Abstract
Background
Since the first cases of COVID-19 were reported in Wuhan, the nutritional status of individuals infected with the virus has not been included in the risk profiles prepared. However, nutritional status, along with other factors, is decisive in the evolution of patients with other infectious diseases. The nutritional status of individuals is considered an indicator of health status. Furthermore, optimal nutritional status transcends the individual, and poor diet in a population can be considered a group risk factor. Evidence exists on the influence that diet has on the immune system and susceptibility to disease.
Objective
To evaluate the nutritional status of patients older than 65 years who were admitted due to COVID-19 and how this has influenced the evolution of patients.
Design
This prospective and observational study was performed in patients with COVID-19 infection confirmed by real-time polymerase chain reaction. Data were collected from the first 24 h of admission. All patients admitted during one month to the wards assigned to COVID-19 infection were included.
Results
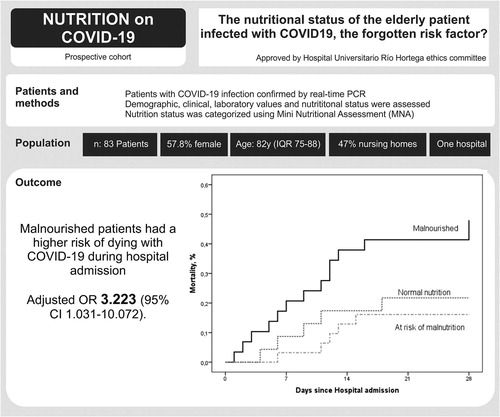
A total of 83 patients were studied. The statistical study of mortality showed associations with age (p = .005), living in a nursing home (p = .022), a high Charlson Comorbidity Index (p = .039), hypertension (p = .032), comorbidities of dementia (p = .019) and cerebral vascular disease (p = .041), and Barthel Index (p = .010). The analysis of the influence of the nutritional state on mortality revealed a statistical association between malnutrition and mortality in the pooled data analysis (p = .005) and analysis by degrees of malnutrition (p = .27).
Conclusions
Malnutrition was a risk factor as powerful as others such as hypertension, age, and different comorbidities. We must evaluate and treat the nutritional status of elderly patients with COVID-19 infection since it directly affects their evolution.
Graphical Abstract
Introduction
Since the first cases of COVID-19 were reported in Wuhan, in China’s Hubei province, the virus has rapidly spread to other countries, causing a global pandemic with high rates of infection and mortalityCitation1,Citation2. From the beginning, physicians have seen the different therapeutic strategies for these patients followed in hospitals and communicated in scientific journals. In parallel, we have assisted in preparing risk profiles for contracting the virus, hospital and intensive care unit admission, and death from COVID-19 infection. In all the published profiles, age, hypertension, male sex, diabetes mellitus, obesity, and previous cardiovascular diseases have been seen as constant risk factors in different countriesCitation3–7. Notably, the nutritional status of individuals infected with COVID-19 has not been included in the risk profiles prepared. However, nutritional status, along with other factors, is decisive in the evolution of patients with other infectious diseases.
The nutritional status of individuals is obviously considered an indicator of health status, in addition to being an element of resistance against intercurrent diseases. Furthermore, optimal nutritional status transcends the individual, and poor diet in a population can be considered a group risk factorCitation7. The concept that nutritional status directly impacts the proper functioning of the immune system is not new. Evidence exists on the influence of diet on the immune system and susceptibility to disease. Deficiencies in specific nutrients or combinations of nutrients can affect the immune system through cell activation, changed production of signaling molecules, and gene expressionCitation8. In addition, the components of the diet are significant determinants of the intestinal microbial composition and, consequently, can shape the characteristics of immune responses in the bodyCitation9. Nutritional deficiencies in energy, protein, and specific micronutrients have been associated with depressed immune function and increased susceptibility to infection. Animal models and epidemiological studies have indicated that deficiencies in specific nutrients, particularly selenium and vitamin E, can lead to reproducible genetic mutations and increased virulence of certain viruses, including the coxsackievirus, poliovirus, and murine influenzaCitation10,Citation11.
Some of the molecular mechanisms of how micronutrients optimize immune function have been describedCitation12. Most micronutrients exhibit pleiotropic functions in support of immune function. Regarding innate immunity, the relevant vitamins and minerals work collectively to support the development and maintenance of physical barriers; antimicrobial protein production and activity; growth, differentiation, and chemotaxis of innate cells; phagocytic and destructive activities of neutrophils and macrophages; and promotion and recovery of inflammationCitation13. This is why nutritional screening of patients admitted to hospitals for different pathologies must be performed. In the case of COVID-19 infection, assessment with different methods such as the Geriatric Nutritional Risk Index (GNRI) or bioelectrical impedance analysis (BIA) is useful to better serve these patientsCitation14,Citation15.
Given this background, this prospective and observational study evaluated the nutritional status of patients older than 65 years who were admitted due to COVID-19. In addition, we aimed to evaluate how this state can influence the evolution of patients.
Materials and methods
Patients
This prospective and observational study was performed in patients with COVID-19 infection confirmed by real-time polymerase chain reaction. Data was collected from the first 24 h of admission. All the data collected belongs to the usual clinical practice of the Río Hortega University Hospital, Valladolid, Spain.
During one month (April 2020), all patients admitted to the wards assigned to attend to respiratory COVID-19 infection were included. Individuals were excluded if it was not possible to obtain data on the relevant study variables or if they had a diagnosis of cancer or a terminal illness, were being followed by the palliative care unit, or had been previously diagnosed with malnutrition. A total of 83 patients were studied.
Demographical data, clinical and laboratory values, and nutritional status
Demographic characteristics, such as patient age, sex, date of birth, and place of residence, were extracted from the electronic medical record. Medical antecedents and pre-admission treatments were obtained from admission reports and the electronic history of primary care. The blood test variables were extracted from the routine tests at admission to the internal medicine units.
Anthropometric data (weight and height) were obtained from the assessment performed by the nursing staff at admission. The assessment of nutritional status was made using the Mini Nutritional Assessment—MNA scale electronic version (https://www.mna-elderly.com/forms/mna_guide_spanish.pdf). The interpretation of the results used the following classification: a total score of ≥12 indicates that the person is well-nourished, 8–11 indicates that the patient is at risk of malnutrition, and ≤7 indicates that the patient is malnourished.
An online calculator was used to determine the Barthel Index (https://www.rccc.eu/ppc/indicadores/Neuro/Barthel.html). A Barthel Index score of 100 indicates independence at a functional level, 99–91 mild dependency, 90–61 moderate dependency, 21–60 severe dependency, and ≤20 total functional dependencyCitation16. The Charlson Comorbidity Index was determined using an electronic calculator on the website of the Andalusian Society of Intensive Medicine and Coronary Units (http://www.samiuc.es/indice-de-comorbilidad-de-charlson-cci/).
Statistical analysis
The sample size was defined as 83 malnourished patients and 84 patients at risk of malnutrition or with normal nutrition. To calculate this sample size, we established a 20% death rate in our patientsCitation17 and an expected increase to 40% in the malnourished group. At the time of the study design, no reported data existed on the COVID-19 death rate in malnourished patients, so to establish the expected mortality rate in this group, we assumed the death rate of the first 20 malnourished patients analyzed as the true mortality. A power of 80% and a significance of 5% were established. Due to the partial end of the COVID-19 epidemic in our area, the sample number was not accomplished. However, due to the achievement of statistical significance, we decided to provide preliminary descriptive statistics. The sample size was equal to the number of patients whose nutritional parameters were studied during the study period.
Due to the low sample size of our study, we used the Shapiro–Wilk test as a preventive assessment to test whether our population followed a normal distribution. Most variables did not follow a normal distribution. The descriptive analysis of the continuous variables is expressed as the median and interquartile range (IQR). Categorical variables are presented as the number of patients (%) with a 95% confidence interval (CI). Categorical data were compared using the Chi-square test or the Fisher exact test when needed. For crosstabs larger than 2 × 2, we used the linear-by-linear association test to assess for trends. Continuous data were compared using the Mann–Whitney rank sum test.
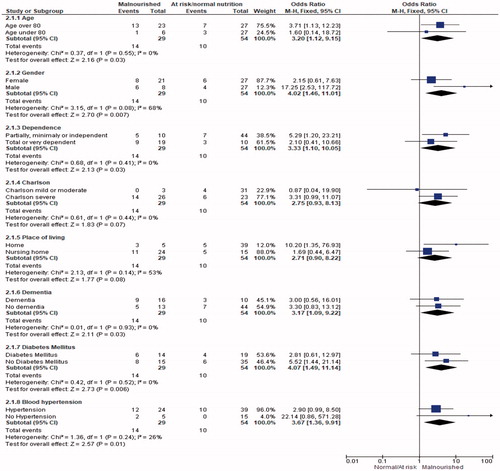
To assess the associations of the MNA with death, we performed simple logistic regression analysis. We performed forward Cox regression analysis with death during admission as the dependent variable and the following nine variables selected as likely related to death: age (≥80 years vs. <80 years), gender, Barthel Index, Charlson Comorbidity Index, place of living (nursing home vs. home), dementia, diabetes mellitus, blood hypertension, and MNA (malnourished vs. at risk of malnutrition or normal nutritional status) to adjusted MNA. A figure was built with the combined risk ratio of MNA and each of the selected variables computed with the Mantel–Haenszel method. To adjust MNA according to gender, age, comorbidity (Charlson Comorbidity Index score plus hypertension), dependency (Barthel Index), dementia, living in a nursing home, and dysphagia, we performed backward stepwise logistic regression. Odds ratios (ORs) are presented with 95% CIs. All tests were two-sided with statistical significance set at p<.05. All analyses were performed with SPSS version 21.0 (IBM SPSS).
Results
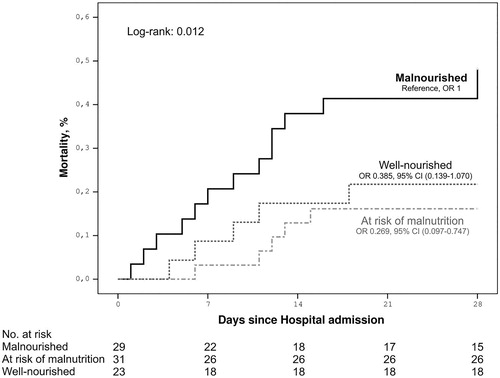
The description of the population studied can be seen in the second column of . The average age of the patients studied was 82 years. Over half were women (57.8%), and almost half lived in nursing homes (47%), with different degrees of dependency. Of the patients, 39.8% were diabetic and 75.9% were hypertensive. The history of medication treatments is shown in Supplementary Table 1. The results of the analytical measurements are shown in Supplementary Table 2. The nutritional status results measured by the MNA show that 34.9% of the patients were malnourished, 37.3% were at risk of malnutrition, and 27.7% were without malnutrition, as shown in .
Table 1. Statistical result of comparing patients who have died and who have survived after COVID19 infection based on clinical variables, comorbidities, dependency.
Table 2. Nutrition measures, MNA test results.
In the statistical study of mortality, patients who were included in the category of “living” were classified at the time of hospital discharge. We showed associations with age (p=.005), living in a nursing home (p=.022), a high Charlson Comorbidity Index (p=.039), hypertension (p=.032), comorbidities of dementia (p=.019) and cerebral vascular disease (p=.041), and Barthel Index (p=.010; ). The analysis of the influence of the nutritional state on mortality revealed a statistical association between malnutrition and mortality in the pooled data analysis (p=.005) and analysis by degree of malnutrition (p=.27; ; ). For the unadjusted MNA, malnourished versus at risk or normal, p=.006, OR = 4.107 (1.509–11.174). For the adjusted MNA, malnourished versus at risk or normal, p=.044, OR = 3.223 (1.031–10.072; ).
Discussion
From the beginning of the COVID-19 pandemic, risk profiles for infection have been developed. Age, vascular risk factors, and previous pulmonary pathologies have been postulated as some of the main risk factors. Different treatments have been proposed to care for infected patients. Strikingly, neither the risk profiles nor the proposed treatments have mentioned the nutritional status of the patient as a risk factor for poor evolution. In the present study, we found a direct relationship between the nutritional status of COVID-19 patients, measured by the MNA, and the risk of dying (p=.005).
The characteristics of the population that we studied are very similar to those in other published case series of COVID-19 infection from different geographic areasCitation4,Citation5. Furthermore, the risk factors for mortality were as shown in the most recent literature, as were the associations we found between mortality and age (p=.005) and hypertension (p=.005). In our cohort of patients, the risk of death was associated with the Barthel Index (p=.010) and the Charlson Comorbidity Index score (p=.035), both reflecting that the patients with the greatest pathology and degree of dependency had the highest risk of mortality. These findings are consistent with the fact that the patients with the highest mortality came from nursing homes (p=.022), where patients have the greatest need for help and number of comorbidities. In addition, patients with greater comorbidities and dependency have a higher risk of malnutrition, a fact clearly observable in our sample. The main hypothesis of our work was verified in the sample, with malnutrition measured by the MNA associated with mortality, in both the analysis of the pooled data (p=.05) and the data stratified by the degree of malnutrition (p=.027).
These results are unsurprising. In advanced age is a high rate of malnutrition, and states of nutritional deficiency harm normal human functioningCitation18, including immune functionCitation19. The higher prevalence of malnutrition in elderly patients makes them more vulnerable to infectious processes, as shown by different series of malnutrition prevalence in hospitalized patients, in addition to how it influences their prognosisCitation20. The results of our sample can be compared with other case series of COVID-19 infection, if we focus on age, comorbidities, vascular risk, and degree of dependency. Articles have hypothesized about the results of the risk that malnutrition confersCitation21–24, but few studies have measured the association. Li et al.Citation25 reported similar results regarding the prevalence of malnutrition and the risk of death. The study by Allard of a cohort of French patients found an incidence of malnutrition similar to ours (38.9 vs. 34.9%), with a risk result for poor evolution and deathCitation26. Our results agree with those of Recinella et al.Citation16, who used a different screening method, the GNRI, to find that the nutritional status assessed was a significant predictor of survival in elderly patients hospitalized for COVID-19. They found that this data, associated with the PaO2/FiO2 ratio, was a good prognostic model in these patients. Further congruent results have been found in other cohorts of patients studied from a nutritional viewpoint with the GNRI and MNACitation27. Regardless of the nutritional screening method, these studies show, like ours, the association between malnutrition and worse infection with COVID-19.
One of the limitations of our study is the inability to analyze the data based on body composition. We could not measure the skeletal muscle mass, fat mass, and fat distribution—important determinants of cardiometabolic risk and severe COVID-19. The conditions of managing these patients during the pandemic and isolation measures prevented obtaining more data that would have enriched our research.
Conclusions
Different risk factors for COVID-19 infection have been investigated, leading to different risk profiles being raised for hospital admission or death. In this study, malnutrition was a risk factor as powerful as others such as hypertension, age, or different comorbidities. We must evaluate and treat the nutritional status of elderly patients with COVID-19 infection since it directly affects their evolution.
Transparency
Declaration of funding
This paper was not funded.
Declaration of interests
All authors declare that there is no conflict of Interest.
Author contributions
Jessica Abadía Otero: Collection of patients and clinical data
Laisa Socorro Briongos: Collection of patients and clinical data, analysis of results
Miriam Gabella Matín: Collection of patients and clinical data
Iciar Usategui Martín: Collection of patients and clinical data
Pablo Cubero Morais: Collection of patients and clinical data
Luis Cuellar Olmedo: analysis of results
Luis Inglada Galiana: analysis of results
Carlos Dueñas Gutiérrez: analysis of results
Juana Carretero Gómez: analysis of results and writing of the paper
Luis Corral Gudino: analysis of results and writing of the paper
José Pablo Miramontes González: study design, analysis of results, and writing of the paper.
Statement of ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki, also under the supervision of the centre's ethics committee, which approved the protocol and supervised its follow-up.
Supplemental Material: Table 2
Download MS Word (13.2 KB)Supplemental Material: Table 1
Download MS Word (12.8 KB)References
- Bogoch II, Watts A, Thomas-Bachli A, et al. Pneumonia of unknown etiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;272:1–3.
- Zhu N, Zhang D, Wang W, et al.; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733.
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95.
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
- Team CC-R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12 – March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386.
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. Erratum in: JAMA. 2020;323(16):1619.
- Stefan N, Birkenfeld AL, Schulze MB, et al. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342.
- Marasco G, Dajti E, Ravaioli F, et al. Clinical impact of sarcopenia assessment in patients with liver cirrhosis. Expert Rev Gastroenterol Hepatol. 2020. DOI:10.1080/17474124.2021.1848542.
- Valdés-Ramos R, Martínez-Carrillo BE, Aranda-González II, et al. Diet, exercise and gut mucosal immunity. Proc Nutr Soc. 2010;69(4):644–650.
- Wypych TP, Marsland BJ, Ubags ND. The impact of diet on immunity and respiratory diseases. Ann Am Thorac Soc. 2017;14(Supplement_5):S339–S47.
- Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22(1):115–125.
- Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133(5 Suppl 1):1463S–1467S.
- Beck M, Handy J, Levander O. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12(9):417–423.
- Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211.
- Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
- Recinella G, Marasco G, Serafini G, et al. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: a monocentric study. Aging Clin Exp Res. 2020;32(12):2695–2701.
- Bianchetti A, Bellelli G, Guerini F, et al. Improving the care of older patients during the COVID-19 pandemic. Aging Clin Exp Res. 2020;32(9):1883–1888.
- Cobos-Siles M, Cubero-Morais P, Arroyo-Jiménez I, et al. Cause-specific death in hospitalized individuals infected with SARS-CoV-2: more than just acute respiratory failure or thromboembolic events. Intern Emerg Med. 2020;15(8):1533–1544.
- Martínez-Martín P, Fernández-Mayoralas G, Frades-Payo B, et al. Validation of the Functional Independence Scale. Gac Sanit. 2009;23(1):49–54.
- Collins N. Dietary regulation of memory T cells. IJMS. 2020;21(12):4363.
- Holder H. Malnutrition in the elderly: a public health concern. Br J Nurs. 2020;29(2):118–119.
- Bellanti F, Lo Buglio A, Quiete S, et al. Comparison of three nutritional screening tools with the new glim criteria for malnutrition and association with sarcopenia in hospitalized older patients. JCM. 2020;9(6):1898.
- Lidoriki I, Frountzas M, Schizas D. Could nutritional and functional status serve as prognostic factors for COVID-19 in the elderly?.Med Hypotheses. 2020;144:109946.
- Soares MJ, Müller MJ. Editorial: Nutrition and COVID-19. Eur J Clin Nutr. 2020;74(6):849.
- Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–875.
- Allard L, Ouedraogo E, Molleville J, et al. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020;12(12):3679.
- Abd-El-Gawad WM, Abou-Hashem RM, El Maraghy MO, et al. The validity of Geriatric Nutrition Risk Index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin Nutr. 2014;33(6):1108–1116.