Abstract
Objectives
Content validation is essential in the development of patient-reported instruments to ensure relevancy and understandability. The aim was to evaluate patient understanding of the Prescription Opioid Misuse and Abuse Questionnaire (POMAQ) using cognitive interviewing among adults with chronic moderate to severe pain.
Methods
This qualitative study involved a one-time in-clinic visit to conduct one-on-one cognitive interviews among participants with chronic moderate to severe pain from four groups: (1) Known Opioid Abuse; (2) Known Abuse of Other Substances (e.g. alcohol, benzodiazepines); (3) Opioid Non-abuse; and (4) No Chronic Opioid Use. Patients were recruited from 6 US clinical centers. Concept elicitation questions regarding misuse and abuse were asked at interview start; the POMAQ was completed via a web interface followed by a cognitive interview regarding POMAQ items and response options.
Results
56 patients were enrolled. Mean age was 48.7 ± 12.3 years; 57% female; 80% Caucasian; mean duration of chronic pain was 11.2 ± 8.2 years with lower back pain predominating at 75%. Overall, the POMAQ was well-understood and received positive feedback. A few (n = 6, 11%) expressed concerns about completing the POMAQ using a secure internet site as they either indicated they were not computer savvy (n = 3, 5%) or were concerned about internet security (n = 3, 5%). Minor wording modifications were made to the POMAQ to enhance clarity and understanding of the POMAQ.
Conclusions
The POMAQ demonstrated content validity among patients with moderate to severe chronic pain and is undergoing psychometric evaluation among a larger cohort of patients.
Introduction
Moderate to severe chronic pain is a significant public health problem in the United States (US) estimated to impact more than 125 million AmericansCitation1. Efficacy of treatment with prescription opioids for adults with moderate to severe chronic pain is supported by clinical evidenceCitation2, yet with long-term use there is an increased risk of opioid physical dependence, problematic patterns of use, and non-therapeutic use, all potentially leading to opioid use disorder (OUD)Citation3. As of 2018, the US is currently experiencing a drug overdose crisis with approximately 90 fatalities occurring daily due to opioid-related overdoses (inclusive of heroin and illicit synthetic opioids such as fentanyl), and it is estimated that two million Americans have an OUD involving prescription opioidsCitation4,Citation5.
In response to these urgent, complex, and intertwined public health priorities of effective chronic pain treatment and prevention of opioid misuse and abuse, the US Food and Drug Administration (FDA) has launched several initiatives to evaluate and improve research methods and measurement in the evaluation of potential new pain therapiesCitation6. Two key measurement challenges in this effort are the absence of a well-validated patient screener to assess intentional prescription opioid misuse and abuse in a chronic pain patient population and the lack of consensus on the definition of misuse, abuse, and related events (MARE)Citation7. To illustrate, a systematic review found a lack of consistent, operationalized definitions of misuse, abuse, and addiction across studies that resulted in estimated rates of patient opioid misuse and abuse ranging from 0.08% to 81.0%Citation3. Despite the lack of precision in measurement, available evidence indicates that misuse may be more prevalent than abuse and that misuse and abuse are distinct behaviors requiring different approaches for prevention and interventionCitation3,Citation5.
While there are a number of widely used patient screeners for opioid risk, none of the existing instruments are validated for use in a chronic pain population to measure the intentionality for opioid misuse/abuse. Reviews of existing patient screening tools, including the Opioid Risk Tool (ORT)Citation8, the Diagnosis, Intractability, Risk, Efficacy (DIRE)Citation9, and Screening Instrument for Substance Abuse Potential (SISAP)Citation10, indicate that these tools are limited in the scope of their assessment questions (e.g. ORT) and lack validation evidence with a chronic pain patient population (i.e. DIRE, SISAP) Citation11. The Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) was developed for a chronic pain population and has demonstrated good psychometric properties (e.g. good reliability, validity)Citation12, but it does not distinguish between misuse and abuse, nor investigate patient intentions regarding these behaviors. The Current Opioid Misuse Measure (COMM) is a well-developed risk assessment screener intended for use with a chronic pain patient population, but the instrument does not evaluate the intentionality of patient misuse behaviorsCitation13,Citation14. These gaps in measurement limit reliable documentation of the scope of misuse and abuse outcomes in patients following long-term opioid therapy thus resulting in missed opportunities for effective preventive intervention for patients engaged in misuse and abuseCitation7,Citation15,Citation16.
In the absence of a “gold standard” measure of prescription opioid misuse/abuse and in support of accurate measurement and reporting of prescription opioid misuse and abuse, the FDA requested, as part of a post-marketing requirement (PMR) for new drug application (NDA) holders of extended release/long-acting (ER/LA) opioids, to collaboratively undertake a study to develop and validate a measure of the opioid-related adverse events of misuse and abuse among patients with chronic pain prescribed long-term opioid therapyCitation17,Citation18. As a result of this request, the Prescription Opioid Misuse and Abuse Questionnaire (POMAQ) was developed as a patient self-report questionnaire. The objective of this study was to qualitatively evaluate the content validity of the POMAQ through cognitive interviews with adult chronic pain patients.
Methods
Definitions of misuse and abuse
To guide the development of the POMAQ, consistent definitions of misuse and abuse were needed. Following a systematic review of definitions of MARE in clinical trials, the Abuse Liability Evaluation for Research, Treatment, and Training (ALERTT) working group was convened by the Analgesic, Anesthetic, and Addiction Clinical Trials, Translations, Innovations, Opportunities, and Networks (ACTTION) partnership to develop consensus recommendations regarding definitions to support prospective, accurate measurement of MAREs in clinical trialsCitation7. Given the definitions for opioid misuse and abuse vary and continue to evolve, those from the ACTTION partnership were used to guide the POMAQ’s development. Misuse was defined as the intentional use of a drug for therapeutic purpose, inappropriately outside label directions or in a way other than prescribed or directed by a healthcare practitioner. This definition includes patients using a drug for a condition different from that for which the drug is prescribed and patients taking more drugs than prescribed or at different dosing intervals. Abuse was defined as the intentional use of a drug for non-therapeutic purpose, repeatedly or sporadically, for the purpose of a positive psychological or physical effect.
Development of the POMAQ
The POMAQ was derived from the Self-Reported Misuse, Abuse and Diversion of Prescription Opioids (SR-MAD)Citation11, which was developed by addiction and outcome measure specialists and refined through patient interviews. It was extensively modified with questions added to address specific PMR research needs and capture all potential prescription opioid misuse and abuse behaviors as well as use and procurement of prescription and non-prescription opioids (and other illicit drugs) and questions regarding diversion of prescription opioids. After content validation by clinical experts, the questionnaire was further evaluated qualitatively by patients as described herein.
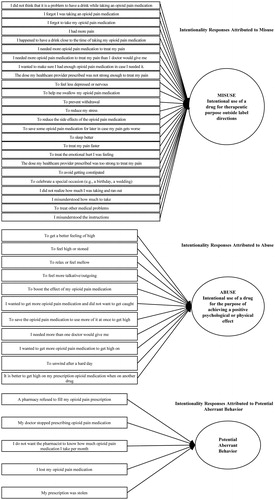
While the full POMAQ is not presented in this paper, illustrates the general stepwise framework of the POMAQ for misuse/abuse measurement moving from patient use in the past year, past three months, and past one month in addition to the patient’s reason for their behavior. If a participant responded affirmatively to a question, they were then asked additional questions regarding the frequency of the behavior and, most importantly, the reason why they performed the behavior so that the intent could be assessed, with the latter questions having shorter recall periods. These three recall periods were derived from prescription opioid patients reporting recall accuracy of a behavior’s frequency within a 30-day timeframe, with the three-months and one-year recall periods capturing longer term duration of the specific behaviorsCitation11. The patient’s intention behind the behavior guided the conceptual framework of the POMAQ (). illustrates the core questions included in the final POMAQ.
Table 1. POMAQ questions.
Content validation
The POMAQ addresses the measurement need for screening misuse/abuse and intentionality but requires evaluation to ensure that the content of the measure is consistent with patients’ experiences and that questions are interpreted as intended and asked in a manner understood by patients as described in the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) guidelines on content validationCitation19,Citation20 and FDA Guidance on patient-reported outcome developmentCitation21. To establish the content validity of a measure, it is important to target the population for whom it will be used to ensure it is “fit for purpose”. To ensure that the POMAQ would be relevant to all chronic pain patients, including those who do not take prescription opioids, after meeting general eligibility requirements, recruitment was conducted from the following four groups:
Group 1 (Known Opioid Abuse): Currently taking opioids (defined as taking a daily opioid dose for at least 7 days prior to screening), have a past history of and/or current diagnosis of opioid abuse or substance use disorder (defined by Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revised [DSM-IV-TR] or DSM-5), with current or history of treatment for opioid abuse or opioid substance use disorder
Group 2 (Known Abuse of Other Substances): Currently taking opioids and have a past history of and/or current diagnosis (defined by DSM-IV-TR or DSM-5 criteria) of non-opioid substance use disorder (e.g. alcohol, benzodiazepines), with current or history of treatment for opioid abuse or opioid substance use disorder
Group 3 (Opioid Non-abuse): Currently taking opioids and do not have a history of opioid abuse or substance use disorder (defined by DSM-IV-TR or DSM-5 criteria) with no treatment history or current treatment seeking
Group 4 (No Chronic Opioid Use): No knowledge of prior and/or current chronic opioid use with no history of opioid abuse or substance use disorder (defined by DSM-IV-TR or DSM-5 criteria) or treatment history.
Study design
This was a cross-sectional, qualitative study involving a one-time in-clinic visit for participants. The study received approval from Chesapeake Institutional Review Board (IRB; IRB # PRO 00012186) and a Certificate of Confidentiality was obtained through the National Institutes of Health (NIH) to protect identifiable research information from forced or compelled disclosure of sensitive information. Participants were recruited from six clinical centers (four research centers, one family medicine practice, one orthopedic center) in a range of geographic regions of the United States (Arkansas, Idaho, Florida, North Carolina, Rhode Island, and Texas). Sites reviewed their patient databases and medical records to ensure that participants met the study criteria and were classified in the appropriate opioid use group; they then approached eligible patients to ascertain interest in participating in the study. Sites recruited five to 15 participants per location for a total of 56 study participants. One-on-one cognitive interviews were conducted in-person by a team of trained qualitative interviewers independent from the clinical recruitment sites among eligible English-speaking adult participants with chronic moderate to severe pain (age 18 years or older) who provided written informed consent.
After a brief interview regarding chronic pain history and experience with opioid medications, as well as open-ended concept elicitation questions to evaluate patient perceptions of opioid misuse and abuse, participants completed the POMAQ via web-based administration. Next, an in-depth semi-structured interview was conducted to assess the participant’s understanding of the instrument questions, response options, and suggested revisions. As the majority of participants would not see the entire POMAQ when completing it via the web (given the inherent response pattern), the paper version was presented during the interviews. Each interview took approximately 90 minutes. Following the interview, patients were asked to complete the Brief Pain Inventory-Short Form (BPI-SF)Citation22 and a sociodemographic form (e.g. age, gender, race, employment, education, comorbid conditions). Sites completed a clinical form about the participant’s pain condition(s), current medications, and other relevant clinical information. Participants were remunerated $100 for their time.
After the first 10 interviews, it was noted that many participants had difficulty completing the entire interview due to fatigue. As such, to reduce participant burden and ensure adequate interview coverage for all POMAQ items, the target sample size was increased from 20–30 to 50–60, and the interview approach was modified such that every other patient enrolled in the study started with the last question of the POMAQ and progressed backward to the first question. All patients responded to the concept elicitation questions regarding misuse and abuse at the start of the interview, and the same number of forward (n = 27) and backward (n = 27) interviews were conducted; of which a subset were full interviews. The mean time to complete the POMAQ online was 12 minutes (range: 3–27 minutes), and the mean duration of the qualitative interview was 92.4 minutes (range: 28–146 minutes). All questions were reviewed by at least seven patients per group. Twenty-one percent (n = 12) of participants were able to complete the full, qualitative, semi-structured interview. Given the questions did not build on each other, this was not felt to introduce bias to the study but ensured that at least seven patients from each of the four groups reviewed every question and set of response options.
Data analysis
Interviews were audio-recorded and transcribed verbatim. Transcripts were reviewed by the research team for content, corrected for obvious transcription errors, and participant identifying information was removed (e.g. references to names, doctors, places). A directed content analysis approach was used to analyze the qualitative concept elicitation interview data and identify, analyze, and report patterns and themes within the data. A coding dictionary was developed after reviewing the first 20 patient interviews and approved by all team members prior to coding the data by two coders. The initial coding of the first three transcripts by the individual coders was reviewed and adjudicated for consistency prior to coding all transcriptsCitation19,Citation23,Citation24. Qualitative cognitive interview data were also tabulated descriptively to present patient responses.
Descriptive analyses of the quantitative data were performed in SAS, version 9.4 (Cary, NC) (e.g. mean, standard deviation [SD], frequency) to characterize the participant sample.
Results
Demographic and clinical characteristics
Fifty-six patients were enrolled and completed the POMAQ while 54 completed the qualitative semi-structured interview. The mean age overall was 48.7 years (). The participants were predominately Caucasian (n = 44, 80%) and female (n = 32, 57%). Almost half (n = 24, 44%) reported receiving disability assistance, and 26% (n = 14) reported full-time employment. Most participants reported some college (n = 26, 47%), secondary/high school (n = 13, 24%), or a college degree (n = 12, 22%) as their highest level of education. No participants had a post-graduate degree. Participant self-report of their current health state varied where most reported “fair” (n = 19, 35%), “good” (n = 15, 27%), or “very good” (n = 12, 22%). The most prevalent comorbid conditions reported were arthritis (n = 30, 55%) and hypertension (n = 17, 31%).
Table 2. Participant-reported demographic characteristics.
Overall, participant responses on the BPI-SF severity items ranged from 4.2 to 7.2 (on a 0–10 scale, higher scores indicate greater pain severity). For the interference items, pain interfered mostly with normal work (score ± SD; 6.1 ± 3.4) followed by general activity (5.8 ± 3.1), walking ability (5.7 ± 3.3), and sleep (5.7 ± 3.0). Participants in the No Chronic Opioid Use group (Group 4) reported the lowest levels of severity and interference on the BPI.
For participants prescribed opioids for pain treatment, the mean length of time taking prescription opioid medication was 5.9 ± 6.4 (years ± SD; range 0.1–25 years) (). For participants engaged in known abuse of opioid pain medication, the mean length of time was 3.4 ± 5.0 years (years ± SD; range 0.2–20 years). More specifically, the mean time for taking prescription opioid medications for participants in the Known Opioid Abuse group (Group 1) was longer at 4.3 ± 6.3 (years ± SD; range 0.5–20 years) than the Known Abuse of Other Substances (Group 2) participants at 2.3 ± 2.1 (years ± SD; range 0.2–7 years). The most frequently reported current opioid medications were oxycodone alone or in combination with another medication (n = 17, 30%), hydrocodone alone or in combination with another medication (n = 16, 29%), morphine (n = 6, 11%) and tramadol (n = 6, 11%) alone or in combination with another medication. No meaningful differences were noted between the forward and backward interview administration groups in terms of responses to the POMAQ.
Table 3. Clinician-reported clinical characteristics.
Participant definitions of misuse and abuse
Participants were asked to describe “misuse” and “abuse” in their own words related to prescription opioid pain medication. The majority (n = 42/56, 75%; Group 1: n = 13/16; Group 2: n = 11/15; Group 3: n = 11/15 Group 4: n = 7/10) commonly described “misuse” as taking more medication than prescribed (e.g. taking 2 pills instead of 1) or on a different dosing schedule than prescribed (e.g. taking every 2 to 4 hours instead of every 6 to 8 hours). Other participants (n = 8/56, 14%; Group 1: n = 3/16; Group 2: n = 4/15; Group 3: n = 1/15) described “misuse” as taking a prescription opioid pain medication “to get high” or for another reason other than to treat pain (note: interviewer questions below are identified in bold text; as some patient quotes were spontaneous, not all quotes include interviewer questions).
And what does the word ‘misuse’ mean to you when related to prescription pain medication? Not using it the way that it’s exactly labeled from a doctor’s office…Abuse is, um, to me, is you knowingly, um, like if it says ‘don’t chew, cut or bite into a tablet’ you knowingly do it, uh, knowing that it’s going to be a stronger dose. Um, or you get medicines from friends of yours, um, because you’ve run out of yours. Um, that would be abuse. Misuse to me would be, um, like I had said earlier, taking two at one—one time instead of taking one because the pain was so bad. (006-010; Group 1, Male, age 58)
What does misuse mean to you when related to prescription pain medication? Not taking it as directed. What about abuse in relation to pain meds? What does that mean to you? Exactly what it says, people abuse it, right…Abuse is you know what your actions are, you know, you know that it’s going to get you high. (001-001; Group 3, Female, age 36)
Approximately half of participants (n = 28/56, 50%; Group 1: n = 8/16; Group 2: n = 11/15; Group 3: n = 7/15; Group 4: n = 2/10) described “abuse” as tampering with the medication, taking it “to get high” or “to get a buzz,” getting the medication from someone other than a healthcare professional, and selling or giving the medication away. Some participants (n = 22/56, 39%; Group 1: n = 8/16; Group 2: n = 3/15; Group 3: n = 6/15; Group 4: n = 5/10) described “abuse” as not taking the medication as prescribed (e.g. taking more than prescribed or on a different dosing schedule than prescribed), and others (n = 8/56, 14%; Group 1: n = 3/16; Group 2: n = 2/15; Group 4: n = 3/10) referred to “abuse” as an indication of an addiction problem.
Misuse reminds me, you know, taking it in any other order than is on the prescription…Abuse would be taking pills in pursuit of an altered state. (002-002; Group 2. Female, age 47)
What does misuse mean to you when related to prescription pain medication? Using it when you don’t need it. Taking it – if it was prescribed for me – you know one pill per day and I know that some people take one, two, three pills a day…More than prescribed. What does abuse mean to you in relation to prescription pain medication? I’ve seen alcoholism – it’s kind of like alcoholism. People get addicted to it and they can’t get off of it… (001-008; Group 4, Male, age 63)
Of note, eight participants (Overall: n = 8/56, 14%; Group 1: n = 3/16; Group 3: n = 3/15; Group 4: n = 2/10) specifically reported misuse to be more of an unintentional act, whereas abuse of opioid pain medications is intentional or deliberate. Seventeen participants (n = 17/56, 30%; Group 1: n = 5/16; Group 2: n = 5/15; Group 3: n = 5/15; Group 4: n = 2/10) reported the terms “misuse” and “abuse” to be similar or the same. Participants for whom English was a second language (n = 6/10; Group 3: n = 3/15 and Group 4: n = 3/15) had some difficulty describing the meaning of “misuse” in their own words.
In summary, most participants were able to describe the meaning of the terms misuse and abuse and agreed that the intentionality responses provided in the POMAQ reflected intentions consistent with prescription opioid misuse and abuse.
POMAQ cognitive interview results
The average time duration to complete the POMAQ via the web-based interface was 12 minutes (range 3 to 27 minutes), and the average patient interview lasted approximately 90 minutes. The majority of participants understood the response options as intended. All but one participant (n = 55/56, 98%; Group 1: 15/16; Group 2: 15/15; Group 3: 15/15; Group 4: 10/10) indicated they understood the instructions. Five participants reported confusion about whether Tylenol was an opioid due to the combination products of Tylenol with codeine. Based on these findings, the instructions were revised for clarity to differentiate the terms “over the counter” and “opioid,” Tylenol with codeine was added to the opioid examples, and “without codeine” was added in example of Tylenol over the counter.
All but one participant stated they were comfortable completing the POMAQ, and none indicated concerns about answering the questions honestly with all participants, stating they were honest when completing the POMAQ. A few (n = 6, 11%) participants reported concerns about completing the POMAQ using a secure internet site as they reported that they were either not computer savvy (n = 3, 5%) or were concerned about internet security (n = 3, 5%).
Recall period
A notable modification of the POMAQ from the SR-MAD was the inclusion of a three-month recall period. In the content validation and pilot testing research of the SR-MADCitation11, all patients indicated they would be able to accurately answer questions over a one-year and 30-day period. A third option of a three-month recall period was included in the POMAQ to measure the occurrence of lower frequency abuse/misuse behaviors. Patients verified the selection of one-year and one-month recall periods and indicated that a three-month interval was a relevant option to capture escalating patterns of use.
Specific behaviors (i.e. the number of times one engaged in a behavior) necessitated a shorter recall period (i.e. 1 month) in order to reduce recall bias, particularly when taking opioid medications. Importantly, patients reported that the three recall periods of the POMAQ (1 year, 3 months and 1 month) were acceptable and accurately captured variation in misuse/abuse patterns over time.
Items
Overall, the 19 items and associated sub-items of the POMAQ were well-understood by participants who reported clearly understanding item meaning, response options, and relevance ( for the 19 core POMAQ items). There were points of refinement suggested by participants regarding item phrasing and response options. For question 7 regarding medications that had been prescribed, one participant recommended including the generic names of all medications as generics are commonly prescribed. Following this feedback, the responses were revised to include generic names and a few uncommon medications were removed to accommodate the longer list.
A response option for question 8 related to accessing opioid pain medication from someone who was not a healthcare provider (i.e. “I took the opioid pain medication from the street of somebody I do not know”) was deleted following participant reports that the phrasing was confusing and a response to this item (“I bought some opioid pain medication from someone on the street”) was modified from “bought” to “got” following participant feedback that “got” could imply buying or stealing.
For question 9 regarding use of “street drugs,” participants indicated common and popular street drugs were missing from the list of options (i.e. bath salts, 5MEO), therefore the list was expanded to include their suggestions. In addition, participants recommended clarifying the response options for question 9c regarding reasons for taking street drugs (i.e. “Getting high on one drug is better when I am on another”) to make it more explicitly relevant to opioids versus combining two street drugs. The response option was revised to, “It is better to get high on my prescription opioid pain medication when on another drug.”
The list of possible response options reflecting reasons for endorsing question 10 (seeing multiple doctors) () was revised to include: (a) I was referred to another doctor; (b) My doctor did not understand my pain level; (c) My doctor thinks I am faking my pain.
Participants reported multiple interpretations to question 19 regarding having a problem with your opioid medication. Participants indicated understanding the meaning of the item as whether or not one “was addicted” or thought they might be addicted to opioids, other participants interpreted this question as experiencing side-effect, and/or “if you had a problem refilling your prescription.” Given that all interpretations could be correct, the question was restructured so that if a patient responded “yes,” they were shown multiple response options (not presented here) as to what type of problem they may be having to reflect patient statements regarding perceived prescription opioid problems.
Discussion
The POMAQ was developed in alignment with the FDA and ISPOR guidance for patient-reported outcome measures to address the lack of a well-validated patient screener to assess intentional prescription opioid misuse and abuse in a chronic pain patient population using a consistent definition of MARECitation21. The POMAQ demonstrated content validity in a chronic pain population and was designed to assess intentionality among misuse/abuse behaviors following the ACTTION definitions, thus filling a critical measurement gap for the assessment of prescription opioid misuse and abuse behaviors. While the definitions and terminology of prescription opioid misuse and abuse continue to evolve, with the most current DSM-5 definition of substance use disorder not reflected in the POMAQ, the behaviors and intention of misuse and abuse remain consistent regardless of definition. The POMAQ fills an unmet need in screening for prescription opioid misuse and abuse to provide documentation as to the scope of misuse and abuse outcomes in patients with chronic pain who are on long-term opioid therapy. The use of a screener such as the POMAQ has the potential to identify patients engaged in misuse and abuse early in therapy so that interventions can be employed to reduce such behaviors.
For a screener to be useful, it must be used by healthcare professionals. Convincing busy healthcare professionals to use screeners required demonstration of usefulness, which the POMAQ currently does not have. A quantitative validation study of the POMAQ has been completed and it is currently being evaluated in a 12-month, longitudinal observational study. While awaiting the longitudinal results, it is important to note that the POMAQ was designed for ease of use with a web interface, streamlining the administration by reducing administrative burden and providing anonymity and security for patient completion to engender greater honesty in responding. This implementation approach should appeal to healthcare practitioners as the POMAQ could be deployed as a monitoring questionnaire for patients on long-term prescription opioid therapy to complete electronically on a quarterly basis. Should misuse or abuse behaviors be reported, alerts could be sent to the healthcare provider, which would trigger a follow-up phone call or visit to further evaluate the patient status. Such information could provide insight into patients who are at risk of adverse consequences.
In addition to the web interface, the POMAQ instructions, recall periods (one year, three months, one month), 19 items, and item response options have all demonstrated content validity and were well-understood by adult patients with chronic and acute pain with and without prior opioid experience. The vast majority of patients reported feeling comfortable completing the instrument via a web-based platform, and none reported concerns about answering the questions honestly. To counter concerns regarding the security of the internet, the POMAQ should only be hosted on secure environments so patients are confident that their anonymity is maintained, allowing them to respond more honestly. Refinements were made to a small set of items and response options in the POMAQ after the qualitative interviews to better capture patients’ preferred phrasing and experiences regarding prescription opioid pain medication use, misuse, abuse, and other drug use.
Qualitative research employs a methodology that provides in-depth, nuanced patient data, but generally on a small sample. As such, these cognitive interview results are understood to reflect an overview of patient experiences but are not intended to be generalizable to all prescription opioid users, given the small sample size. Other limitations of this research may include an unaccounted order effect by administering the interview guide in forward and backward assignment, given the length of the interview, although the distribution of participants across opioid groups and interview response assignment should mitigate any administration order effect response bias. As with all research on sensitive topics, the results are based on presumed honest responses from the participants. Importantly, the goal of the cognitive interview was to understand how patients interpreted the questions and if they understood what was being asked, which was demonstrated.
Conclusions
The POMAQ has demonstrated content validity among patients with chronic pain (e.g. questions were interpreted as intended, phrased in a manner understood by patients). In alignment with FDA guidanceCitation21, psychometric evaluation to understand the statistical properties of the screener with a cohort of patients with chronic pain who are currently taking prescription opioids has recently been completed as the next stage of development of the POMAQ and will be presented in subsequent publications. Establishing the content validity of the POMAQ is the first step to support its use in post-approval and long-term follow-up studies, which could support the accurate assessment of the incidence, prevalence, and persistence of prescription opioid misuse and abuse.
Transparency
Declaration of funding
This project was conducted as part of a Food and Drug Administration (FDA)-required post-marketing study for extended-release and long-acting opioid analgesics and was funded by the Opioid Postmarketing Consortium (OPC) consisting of the following companies: Allergan; Assertio Therapeutics, Inc.; BioDelivery Sciences, Inc.; Collegium Pharmaceutical, Inc.; Daiichi Sankyo, Inc.; Egalet Corporation; Endo Pharmaceuticals, Inc.; Hikma Pharmaceuticals USA Inc.; Janssen Pharmaceuticals, Inc.; Mallinckrodt Inc.; Pernix Therapeutics Holdings, Inc.; Pfizer, Inc.; Purdue Pharma, LP.
Declaration of financial/other relationships
Karin S. Coyne and Anne Brooks are employees of Evidera, a company that received funding from the Opioid Postmarketing Consortium (OPC) to conduct this study. At the time of the study, Alexandra I. Barsdorf was employed by Pfizer, a member company of the OPC. At the time of the study, Jean-Yves Mazière was an employee of PPD, a parent company to Evidera and is now an employee of Astellas. Renee F. Pierson is an employee of Janssen, a member company of the OPC. Sidney H. Schnoll is an employee of Pinney Associates, a company that received funding from the OPC for time spent consulting on this study. Stephen F. Butler received funding from the OPC for time spent consulting on this study. All aspects of the study design, interpretation, and decision to submit for publication were determined by the authors.
Author contributions
All authors were involved in the conception and design of the study, analysis and interpretation of the data, drafting of the manuscript, and revising it critically for intellectual content. All authors provided final approval of the version to be published.
Acknowledgements
We thank Naomi Knoble, PhD, for her writing assistance and Kawthar Nakayima, Amara Tiebout, and Michael Grossi for their production and editorial assistance.
References
- Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharmacol Toxicol. 2018;58:143–159.
- Meske DS, Lawal OD, Elder H, et al. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. JPR. 2018;11:923–934.
- Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569–576.
- Lipari RN, Hughes A. How people obtain the prescription pain relievers they misuse. In: The CBHSQ Report. Rockville (MD): Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2017.
- National Academies of Sciences and Medicine, editor. Pain management and the opioid epidemic: balancing societal and individual benefits and risks of prescription opioid use. Washington (DC): The National Academies Press; 2017.
- Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press; 2011.
- Smith SM, Dart RC, Katz NP, Nov, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154(11):2287–2296.
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432–442.
- Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7(9):671–681.
- Coambs R, Jarry J, Santhiapillai A, et al. The SISAP: a new screening instrument for identifying potential opioid abusers in the management of chronic nonmalignant pain within general medical practice. Pain Res Manage. 1996;1(3):155–162.
- Setnik B, Roland CL, Barsdorf AI, et al. The content validation of the Self-Reported Misuse, Abuse and Diversion of Prescription Opioids (SR-MAD) instrument for use in patients with acute or chronic pain. Curr Med Res Opin. 2017;33(6):1067–1076.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9(4):360–372.
- Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the current opioid misuse measure. Development and validation of the current opioid misuse measure. Pain. 2007;130(1–2):144–156.
- Smith SM, Paillard F, McKeown A, et al. Instruments to identify prescription medication misuse, abuse, and related events in clinical trials: an ACTTION systematic review. J Pain. 2015;16(5):389–411.
- Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):131–146.
- Passik SD, Kirsh KL, Casper D. Addiction-related assessment tools and pain management: Instruments for screening, treatment planning, and monitoring compliance. Pain Med. 2008;9(Suppl 2):S145–S166.
- Food and Drug Administration, Department of Health and Human Services. Postmarketing Requirements under 505(o) (Study 3033-3). Available from: https://www.fda.gov/media/95546/download.
- Coplan PM, Cepeda MS, Petronis KR, et al. Postmarketing studies program to assess the risks and benefits of long-term use of extended-release/long-acting opioids among chronic pain patients. Postgrad Med. 2020;132(1):44–51.
- Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1-eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–977.
- Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2-assessing respondent understanding. Value Health. 2011;14(8):978–988.
- Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Fed Regist. 2009;74:65132–65133.
- Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994;23(2):129–138.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288.