Abstract
Objective
Despite the availability of multiple screening modalities for early detection of colorectal cancer (CRC), participation rates remain well below guideline recommendation goals in the United States. This study analyzed and compared recent national trends in utilization of CRC screening modalities using Medicare claims data.
Method
Medicare claims data for CRC screening during 2014–2018 were aggregated and analyzed by CPT code frequency. Changes in CRC screening test frequencies during the analysis period were measured via generalized linear models and analysis of compound annual growth rates (CAGRs).
Results
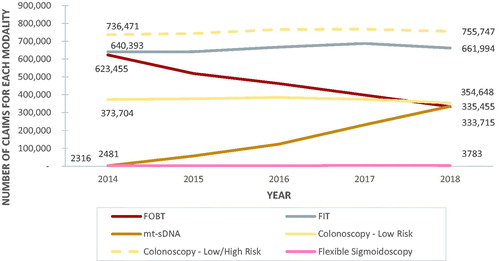
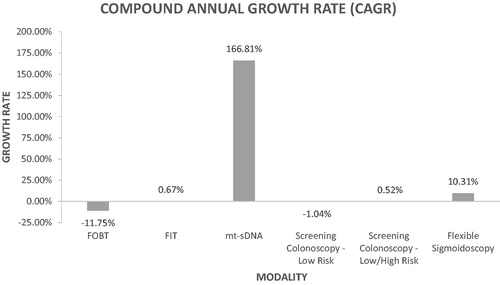
Utilization of the mt-sDNA test increased significantly over time (from 2481 claims in 2014 to 335,455 claims in 2018; p < .001), in contrast to the other analyzed CRC screening tests. The CAGR was higher for mt-sDNA (166.81%) than for COL (0.52%), FOBT (–11.75%), and FIT (0.67%).
Conclusions
Utilization of the mt-sDNA test for average-risk CRC screening has increased rapidly since its approval in 2014. These data support growing patient and provider interest in the mt-sDNA test as a non-invasive option for average-risk CRC screening.
Objective
Colorectal cancer (CRC) remains the second-leading cause of cancer-related death in the United States (US) despite being highly preventable and curable through effective screening programsCitation1. The US National Colorectal Cancer Roundtable has established a goal for CRC screening adherence of at least 80% among adults 50 and older, but current rates remain well below this targetCitation2,Citation3. The objective of this analysis was to analyze and compare recent trends in CRC screening modalities using publicly available Medicare claims data to inform ongoing efforts to achieve the national CRC screening adherence target of > 80%.
Methods
Medicare claims data for CRC screening from 2014 to 2018Citation4 were aggregated and analyzed by CPT code frequency for colonoscopy [COL: G0121 (colorectal cancer screening; colonoscopy on individual not meeting the criteria for high risk), G0105 (colorectal cancer screening; colonoscopy on individual at high risk)], flexible sigmoidoscopy [FSIG: G0104], fecal occult blood test [FOBT: 82270, G0107], fecal immunochemical test [FIT: G0328], and multi-target stool DNA [mt-sDNA: G0464, 81528]. Since CT colonography is not covered by Medicare, this screening modality was not included in our analyses. A generalized linear model was used to examine differences in CRC screening test frequencies during the analysis period. Compound annual growth rate (CAGR) and year-over-year (Y/Y) growth rates were also analyzed for each screening modality over the analysis period (2014–2018). The study analyses were performed in November 2019, with statistical analyses performed in R version 3.6.3.
Results
FSIG represented < 1% of all CRC screening tests over the entire analysis period and was not further considered in the analyses. COL (including both low and high-risk codes) accounted for the most CRC screening tests over the entire analysis period (>700,000 claims from 2014 to 2018; ). Utilization of the mt-sDNA test increased significantly over time (from 2481 claims in 2014 to 335,455 claims in 2018; p < .001; ), as compared to the other analyzed CRC screening tests. Conversely, FOBT exhibited the most striking decline in utilization (from 623,455 claims in 2014 to 333,715 claims in 2018; p < .001; ). COL was relatively stable throughout the analysis period (from 373,704 claims in 2014 to 354,648 claims in 2018 for low-risk claims only; 736,471 claims in 2014 to 755,747 in 2018 for combined low- and high-risk claims; p > .05 for both; ). As shown in , the CAGR was higher for mt-sDNA (166.81%) than for COL (0.52%), FOBT (–11.75%), and FIT (0.67%).
Discussion
Based on this analysis of publicly available Medicare claims data from 2014 to 2018, utilization of the mt-sDNA test for average-risk CRC screening has increased rapidly since it received FDA approval in August 2014, while utilization of other endorsed CRC screening modalities has either declined (FOBT) or remained relatively stable (COL, FIT) over the same time period. These data reveal growing patient and provider interest in the molecularly-based mt-sDNA test as a non-invasive option for average-risk CRC screening. Given that some studies have found that stool-based screening modalities increase the likelihood of screening completion by up to 10%Citation5, and the national 80% CRC screening goal has not yet been achieved, this increase in mt-sDNA may be useful in accelerating progress toward the 80% adherence target. Increased adherence to guideline-endorsed strategies would enhance the programmatic effectiveness of average-risk CRC screening, with consequent reductions in CRC incidence and mortality.
The main limitation of our analysis is that screening COL is underestimated by our methodology, as screening COLs resulting in biopsy or other intervention during the procedure are likely not captured with the applied codes. Despite the potential underestimation, screening rates for COL appeared to be relatively constant over time (). In addition, we note that since CTC is not covered by Medicare, this screening modality was not included in our analyses. Finally, further investigation using data from other sources, including those that include non-Medicare patients, are encouraged to confirm and extend these findings.
In summary, increased uptake in mt-sDNA may have a considerable public health impact by advancing the 80% national CRC screening target, with consequent reductions in both CRC incidence and mortality.
Transparency
Declaration of interest
A.B. Ozbay and Marcus Parton are employees of Exact Sciences Corporation. P.J. Limburg serves as Chief Medical Officer for Screening at Exact Sciences through a contracted services agreement with Mayo Clinic. Dr. Limburg and Mayo Clinic have contractual rights to receive royalties through this agreement. L.J. Finney Rutten is a consultant to Exact Sciences Corporation through a contractual agreement between Mayo Clinic and Exact Sciences Corporation. Dr. Kisiel is listed as an inventor of intellectual property jointly owned by Mayo Clinic and Exact Sciences. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Society AC. Cancer Facts and Figures 2019. 2019, November 11. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf.
- Levin TR. Colorectal cancer screening: 80% by 2018. Colonoscopists simply cannot do it alone. Gastrointest Endosc. 2016;83(3):552–554.
- Working toward the shared goal of 80% screened for colorectal cancer by 2018. 2019, November 7. Available from: https://nccrt.org/what-we-do/80-percent-by-2018/.
- Part B national summary data file. Centers for Medicare and Medicaid Services; 2019.
- Liles EG, Perrin N, Rosales AG, et al. Change to FIT increased CRC screening rates: evaluation of a US screening outreach program. Am J Manag Care. 2012;18(10):588–595.