Abstract
Objectives
To evaluate the efficacy and safety of oral sitafloxacin versus oral moxifloxacin in the treatment of Chinese adults with community-acquired pneumonia (CAP).
Patients and methods
This is a multicenter, randomized, open-label, positive-controlled clinical trial (chinadrugtrials.org.cn identifier: CTR20130046). CAP patients received sitafloxacin tablets 100 mg once daily (qd) or 100 mg twice daily (bid) to compare with moxifloxacin tablets 400 mg qd, for 7–10 days. The primary outcome was non-inferiority of sitafloxacin to moxifloxacin in clinical cure rate at test of cure (TOC) visit in per-protocol set (PPS).
Results
A total of 343 patients were randomized (sitafloxacin 100 mg qd, n = 117; sitafloxacin 100 mg bid, n = 116; moxifloxacin, n = 110), 291 patients were included in the PPS (sitafloxacin 100 mg qd, n = 96; sitafloxacin 100 mg bid, n = 94; moxifloxacin, n = 101). The clinical cure rate was 94.8% in the sitafloxacin 100 mg qd group, 96.8% in the sitafloxacin 100 mg bid group and 95.0% in the moxifloxacin group. At the TOC visit, the microbiological success rate was 97.0% (32/33) in the sitafloxacin 100 mg qd group, 97.1% (34/35) in the sitafloxacin 100 mg bid group and 94.9% (37/39) in the moxifloxacin group in the microbiological evaluable set (MES). The incidence of study-drug-related adverse events (AEs) was 23.3% (27/116) in the sitafloxacin 100 mg qd group, 29.8% (34/114) in the sitafloxacin 100 mg bid group and 28.2% (31/110) in the moxifloxacin group (p > .05). The common AEs related to study drug were dizziness, nausea, diarrhea, increased platelet count and alanine transaminase (ALT) elevation. All the AEs resolved completely after discontinuation of study drug.
Conclusion
Sitafloxacin 100 mg qd or 100 mg bid for 7–10 days is not inferior to moxifloxacin 400 mg qd for 7–10 days in clinical efficacy for adult CAP patients. Sitafloxacin provides a safety profile comparable to moxifloxacin.
Introduction
Community-acquired pneumonia (CAP) is one of the commonest infectious diseases threatening human health and life. It is also one of the major causes of mortality due to infectious diseases in the worldCitation1–3. The distribution and antibiotic resistance of CAP pathogens not only vary with country and geographical region, but also change over time. However, the most common pathogens are still Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis, followed by Klebsiella pneumoniae and Staphylococcus aureus. Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella pneumophila are also important atypical pathogens of CAPCitation4–6.
At the present time, CAP treatment is becoming more and more challenging and troublesome because the pathogens of CAP are increasingly resistant to the primary antimicrobial therapies such as penicillins and macrolides. CHINET surveillance of bacterial resistance in 2017 across China reported that of the 1288 strains of non-meningitis S. Pneumonia isolated from adults, nearly all (100%) of the penicillin-nonsusceptible strains, including penicillin-intermediate S. pneumoniae (PISP) and penicillin-resistant S. pneumoniae (PRSP), were resistant to erythromycinCitation7. In recent years, newer fluoroquinolones have become one of the important treatments for CAP in adults because these fluoroquinolones have good antimicrobial activity against key CAP pathogens such as S. pneumoniae (including penicillin-nonsusceptible strains) and H. influenzae, as well as M. pneumoniae and other atypical pathogens. In the guidelines published by the American Thoracic Society and the Infectious Diseases Society of America on CAP in adults, empirical fluoroquinolone monotherapy is recommended for CAP in adult in patients who have pulmonary or heart disease, or other underlying disease and outpatients with complicated CAPCitation2.
Sitafloxacin is a novel fluoroquinolone antimicrobial agent developed by Daiichi Sankyo Company Limited. It was approved and launched onto the Japan market in 2008. Sitafloxacin works primarily by inhibiting the activity of DNA gyrase and topoisomerase IV with broad spectrum antimicrobial activity against gram-positive bacteria such as S. pneumoniae and S. aureus, and gram-negative organisms including H. influenzae and K. pneumoniae, as well as atypical pathogens. Sitafloxacin has shown better antimicrobial effects than levofloxacin and moxifloxacinCitation8–15. Furthermore, sitafloxacin has a potent inhibitory effect on the activity of the enzymes encoding the mutation of the quinolone resistance-determining region (QRDR)Citation10. Therefore, for strains resistant to other fluoroquinolones, sitafloxacin still has powerful antimicrobial activity. Both the frequency of naturally emerging resistant strains and in vitro assay of acquired resistance indicate that the emergence of resistant strains is less likely for sitafloxacin than for other fluoroquinolonesCitation16.
The dosage of sitafloxacin is usually 50 mg bid, or 100 mg qd or bid in adults. A clinical trial conducted in Japan showed that sitafloxacin treatment resulted in a clinical cure rate of 92.5% in patients with CAP or acute exacerbation of chronic respiratory diseases, while levofloxacin achieved a clinical cure rate of 92.1%Citation17. Another study reported that sitafloxacin treatment achieved a clinical cure rate of 93.3% in mild to moderate CAP patients, while tosufloxacin showed a clinical cure rate of 89.6%Citation18.
This study was a multicenter, randomized, open-label, parallel group study to evaluate the efficacy and safety of sitafloxacin in Chinese CAP adults. The study was conducted during the period from 20 December 2012 to 8 February 2014. The results will be used to support the clinical use of sitafloxacin in clinical practice.
Methods
Study design
This is a multicenter, randomized, open-label, parallel group clinical trial designed to evaluate the efficacy and safety of sitafloxacin tablets versus moxifloxacin in treating adults with CAP (chinadrugtrials.org.cn identifier: CTR20130046). The patients were randomized at 26 study centers across China in a ratio of 1:1:1 to receive sitafloxacin 100 mg once daily (qd), sitafloxacin 100 mg twice daily (bid) or control drug moxifloxacin 400 mg qd. The patients took the allocated drugs orally for 7–10 days. The randomization was performed centrally via an online interactive system. A unique subject identifier was assigned to each patient. The containers were numbered sequentially to keep the sequence of random allocation. Study protocol and informed consent form were approved by the Ethics Committee of each participating study center under the principle of the Declaration of Helsinki before initiation of study. The informed consent form was signed by every subject or his/her legal representative before entry into study. The original protocol was in supplemental data.
Inclusion and exclusion criteria
Eligible patients met all of the following inclusion criteria: male or female patient, 18–70 years of age (inclusive); did not receive antimicrobial agents or received other antimicrobial agent (excluding fluoroquinolones) but less than 24 h and still with symptoms of infection within 48 h before administration of study drug. CAP diagnosis was established in accordance with the following diagnostic criteria: (1) chest X-ray revealed apparent signs of acute inflammatory infiltration within 48 h before administration of study drug; (2) presence of purulent sputum, i.e. containing at least 25 white blood cells (WBCs) and less than 10 squamous epithelial cells (SEC) per low power field (LPF) on direct smear and microscopic examination; (3) presence of fever (oral temperature ≥37.5 °C, or axillary temperature ≥37 °C, or rectal temperature ≥38 °C) and/or increased peripheral WBCs (>10*109/L) or neutrophils percentage >70%; (4) have two or more of the following manifestations: cough; signs of lung consolidation evidenced by bronchial sound and/or local wet rales; dyspnea/tachypnea; chest pain/discomfort.
Patients were excluded if any of the following exclusion criteria were present: clinically diagnosed with pneumonia but requiring invasive mechanical ventilation or with septic shock requiring vasopressorCitation2; had a history of bronchial obstruction and subsequent pneumonia or bronchiectasis (excluding chronic obstructive pulmonary disease); presence of primary lung cancer or pulmonary metastasis of malignant tumor; patients with pulmonary cystic fibrosis, AIDS, known or suspected pneumocystis pneumonia or active tuberculosis; immunocompromised patients receiving glucocorticoids (total equivalent prednisone dose 20 mg/d for more than 2 weeks) or immunosuppressive agent; abnormal liver function tests, such as aspartate transaminase (AST) and/or alanine transaminase (ALT) >3 × upper limit of normal (ULN), and/or total bilirubin >2 × ULN; moderate or severe renal dysfunction evidenced by endogenous creatinine clearance rate <50 mL/min; cancer or other malignant disease; history of epilepsy or other central nervous system disorders; history of myasthenia gravis; QT elongation or serious heart disease.
Clinical data were collected, including patient age, sex, underlying diseases, symptoms and signs, clinical outcomes, laboratory tests such as hematologic tests, sputum smear and culture, blood culture, serological test, urinary antigen testing, biochemical assays, urinalysis, electrocardiogram (ECG), chest X-ray and other relevant information, and entered into the study-specific database.
Evaluation of clinical and microbiological efficacy
Efficacy evaluation included clinical efficacy, microbiological efficacy and comprehensive efficacy. The primary efficacy outcome was defined as the clinical cure rate at test of cure (TOC) visit (7–14 days after the end of treatment). The other efficacy evaluation was considered as secondary efficacy outcomes.
Clinical efficacy was evaluated at visit 3 (end/discontinuation of treatment, i.e. 2 days after end of treatment) and visit 4 (test of cure, 7–14 days after end of treatment), respectively. Clinical efficacy was evaluated as cure or failure. Clinical cure was confirmed if all the baseline clinical symptoms and signs were resolved or normalized after treatment, AND chest X-ray indicated cure or improvement, laboratory tests were normalized or recovered to corresponding baseline level, AND systemic antimicrobial therapy was not required for the target indication. Clinical failure was defined if the baseline clinical symptoms and signs were persistent or aggravated, or not resolved completely after treatment; OR the disease had new symptom or sign AND/OR other antimicrobial agent was used to treat the disease under study.
Microbiological efficacy was evaluated at visit 3 and visit 4 in clinically evaluable patients who had baseline pathogen isolated and post-treatment follow-up data. Microbiological efficacy was evaluated as eradication, presumed eradication, persistent or presumed persistent. The cases of eradication and the cases of presumed eradication were combined to calculate the microbiological success rate. The bacterial isolates were tested by Kirby–Bauer method for their susceptibility to sitafloxacin and moxifloxacin. The minimum inhibitory concentrations (MICs) of sitafloxacin and moxifloxacin against the baseline isolates were determined by agar dilution method. The results of susceptibility testing were interpreted according to the Clinical & Laboratory Standards Institute breakpoints in 2014.
Comprehensive efficacy was evaluated at visit 4 only in the patients with baseline isolates, combining clinical efficacy and microbiological efficacy. Comprehensive efficacy was assessed as cure or failure. Cure was defined as clinical cure, AND microbiological eradication or presumed eradication at visit 4. Failure was defined as clinical failure, OR microbiological persistent or presumed persistent at visit 4.
Safety evaluation
The study subjects were observed closely to document clinical adverse events (AEs) and laboratory abnormalities in detail. The adverse reactions/events were evaluated in terms of severity and their relatedness to the study drug according to well recognized standards. The AEs definitely, probably or possibly related to study drug were combined (collectively considered as adverse drug reactions) to calculate the incidence of adverse drug reactions.
Statistical analysis
The data of this study were analyzed with SAS 9.2 software. Clinical efficacy was tested using a non-inferiority design. If the difference in clinical cure rate between the sitafloxacin group and moxifloxacin is greater than −10% in terms of its lower limit of 95% CI, sitafloxacin is considered non-inferior to moxifloxacin in clinical efficacy. Microbiological efficacy was compared by the difference between groups, which was tested by two-sided test. The significance level for the hypothesis test was .05. If the p value is not higher than .05 for the comparison between three groups, it can be inferred that the difference between treatment groups is statistically significant. It is assumed that both sitafloxacin 100 mg qd and moxifloxacin treatment groups will result in a clinical efficacy rate of 93% at TOC visit. If α is set as .05 at two-sided test, the power of the test is 80% and the non-inferiority margin is −10%, assuming patient dropout rate of 10%, then the sample size in each treatment group will be 115 patients. In addition, it is estimated that the clinical cure rate in the sitafloxacin 100 mg bid group at TOC visit will not be lower than that of the sitafloxacin 100 mg qd group. So the sample size required for the sitafloxacin 100 mg bid group will not be more than 115. To sum up, the total sample size was 345 patients, specifically 115 patients in each of the sitafloxacin 100 mg qd group, the sitafloxacin 100 mg bid group and the moxifloxacin group.
The full analysis set (FAS) included all randomized patients, except those who seriously violated good clinical practice (GCP) guidelines, did not receive any study drug, did not have study data after randomization or did not have the specific target indication of this study. The per-protocol set (PPS) was defined as all the patients in the FAS who had a clinical efficacy evaluation at TOC visit AND did not have major violation of study protocol in terms of inclusion or exclusion criteria, concomitant medications or treatments, dosage (actual administered dose was 80%–120% of the nominal dosage), or no data were available at visits after taking study drug. The discontinued patients were also included in the PPS even if their disease got worse or did not improve after treatment for more than 72 h AND clinical efficacy was judged as “failure”. The microbiologically evaluable set (MES) was defined as all the patients in the PPS who had baseline pathogenic isolates, AND post-treatment follow-up data. The safety set (SS) included all randomized patients who had received at least one dose of study drug.
Results
Study population
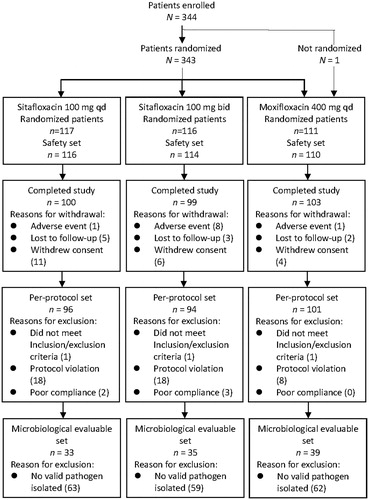
This study was conducted in 26 study centers across China. A total of 344 CAP patients were enrolled, and 343 of these patients were assigned via central randomization system to receive sitafloxacin 100 mg qd (n = 117), sitafloxacin 100 mg bid (n = 116) or moxifloxacin 400 mg qd (n = 110). Most of the patients were enrolled in the emergency room. One patient assigned to the moxifloxacin group was not randomized and so excluded from analysis.
Finally, a total of 340 patients were included in the SS. There were 326 patients in the FAS, specifically 111 patients in the sitafloxacin 100 mg qd group, 109 patients in the sitafloxacin 100 mg bid group and 106 patients in the moxifloxacin group. The PPS included 291 patients, 96 patients in the sitafloxacin 100 mg qd group, 94 patients in the sitafloxacin 100 mg bid group and 101 patients in the moxifloxacin group. There were 107 patients in the MES, including 33 cases in the sitafloxacin 100 mg qd group, 35 cases in the sitafloxacin 100 mg bid group and 39 cases in the moxifloxacin group ().
Baseline characteristics of patients
When the FAS was considered, the baseline characteristics of patients were well balanced and comparable among the three treatment groups (sitafloxacin 100 mg qd, sitafloxacin 100 mg bid and moxifloxacin 400 mg qd) in terms of age, sex, body weight, height, body mass index, smoking and drinking status. The results in the PPS were similar to those in the FAS. The main underlying diseases were hypertension and diabetes mellitus in the three groups in the FAS, which was consistent with the results in the PPS ().
Table 1. Demographic and baseline characteristics of patients in the full-analysis set.
In the FAS population, 288 (88.3%) patients had fever, all the 326 (100%) patients reported productive cough, 174 (53.4%) patients had dyspnea/tachypnea, 207 (63.5%) patients reported chest pain/discomfort and 245 (75.2%) patients had signs of lung consolidation. The symptoms and signs associated with pneumonia were comparable between the sitafloxacin 100 mg qd, sitafloxacin 100 mg bid and moxifloxacin groups. Baseline sputum/blood culture was positive in 33.3% (37/111) of patients in the sitafloxacin 100 mg qd group, 35.8% (39/109) in the sitafloxacin 100 mg bid group and 38.7% (41/106) in the moxifloxacin group. The results in the PPS were comparable to the FAS results.
Efficacy results
The clinical cure rate in the sitafloxacin 100 mg qd group was 94.2% at visit 3 and 94.9% at visit 4 in the FAS population. The corresponding clinical cure rate was 95.0% and 94.9% in the sitafloxacin 100 mg bid group, and 95.2% and 95.1% in the moxifloxacin group. In the PPS population, the sitafloxacin 100 mg qd treatment group showed a clinical cure rate of 93.7% at visit 3 and 94.8% at visit 4, while the clinical cure rate was 96.8% in the sitafloxacin 100 mg bid group and 95.0% in the moxifloxacin group at both visit 3 and visit 4. The primary efficacy outcome of this study was the clinical cure rate at visit 4 in the PPS population. When the sitafloxacin 100 mg qd group was compared with the moxifloxacin group, the difference in clinical cure rate between these two groups (sitafloxacin 100 mg − moxifloxacin group) was −0.3% (95% CI: −6.4%, 5.9%). The lower limit of 95% CI was greater than −10%. Similarly, when the sitafloxacin 100 mg bid group was compared with the moxifloxacin group, the difference in clinical cure rate between these two groups (sitafloxacin 100 mg bid − moxifloxacin) was 1.8% (95% CI: −3.8%, 7.3%). The lower limit of 95% CI was also greater than −10%. Statistical analysis indicated that the clinical cure rate in both the sitafloxacin 100 mg qd group and the sitafloxacin 100 mg bid group was non-inferior to that in the moxifloxacin group at TOC visit in the PPS population ().
Table 2. Clinical efficacy of sitafloxacin and moxifloxacin in the full analysis set and the per-protocol set.
Clinical efficacy was also analyzed in terms of pathogenic bacterial species. Sitafloxacin resulted in a good clinical cure rate for CAP caused by S. pneumoniae, S. aureus, Haemophilus spp., Klebsiella spp., and atypical pathogens such as M. pneumoniae, C. pneumoniae and L. pneumophila ().
Table 3. Clinical efficacy of sitafloxacin and moxifloxacin in infections caused by specific pathogens at test of cure visit in per-protocol set.
Microbiological efficacy was analyzed at visit 3 and visit 4 in the MES population. The outcome at visit 4 in the MES population was primary microbiological efficacy. In the MES population, the microbiological success rate was 97.0% (32/33) in the sitafloxacin 100 mg qd group, 97.1% (34/35) in the sitafloxacin 100 mg bid group and 94.9% (37/39) in the moxifloxacin group (p > .05) ().
Table 4. Microbiological efficacy of sitafloxacin and moxifloxacin at visit 3 and visit 4 in the microbiologically evaluable set.
The baseline pathogenic isolates, including S. pneumoniae, H. influenzae and S. aureus, were eradicated completely by sitafloxacin or moxifloxacin treatment. The strains not eradicated were K. pneumoniae and other Enterobacteriaceae (1 strain in the sitafloxacin 100 mg qd group, 1 strain in the sitafloxacin 100 mg bid group and 3 strains in the moxifloxacin group).
Comprehensive efficacy was analyzed at visit 4 in patients with baseline pathogenic isolates and evaluated as cure or failure. The comprehensive cure rate was 93.9% (31/33, 95% CI: 79.8%–99.3%) in the sitafloxacin 100 mg qd group, 97.1% (34/35, 95% CI: 85.1%–99.9%) in the sitafloxacin 100 mg bid group and 92.3% (36/39, 95% CI: 79.1%–98.4%) in the moxifloxacin group.
Minimum inhibitory concentration of sitafloxacin and moxifloxacin against baseline pathogens
Kirby–Bauer disk susceptibility testing showed that the baseline pathogens had comparable pattern and rate of susceptibility to sitafloxacin and moxifloxacin in the three groups. Agar dilution method was used to determine the MICs of sitafloxacin and moxifloxacin against baseline pathogens (). Sitafloxacin showed highly antimicrobial activity against S. pneumoniae, S. aureus, H. influenzae and K. pneumoniae. The MIC90 value and MIC range of sitafloxacin were from ≤0.06 mg/L to 0.125 mg/L against S. pneumoniae, S. aureus and H. influenzae. The MIC90 value was 0.25 mg/L against K. pneumoniae. Sitafloxacin had lower but still good activity against Pseudomonas spp. (MIC90= 2 mg/L). Sitafloxacin was better than moxifloxacin in activity against H. influenzae, S. aureus and Pseudomonas spp., and comparable to moxifloxacin in activity against other baseline isolates ().
Table 5. The minimum inhibitory concentrations of sitafloxacin and moxifloxacin against major baseline isolates.
Safety results
A total of 340 patients were evaluable for safety, including 116 patients in the sitafloxacin 100 mg qd group, 114 in the sitafloxacin 100 mg bid group and 110 in the moxifloxacin 400 mg group. The overall incidence of AEs was 34.5% (40/116) and the incidence of drug-related AEs was 23.3% (27/116) in the sitafloxacin 100 mg qd group. The corresponding incidence was 45.6% (52/114) and 29.8% (34/114) in the sitafloxacin 100 mg bid group, and 39.1% (43/110) and 28.2% (31/110) in the moxifloxacin 400 mg qd group. The incidence of drug-related AEs (including clinical AEs, laboratory abnormalities, abnormal ECG findings) did not show a significant difference between the three groups (p > .05).
Drug-related AEs in this study included clinical AEs, laboratory abnormalities and abnormal ECG findings. In the sitafloxacin 100 mg qd group, the incidence of drug-related clinical AEs was 16.4% (19/116); the most frequently reported one was dizziness (4.3%). The incidence of drug-related laboratory abnormalities was 8.6% (10/116), mainly increased platelet count (2.6%). Drug-related abnormal ECG finding (sinus arrhythmia) was found in 2 patients. In the sitafloxacin 100 mg bid group, the incidence of drug-related clinical AEs was 20.2% (23/114), mainly nausea and diarrhea (both 3.5%). The incidence of drug-related laboratory abnormalities was 12.3% (14/114), mainly ALT elevation (7.0%), followed by AST elevation and γ-glutamyltransferase increase (both 2.6%). Drug-related abnormal ECG findings were found in 2 patients (sinus bradycardia, supraventricular extrasystole). In the moxifloxacin group, the incidence of drug-related clinical AEs was 20.9% (23/110), mainly nausea (8.2%), dizziness (5.5%), fatigue (4.5%) and vomiting (3.6%). The incidence of drug-related laboratory abnormalities was 8.2% (9/110), mainly AST elevation (3.6%) and ALT elevation (2.7%). Drug-related abnormal ECG findings (sinus bradycardia) were found in 3 patients ().
Table 6. Adverse events and laboratory abnormalities related to study drug occurred in at least 2% of patients in each group in the safety set.
Five cases of serious AE (SAE) were reported in 3 patients during the study. All SAEs improved after treatment. Only one case of SAE (degree I atrioventricular block) in the sitafloxacin 100 mg qd group was possibly related to the study drug. The ECG of this patient recovered to normal 8 days after end of study treatment. The severity of drug-related AEs was summarized in cases by group, specifically, mild and moderate AEs accounted for 97.8% (45/46) and 2.2% (1/46) respectively in the sitafloxacin 100 mg qd group; 80.9% (55/68) and 19.1% (13/68) respectively in the sitafloxacin 100 mg bid group; and 94.6% (53/56) and 5.4% (3/56) respectively in the moxifloxacin group. The above results indicate that oral sitafloxacin 100 mg qd or bid is well tolerated in adult CAP patients. Most of the drug-related clinical AEs and laboratory abnormalities are mild in severity. The most common AEs are gastrointestinal disorders and liver enzyme elevation. Fluoroquinolone-associated impaired blood glucose and unexpected AE were not observed. Eight patients discontinued treatment due to drug-related AEs in this study, including 7 in the sitafloxacin 100 mg bid group and 1 in the moxifloxacin group. Their AEs resolved after discontinuation.
Discussion
Previous studies have shown that sitafloxacin has broad spectrum antimicrobial activity in vitro and in vivo. Sitafloxacin is highly active against most gram-positive bacteria, gram-negative organisms, anaerobes and atypical pathogens. Several studies in JapanCitation8–11,Citation19–21 demonstrated that sitafloxacin was superior to levofloxacin, ciprofloxacin, tosufloxacin, trovafloxacin, moxifloxacin, garenoxacin and gatifloxacin in in vitro antimicrobial activity against most bacterial pathogens. Its MIC90 value (mg/L) was 0.06–0.5 against methicillin-susceptible S. aureus, 0.5–2 against methicillin-resistant S. aureus, 0.03–0.06 against S. pneumoniae, 0.004 against H. influenzae, 0.008–0.015 against M. catarrhalis and 0.12–0.25 against K. pneumoniae. Studies have indicated that the antimicrobial activity of sitafloxacin against S. pneumoniae and E. coli is not affected by their resistance to other fluoroquinolones, suggesting no cross-resistance to sitafloxacin in these two bacterial speciesCitation10. Oral administration of sitafloxacin 100 mg qd can result in an effective antimicrobial level of sitafloxacin in plasma against S. aureus, S. pneumoniae, E. coli, H. influenzae and M. catarrhalis. Sitafloxacin penetrates well into alveolar epithelial lining fluid in critically ill patients with pneumonia after a single oral dose of 200 mgCitation22. But sitafloxacin 100 mg bid should be used for infections caused by P. aeruginosaCitation10.
The pathogen spectrum in this series of CAP patients is similar to other reports from China and foreign countries, primarily S. pneumoniae, H. influenzae, S. aureus and K. pneumoniae. The MIC90 value of sitafloxacin against these strains was 0.125, 0.125, ≤0.06 and 0.25 mg/L, respectively. A pharmacokinetic study has shown that the Cmax of sitafloxacin is (1.00 ± 0.14) mg/L and AUC is (5.55 ± 1.22) mg·h/L after an oral dose of 100 mg under fasted conditionCitation23. Pharmacokinetic–pharmacodynamic (PK-PD) study indicates that fluoroquinolones including sitafloxacin are concentration-dependent antimicrobial agents, the efficacy of which is correlated with Cmax/MIC and AUC/MIC ratios. Sitafloxacin 100 mg qd or bid can provide an adequate Cmax/MIC (≥8) and AUC/MIC (≥30) ratio against major CAP pathogens, suggesting that both dosing regimens of sitafloxacin can result in good clinical and microbiological efficacy for CAP patients. After single-dose and multiple-dose administration of sitafloxacin tablets, the mean plasma sitafloxacin concentration and total exposure are slightly higher, and terminal T1/2 is slightly longer, suggesting slower elimination of sitafloxacin, in healthy Chinese subjects than in healthy Japanese subjects. But the cumulative urinary excretion rate of sitafloxacin is similar in Chinese and Japanese subjects. The PK parameters of sitafloxacin do not show significant difference between healthy Chinese subjects and Japanese subjectsCitation24,Citation25.
Clinical studies of sitafloxacin in patients with infection have proved the overall good safety and tolerability of sitafloxacin. Combined safety analysis of clinical studies in Japanese subjects with respiratory tract infections, urinary tract infections, ear, nose and throat infections, oral infections, and reproductive system infections reported that, of the 1220 patients, 409 (33.5%) developed AEs. The main AEs were diarrhea, loose stool, headache, liver enzyme elevation and eosinophilia. In post-marketing clinical studies, 148 (4.4%) of 3331 patients experienced AEs. The main AEs were diarrhea, loose stool, skin rash and liver enzyme elevationCitation26. The incidence of common AEs was 16.7% for gastrointestinal disorders and 16.4% for laboratory abnormalities. The incidence of diarrhea was 12.5%. ALT elevation was reported in 5.95% of patients. No severe AE was reported. The incidence of moderate AEs was 3.7% (45/1220), mainly diarrhea (1.2%, 15/1220). The incidence of mild AEs was 31.1% (380/1220). The incidence of AEs was 38.9% (222/570) in patients with respiratory tract infections. A recent study reported that sitafloxacin shows comparable efficacy and safety to garenoxacin for the treatment of pneumonia in elderly patientsCitation27.
In the present study, the safety profiles in the sitafloxacin 100 mg qd group and the sitafloxacin 100 mg bid group are similar to that in the moxifloxacin group for treating CAP patients. Most of the drug-related clinical AEs and laboratory abnormalities in this study were mild and well tolerated. The majority of the AEs were mild in severity in both the sitafloxacin 100 mg qd group (97.8%) and the sitafloxacin 100 mg bid group (80.9%). Seven patients in the sitafloxacin 100 mg bid group discontinued treatment due to drug-related AEs. None of the patients in the sitafloxacin 100 mg qd group discontinued treatment. Sitafloxacin 100 mg qd can provide comparable efficacy and better safety than sitafloxacin 100 mg bid. The PK data in Chinese subjects also show that sitafloxacin is absorbed quickly after oral administration. Linear PK and good tolerability of sitafloxacin is evident within the dose range from 50 to 200 mgCitation24.
In addition, a post-marketing study in 3225 Japanese patients demonstrates that the overall efficacy rate of sitafloxacin is 92.9% in treating infections. When sitafloxacin is used to treat the 513 patients who failed prior antimicrobial treatment, including penicillins, cephalosporins, macrolides, other fluoroquinolone monotherapy or combination therapy, the clinical efficacy rate is up to 89.5%. This result further underpins the broad spectrum and potent antimicrobial activity of sitafloxacinCitation28.
In conclusion, the dosing regimen of sitafloxacin tablets 100 mg qd and sitafloxacin 100 mg bid can provide good clinical and microbiological efficacy in treating adult CAP caused by bacteria and atypical pathogens. The adverse reactions are rare, mild and transient. Sitafloxacin can be used to treat CAP in adults. The proposed dosing regimen is 100 mg qd or bid, for 7–10 days.
Transparency
Declaration of funding
This study was supported by Daiichi Sankyo (China) Holdings Co. Ltd. The design, conduct and conclusions of the study, as well as the analysis and interpretation of study data, are the responsibility of the authors, not affected by Daiichi Sankyo.
This work was supported by grants from the Major Research and Development Project of Innovative Drugs, Ministry of Science and Technology of China (grant number 2017ZX09304005).
Declaration of financial/other relationships
No potential conflict of interest was reported by the authors.
Author contributions
Y.Z. designed the study and was the coordinating investigator. Y.L. wrote the first manuscript which was read, commented and amended by all the other authors. All authors were involved in all diverse phases of the study (including enrolment of patients, conduct of study, collection and analysis of study data) and approval of the manuscript.
Study Protocol Synopsis - English Translation from Chinese text
Download PDF (289.7 KB)CONSORT 2010 checklist when reporting clinical trial CTR20130047
Download MS Word (219 KB)Acknowledgements
The authors would like to thank the other participating investigators (Dr. Feng Wang from the Department of Infectious Diseases, First Hospital of Jilin University and Jianpeng Zhang from the Department of Respiratory Medicine, Third Medical Center of the PLA General Hospital) in this study not listed as authors for their support and cooperation.
Data availability statement
The datasets generated in this study and the protocol are available from the corresponding author on reasonable request.
References
- Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–iii55.
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67.
- Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427.
- Welte T, Köhnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ network. Semin Respir Crit Care Med. 2009;30(2):127–135.
- Tao LL, Hu BJ, He LX, et al. Etiology and antimicrobial resistance of community-acquired pneumonia in adult patients in China. Chin Med J. 2012;125(17):2967–2972.
- Carbonara S, Monno L, Longo B, et al. Community-acquired pneumonia. Curr Opin Pulm Med. 2009;15(3):261–273.
- Hu FP, Guo Y, Zhu DM, et al. [CHINET 2017 surveillance of bacterial resistance in China]. Chin J Infect Chemother. 2018;18:241–251.
- Yamaguchi K, Ohno A, Ishii Y, et al. In vitro susceptibilities to levofloxacin and various antibacterial agents of 12,919 clinical isolates obtained from 72 centers in 2007. Jpn J Antibiot. 2009;62:346–370.
- Amano A, Matsuzaki K, Kishi N, et al. In vitro activity of sitafloxacin against clinical isolates in 2009. Jpn J Antibiot. 2010;63:411–430.
- Kanda H, Kurosaka Y, Fujikawa K, et al. In vitro and in vivo antibacterial activity of sitafloxacin. Jpn J Chemother. 2008;56 (Suppl 1):1–17.
- Yamamoto N, Fujita J, Shinzato T, et al. In vitro activity of sitafloxacin compared with several fluoroquinolones against Streptococcus anginosus and Streptococcus constellatus. Int J Antimicrob Agents. 2006;27(2):171–173.
- Mikamo H, Kawazoe K, Sato Y, et al. Therapeutic effects of a new quinolone, DU-6859a, on polymicrobial infections in a newly designed model of rat uterine endometritis. J Antimicrob Chemother. 1998;41(1):131–133.
- Fukuda Y, Yanagihara K, Ohno H, et al. In vivo efficacies and pharmacokinetics of DX-619, a novel des-fluoro(6) quinolone, against Streptococcus pneumoniae in a mouse lung infection model. Antimicrob Agents Chemother. 2006;50(1):121–125.
- Nakamura S, Yanagihara K, Araki N, et al. In vivo efficacy of sitafloxacin in a new murine model of non-typeable Haemophilus influenzae pneumonia by sterile intratracheal tube. Int J Antimicrob Agents. 2009;34(3):210–214.
- Kurosaka Y, Ishida Y, Yamamura E, et al. Therapeutic efficacy of sitafloxacin against Pseudomonas aeruginosa in a rat model of urinary tract infection. Drugs. 1999;58(Suppl 2):412–414.
- Gillian MK. Sitafloxacin in bacterial infections. Drugs. 2011;71:731–744.
- Kobayashi H, Watanabe A, Nakata K, et al. [Double-blind comparative study of sitafloxacin versus levofloxacin in patients with respiratory tract infection]. Jpn J Chemother. 2008;56(Suppl 1):36–48.
- Saito A, Watanabe A, Aoki N, et al. Phase III double-blind comparative study of sitafloxacin versus tosufloxacin in patients with community-acquired pneumonia. Jpn J Chemother. 2008;56(Suppl 1):49–62.
- Amano A, Matsuzaki K, Kishi N, et al. In vitro activity of sitafloxacin against clinical isolates in 2012. Jpn J Antibiot. 2013;66:311–330.
- Horii T, Suzuki Y, Monji A, et al. Detection of mutations in quinolone resistance-determining regions in levofloxacin- and methicillin-resistant Staphylococcus aureus: effects of the mutations on fluoroquinolone MICs. Diagn Microbiol Infect Dis. 2003;46(2):139–145.
- Schmitz FJ, Fluit AC, Brisse S, et al. Molecular epidemiology of quinolone resistance and comparative in vitro activities of new quinolones against European Staphylococcus aureus isolates. FEMS Immunol Med Microbiol. 1999;26(3–4):281–287.
- Paiboonvong T, Nosoongnoen W, Sathirakul K, et al. Pharmacokinetics and penetration of sitafloxacin into alveolar epithelial lining fluid in critically ill Thai patients with pneumonia. Antimicrob Agents Chemother. 2019;63(10):e00800–19.
- Nakashima M, Uematsu T, Kosuge K, et al. Pharmacokinetics and tolerance of DU-6859a, a new fluoroquinolone, after single and multiple oral doses in healthy volunteers. Antimicrob Agents Chemother. 1995;39(1):170–174.
- Wu X, Fan Y, Zhang J, et al. Pharmacokinetics of oral sitafloxacin in healthy Chinese subjects. Chin J Infect Chemother. 2018;18:352–359.
- Wu G, Wu L, Hu X, et al. Pharmacokinetics and safety of sitafloxacin after single oral doses in healthy volunteers. Int J Clin Pharmacol Ther. 2014;52(12):1037–1044.
- Sitafloxacin insert package. Tokyo (Japan): Daiichi Sankyo Company, Limited; 2020.
- Miyazaki T, Nakamura S, Hashiguchi K, et al. The efficacy and safety of sitafloxacin and garenoxacin for the treatment of pneumonia in elderly patients: a randomized, multicenter, open-label trial. J Infect Chemother. 2019;25(11):886–893.
- Matsumoto T, Uchino K, Yamaguchi H, et al. Study on the safety and efficacy of sitafloxacin-results of the use-results survey. Jpn J Antibiot. 2011;64:319–337.