use. Support Care Cancer. 1997;5(6):451–456.
- Yanni LM, Weaver MF, Johnson BA, et al. Management of chronic nonmalignant pain: a needs assessment in an internal medicine resident continuity clinic. J Opioid Manag. 2008;4(4):201–211.
- Roth RS, Geisser ME, Williams DA. Interventional pain medicine: retreat from the biopsychosocial model of pain. Transl Behav Med. 2012;2(1):106–116.
- Cheatle MD, Klocek JW, McLellan AT. Managing pain in high-risk patients within a patient-centered medical home. Transl Behav Med. 2012;2(1):47–56.
- Food and Drug Administration. Public meeting on patient-focused drug development for chronic pain 2018. [cited 2018 September 18]. Available from: https://www.fda.gov/downloads/Drugs/NewsEvents/UCM612498.pdf
- et al. Hays RD, Sherbourne CD, Spritzer KL, editors. A microcomputer program (sf36. exe) that generates SAS Code for scoring the SF-36 Health Survey. 22nd Annual SAS Users Group International Conference.; 1996.
