Abstract
Objective
We compared the effectiveness and safety outcomes of non-vitamin K antagonist oral anticoagulants (NOACs) and warfarin in patients with AF and VHD, as these patients have been partially excluded from clinical trials.
Methods
This retrospective cohort study was conducted using the National Health Insurance Research Database in Taiwan. Patients with AF and VHD and above 20 years of age, who were prescribed oral anticoagulants such as warfarin, dabigatran, rivaroxaban, and apixaban, were included. Propensity score matching was performed to balance intergroup differences. The Cox proportional hazards model was used to compare the effectiveness and safety of warfarin and NOACs.
Results
We included 5833 NOAC-warfarin pairs, 3001 dabigatran-warfarin pairs and 2595 rivaroxaban-warfarin pairs. Warfarin and NOACs had similar risk of ischemic stroke (hazard ratio [HR], 0.92; 95% confidence interval [CI], 0.79–1.06; p = .25) and bleeding events (HR, 0.90; 95% CI, 0.78–1.02; p = .10). NOACs showed reduced risk of venous thromboembolism, intracranial hemorrhage, and mortality (HR and 95% CI, 0.39 [0.20–0.77], p = .01; 0.62 [0.45–0.84], p < .01; and 0.47 [0.41–0.53], p < .01, respectively). The benefit of NOACs in reducing the risk of venous thromboembolism was mainly driven by dabigatran, and the benefit of reducing the risk of intracranial hemorrhage and mortality was observed in both dabigatran and rivaroxaban users.
Conclusions
NOACs had a comparable risk of ischemic stroke and bleeding in patients with AF and VHD, and reduced the risk of venous thromboembolism, intracranial hemorrhage, and mortality, compared to warfarin. Therefore, NOAC is an effective and safe alternative to warfarin in these patients.
Introduction
Non-vitamin K antagonist oral anticoagulants (NOACs) have been reported as comparably effective to warfarin in preventing thromboembolism and reducing the risk of intracranial bleeding in patients with atrial fibrillation (AF)Citation1–4. Valvular heart disease (VHD) is a condition that often coexists with AFCitation5. However, VHD is currently not well defined, and its inclusion criteria vary across different trials. In general, patients with mechanical heart valve replacement and mitral stenosis of moderate to severe intensity have been excluded from clinical trialsCitation1–4. In certain landmark trials, such as the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial for apixaban, and the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial for edoxaban, the inclusion criteria were less stringent and enrolled patients with bioprosthetic valve replacement or valve repairCitation2,Citation3.
The effectiveness and safety of NOACs have been compared to warfarin in patients with AF and VHD in previous studies. The meta-analyses of clinical trial data show that NOACs reduces the risk of stroke and systemic thromboembolism compared to warfarin. NOACs, including dabigatran, apixaban, and edoxaban, but excluding rivaroxaban, reduce the risk of major bleeding and intracranial hemorrhage (ICH)Citation6. A study conducted in the United States demonstrated that NOACs reduces mortality and non-gastrointestinal (GI) bleeding, but does not alter the risk of ischemic stroke and GI bleeding compared to warfarinCitation7. Another study in the United States demonstrated no difference between NOACs and warfarin in reducing the risk of stroke and ICHCitation8. The risk of bleeding associated with NOACs use is higher in the Asian population than that in other ethnicitiesCitation9. Thus, it remains unclear if the results from a previous study conducted mainly in the Caucasian population can be extrapolated to the Asian population. Moreover, the proportion of enrolled patients with VHD in clinical trials is low, ranging from 13% to 22%Citation1–4, and the results from real-world data have been inconsistent. Although a study in Korean patients compared the effectiveness and safety of NOACs to that of warfarin in patients with AF and VHD, the outcomes of individual NOAC have not been investigatedCitation10.
The purpose of this study was to investigate the effectiveness and safety of NOACs in Asian patients with both AF and VHD. In addition, we compared the clinical outcomes of NOACs and warfarin users as well as the effectiveness and safety of individual NOACs to warfarin.
Methods
Data source
The data used in this study were retrieved from the National Health Insurance Research Database (NHIRD) that covers more than 99.9% (approximately 24 million beneficiaries) population in TaiwanCitation11. The database (NHIRD) contains detailed information on each inpatient care, outpatient visit, and prescription drug utilization, and all information is de-identified. Data from the years 2007 to 2015 served as the data source in this study. The study protocol was reviewed and approved by the Research Ethics Committee of the National Taiwan University Hospital.
Study population
This was a nationwide, population-based, retrospective cohort study. Patients with AF and VHD, registered between January 1, 2007, and December 31, 2015, were included. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to identify patients with AF and VHD. At least one inpatient and two outpatient diagnoses were required to confirm the classification codes for patients. To further validate the diagnosis of VHD, at least one cardiac echocardiography between two VHD diagnoses was necessary. The detailed ICD-9-CM codes for the identification of participants are listed in Table S1Citation12,Citation13.
Participants with AF and VHD aged above 20 years, who were newly prescribed oral anticoagulant agents (warfarin, dabigatran, rivaroxaban, or apixaban), were enrolled in the study. Patients were assigned treatment groups according to their first oral anticoagulant prescription from January 2012 to December 2015. The first NOAC (dabigatran) has been reimbursed in Taiwan since June 2012. Edoxaban was not included because it has not been reimbursed till the end of the study period. Patients with pregnancy, cancer, or end-stage renal disease requiring dialysis were excluded.
We first compared composite outcomes of NOACs to the outcomes of warfarin, followed by subgroup analyses of individual NOACs to warfarin. Apixaban was not included in the subgroup analysis owing to its small sample size. The index date was defined as the first date of either NOAC or warfarin prescription date after January 1, 2012. Since the database does not provide information on the severity of mitral stenosis and the types of heart valve replacement, we stratified all participants into severe and non-severe VHD groups, to analyze the effect of inclusion or exclusion of patients with mitral stenosis or valvular replacement on the study outcomes. The severe VHD group included participants with mitral stenosis or valvular replacement, and the non-severe VHD group included all other participants.
Study outcomes
In this study, we investigated the effectiveness and safety outcomes as composite events and then evaluated the outcomes separately. Effectiveness outcomes included ischemic stroke, venous thromboembolism (VTE), and transient ischemic attack (TIA). Ischemic stroke and TIA were defined by a primary diagnosis, either from hospital discharge or an emergency department visit. VTE, including deep vein thrombosis and pulmonary embolism, was defined by a primary diagnosis in an in-patient setting. The safety outcomes were assessed based on bleeding events of ICH and GI. All bleeding events were defined by one primary diagnosis from a hospital discharge or an emergency department visit. The detailed ICD-9-CM codes are listed in Table S1. In addition to effectiveness and safety outcomes, we also evaluated the risk of mortality as an independent outcome in the present study.
Follow-up period
The follow-up period was from the index date to the first occurrence of any of the events including the outcome of interest, switching to other oral anticoagulants, anticoagulant discontinuation, death, or end of the study (December 31, 2015). Discontinuation of oral anticoagulant therapy was defined as a gap of > 42 days between the last day’s supply and the next prescription. Since medications have carry-over effects, a three-day grace period was given to patients who had been censored due to medication switch or discontinuation.
Covariates
Confounding factors were collected and adjusted using multivariate analysis and were inclusive of age at the index date, sex, comorbidities, concomitant medications, and the CHA2DS2-VASc scoreCitation8,Citation13–15. Comorbidities were identified with at least one diagnosis within 12 months prior to the index date, including congestive heart failure, hypertension, diabetes mellitus, myocardial infarction, peripheral artery disease, renal disease, liver disease, ischemic stroke, VTE, TIA, ICH, GI bleeding, and other bleeding events. Concomitant medications were identified based on prescription records within 3 months preceding the index date, including drugs that interact with warfarin or NOACs, such as antiplatelet agents, class III antiarrhythmic drugs, digoxin, beta-blockers, non-dihydropyridine calcium channel blockers, agents acting on the renin-angiotensin system, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, nonsteroidal anti-inflammatory drugs, histamine receptor antagonists, and proton pump inhibitors. CHA2DS2-VASc scoreCitation16 was used to estimate the risk of stroke and was categorized as low risk (0 points), moderate risk (1 point), and high risk (≥2 points). The HAS-BLED scoreCitation17 for estimating bleeding risk was calculated but not included in the adjusted analysis owing to the lacking data in the NHIRD related to international normalized ratio, alcohol abuse, and laboratory tests of renal and liver functions.
Statistical analysis
We performed 1:1 propensity score matching (PSM) to analyze NOAC and warfarin users, using calipers of width equal to 0.2 of the standard deviation of the logit of the propensity scoreCitation18 The absolute standardized mean difference was used to determine the balance of characteristics between the study groups. No significant imbalance was defined when the absolute standardized mean difference was < 10%Citation19. Poisson regression was applied to calculate the incidence rate ratios. The Cox proportional hazards model was used to determine the association between oral anticoagulants and outcomes of interest. The statistical test results were considered significant with a two-sided p value of < .05. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Sensitivity analysis
Two sensitivity analyses were conducted to check the robustness of our findings. First, we re-analyzed the data in the absence of a three-day grace period in patients who discontinued or switched to other oral anticoagulants, to avoid potential bias from exposure misclassification. Second, we completed the analysis without censoring patients who switched to different NOACs. As our primary analysis evaluated NOACs as a group, we did not censor those patients who switched to other NOACs in the sensitivity analysis, to avoid misclassification.
Results
Study population
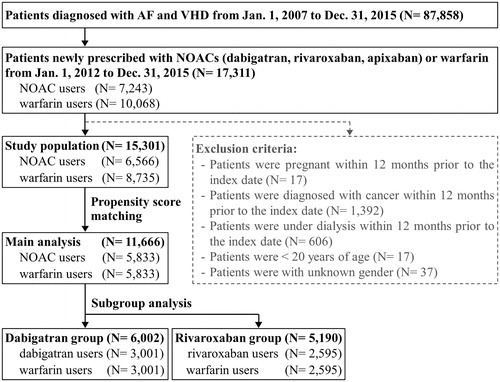
We identified 17,311 participants diagnosed with AF and VHD and were newly prescribed either NOACs or warfarin between January 1, 2012, and December 31, 2015. There were 6,566 NOAC users and 8,735 warfarin users after applying the exclusion criteria. PSM resulted in 5,833 pairs of NOAC and warfarin users. In subgroup analyses stratified by individual NOACs, there were 3,001 dabigatran-warfarin pairs and 2,595 rivaroxaban-warfarin pairs. depicts the participant-enrollment process. The baseline characteristics of warfarin and NOAC users are shown in . Before applying PSM, NOAC users were older (77 vs. 71 years old), had a higher mean CHA2DS2-VASc score (4.0 vs. 3.4 points), a lower mean HAS-BLED score (2.6 vs. 3.0 points), and higher prevalence of hypertension (71% vs. 62%) and ischemic stroke (19% vs. 12%) than warfarin users. Moreover, a higher proportion of NOAC users were on concomitant medications including agents acting on the renin-angiotensin system (56% vs. 51%), antiplatelet drugs (70% vs. 60%), and statins (21% vs. 15%) than warfarin users. After applying PSM, all baseline characteristics were well balanced with the absolute standardized mean difference values less than 0.1.
Table 1. Baseline characteristics of the study population before and after propensity score matching.
Study outcomes
The median follow-up period was 6.0 months and 7.7 months for the NOAC and warfarin groups, respectively. The follow-up duration for the dabigatran and rivaroxaban groups was 6.5 and 5.7 months, respectively. The comparative effectiveness of NOACs and warfarin is shown in . NOAC users showed similar risk of thromboembolic events (hazard ratio [HR], 0.89; 95% confidence interval [CI], 0.77–1.02, p = .09), ischemic stroke (HR, 0.92; 95% CI, 0.79–1.06, p = .25), and TIA (HR, 1.18; 95% CI, 0.81–1.72, p = .39) compared to warfarin users. NOAC users had a significantly lower risk of VTE (HR, 0.39; 95% CI, 0.20–0.77, p = .01) than warfarin users. The safety outcomes of NOACs compared to warfarin are shown in . Compared to warfarin users, NOAC users showed a similar risk of composite safety outcome (HR, 0.90; 95% CI, 0.78–1.02, p = .10) and a similar risk of GI bleeding (HR, 1.01; 95% CI, 0.84–1.20, p = .95). NOACs significantly decreased the risk of ICH (HR, 0.62; 95% CI, 0.45–0.84, p < .01) compared to warfarin. The comparative risk of mortality between NOAC and warfarin users is presented in . Overall, NOACs were associated with a lower risk of death than warfarin (HR, 0.47; 95% CI, 0.41–0.53, p < .01).
Figure 2. Effectiveness of NOACs compared to warfarin in Asian patients with atrial fibrillation and valvular heart disease.
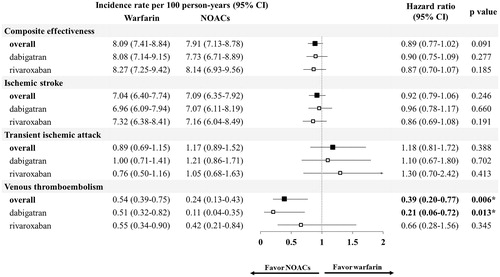
Figure 3. Safety of different NOACs compared to warfarin in Asian patients with atrial fibrillation and valvular heart disease.
Table 2. Risk of mortality using non-vitamin K antagonist oral anticoagulants in comparison to warfarin in patients.
Outcomes of individual NOACs
The effectiveness and safety of individual NOACs were compared to warfarin and the results are shown in and . Regarding the effectiveness outcomes, most of the results were in line with the main analyses; all NOACs showed a comparable risk of thromboembolic events, ischemic stroke, and TIA to warfarin. Nevertheless, discordance was observed for VTE risk; dabigatran significantly reduced the risk (HR, 0.21; 95% CI, 0.06–0.72, p = .01), but rivaroxaban did not (HR, 0.66; 95% CI, 0.28–1.56, p = .35, respectively), as compared to warfarin. Regarding safety outcomes, all NOACs displayed comparable composite outcomes of the risk of bleeding and GI-bleeding events. Both dabigatran and rivaroxaban users were observed with reduced risk of ICH (HR, 0.63; 95% CI, 0.41–0.98; p = .04 and HR, 0.57; 95% CI, 0.36–0.89, p = .01, respectively) compared to warfarin. Moreover, both dabigatran and rivaroxaban were associated with a lower risk of mortality (HR, 0.36; 95% CI, 0.30–0.45, p < .01 and HR, 0.61; 95% CI, 0.50–0.74, p < .01, respectively) than warfarin.
Outcomes of severe and non-severe VHD groups
The outcomes of NOACs compared to warfarin in users with different VHD severities are listed in Table S2. A total of 11,364 participants were grouped in non-severe VHD, including 5,598 warfarin users and 5766 NOAC users. A total of 302 participants were grouped in severe VHD, including 231 participants with mitral stenosis and 71 participants who underwent valvular surgery. Stratified by treatment in severe VHD group, 235 participants were warfarin users and 67 were NOAC users. PSM was not performed between the warfarin and NOAC groups in this subgroup analysis owing to the small sample size, but all the imbalanced baseline characteristics were adjusted in the regression model. There was no significant imbalance recognized between baseline characteristics of non-severe VHD patients receiving NOACs and warfarin. In the severe VHD group, NOAC users had higher mean CHDA2DS2-VASc (3.82, standard deviation [SD]: 1.69 versus 3.73, SD: 1.62) and HAS-BLED scores (2.79, SD: 1.11 versus 2.76, SD: 1.17) and a lower proportion of valve surgery (16% versus 26%) than that in warfarin users.
Similar to the main analysis, NOACs showed comparable risk of thromboembolism and bleeding to warfarin (HR, 0.87; 95% CI, 0.76–1.00, p = .06 and 0.90, 95% CI, 0.79–1.03, p = .14, respectively), but reduced risk of VTE (HR, 0.37; 95% CI, 0.19–0.74, p = .005), ICH (HR, 0.62; 95% CI, 0.46–0.86, p = .003) and mortality (HR, 0.46; 95% CI, 0.40–0.53; p ≤ .01) in the non-severe VHD group. Overall, NOACs showed similar effectiveness (HR, 1.49; 95% CI, 0.48–4.56, p = .49) and mortality (HR, 0.84; 95% CI, 0.29–2.43, p = .74) as compared to warfarin in the severe VHD group. No safety outcomes were observed during the follow-up period in the severe VHD group; therefore, comparative data are not available for this group.
Sensitivity analysis
Most of the results of the sensitivity analyses were similar to those of the main analysis. After discounting the grace period of anticoagulants (3 days), NOACs still displayed a similar composite outcome of thromboembolic or bleeding risk, and reduced VTE and ICH risk, compared to warfarin. In the sensitivity analysis that did not censor patients switching to other NOACs, the primary outcome of reduced VTE risk in NOAC users compared to warfarin users became non-significant in this analysis.
Discussion
In this study, we investigated the effectiveness and safety of NOACs compared to warfarin in Asian patients with AF and VHD. The main findings were as follows: 1) NOACs showed comparable risk of ischemic stroke, TIA, composite bleeding events and GI bleeding, but reduced risk of VTE, ICH and mortality, compared to warfarin; and 2) the outcome of NOACs in reducing the risk of VTE was mainly driven by dabigatran, whereas the outcome of NOACs in reducing the risk of ICH was driven by both dabigatran and rivaroxaban.
VHD is currently not well defined, and the acceptable inclusion criteria for VHD have varied widely across different studiesCitation1–4. Both the American Heart Association and European Society of Cardiology are averse to the use of NOACs in patients with mechanical heart valve or mitral stenosis of moderate to severe intensityCitation20,Citation21 because these patients have been generally excluded from clinical trialsCitation1–4. In addition, the efficacy and safety of dabigatran have been proven to be inferior to warfarin in patients with mechanical heart valves, according to the report of Randomized Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Replacement (RE-ALIGN) trialCitation22. A meta-analysis of clinical trial data shows that the patients with VHD have a higher risk of all-cause mortality and major bleeding than those without VHD, but a similar risk of stroke, systemic thromboembolism, and ICH exist. However, NOACs have been reported to reduce the risk of stroke, systemic embolism, and ICH compared to warfarin in patients with VHDCitation6.
Results from observational studies have been inconsistent. Briasoulis et al.Citation7 used Medicare beneficiaries in the United States to investigate the outcomes of dabigatran and rivaroxaban in patients with AF and VHD. The results revealed that NOACs reduced the risk of death and non-GI bleeding, but had a similar risk of stroke and GI bleeding compared to warfarin. The results for individual NOACs were consistent, and the differences in effectiveness and safety outcomes for dabigatran and rivaroxaban versus warfarin were not significant. Moon et al.Citation10 used the Korean National Health Insurance Service Database to investigate a similar subject and observed that NOACs reduced both bleeding and thromboembolism risk, including GI bleeding, major bleeding, fetal ICH, and ischemic stroke; however, their results were not consistent with our findings. The reasons for inconsistent results across different studies are multifocal. First, the characteristics of enrolled participants differed in different studies. Moon et al.Citation10 enrolled only those patients with AF, who were under treatment with NOACs for primary stroke prevention; moreover, about one-third of the participants were younger than 65 years. In contrast, 10% of participants in the study by Briasoulis et al.Citation7 and 16% in the current study had a history of stroke. More than 85% of participants in our study were above 65 years of age. The risk of ischemic stroke and bleeding increases with advanced age and stroke historyCitation23. The relatively low-risk group in the study by Moon et al.Citation10 may explain the relatively promising findings. Second, the outcomes have been defined differently in studies; for example, the risk of ICH as an outcome was not investigated in the study by Briasoulis et al.Citation7, and the risk of VTE as an outcome was investigated only in our studyCitation10. Lastly, NOAC utilization patterns varied in different studiesCitation7,Citation10. As suggested by the present study, different NOACs may have different risk-benefit profiles, different patterns of NOAC treatment may also lead to different findings. Despite inconsistent findings, the use of NOACs in patients with AF and VHD has been recognized as a superior alternative to warfarin owing to their comparable or even lower risk of thromboembolism and bleedingCitation7,Citation10.
The effectiveness and safety of individual NOACs are important concerns in clinical practice. In a meta-analysis, the risk reduction of major bleeding and ICH by NOACs in patients with AF and VHD was mainly driven by dabigatran, apixaban, and edoxaban, but not rivaroxaban. The risk of major bleeding increased in patients with VHD taking rivaroxaban, although the risk of ICH was comparable to that of warfarinCitation6. Our data also revealed differences in the effectiveness of different NOACs. The benefit of reducing VTE risk was mainly driven by dabigatran, but the benefit in reducing the risk of ICH and mortality was observed in patients taking dabigatran and rivaroxaban.
This study had several limitations. First, the results for echocardiography were not available in the NHIRD, and thus we were unable to clearly define the severity of VHD among the participants in our study. Therefore, we performed a subgroup analysis by excluding all participants with mitral stenosis and valve replacement surgery. The results were in line with the main analysis. Nevertheless, the proportion of participants with valve replacement and mitral stenosis (included in the severe VHD group) was small; therefore, our data were limited to reflect the effectiveness and safety of NOAC for this group compared to that of warfarin. Second, the international normalized ratio was not available in the NHIRD; therefore, we were unable to monitor the quality of warfarin therapy. In addition, the actual medication-taking behavior of patients was unavailable in the NHIRD; thus, we followed the prescription-filling records as a proxy for medication adherence. On the brighter side, our data reflected the actual setting of the use of the medication in the real world, as these issues also exist in clinical practice.
This study also had several strengths. First, it provides real-world data regarding the effectiveness and safety of NOAC in Asian patients with AF and VHD. We enrolled participants taking medications for both primary and secondary prevention, which is closer to real-world practice, making the results more comparable to a clinical setting. Second, we investigated each NOAC individually to determine if the results of the outcomes changed across different drugs. Lastly, we performed an as-treated analysis and censored follow-up once the participants changed their anticoagulant therapy to avoid misclassification. The design improved the robustness of our findings.
Conclusion
Our data showed that NOAC was as effective as warfarin in preventing ischemic stroke and had a similar risk of bleeding in patients with AF and VHD. Moreover, NOAC reduced the risk of VTE, ICH, and mortality compared to warfarin. Given the limited number of patients with severe VHD in the present study, further research is needed to confirm the efficacy and safety of NOACs in this subgroup.
Transparency
Declaration of funding
This study was funded by research grants from the Ministry of Science and Technology in Taiwan (MOST 106-2320-B-002 − 032 -MY3; MOST 107-2636-B-002-002). The funder had no role in the research design and result interpretation.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Author contributions
Conception and design: HJ Li, SY Lin, FJ Lin, CS Hung, CC Wang; Data analysis: HJ Li; Interpretation of the data: HJ Li, SY Lin, FJ Lin, CS Hung, CC Wang; Drafting of the paper: HJ Li, SY Lin; Revising the paper critically for intellectual content: FJ Lin, CS Hung, CC Wang; Final approval of the version to be published: HJ Li, SY Lin, FJ Lin, CS Hung, CC Wang; All authors agree to be accountable for all aspects of the work.
Supplemental Material: Table 2
Download MS Word (16.4 KB)Supplemental Material: Table 1
Download MS Word (19.4 KB)Acknowledgements
We would like to acknowledge the support from the Stroke Center and Department of Neurology, National Taiwan University Hospital. We thank Taylor & Francis Editing Services and Hsiao-Hang Lin for her editorial service for this article.
Data availability statement
The data that support the findings of this study are available from the Ministry of Health and Welfare of Taiwan. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://www.mohw.gov.tw/mp-2.html with the permission of the Ministry of Health and Welfare of Taiwan.
References
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. The Lancet. 2006;368(9540):1005–1011.
- Pan KL, Singer DE, Ovbiagele B, et al. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(7):e005835.
- Briasoulis A, Inampudi C, Akintoye E, et al. Safety and efficacy of novel oral anticoagulants versus warfarin in medicare beneficiaries with atrial fibrillation and valvular heart disease. J Am Heart Assoc. 2018;7(8):e008773.
- Noseworthy PA, Yao X, Shah ND, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease. Int J Cardiol. 2016;209:181–183.
- Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44(7):1891–1896.
- Moon I, Lee SR, Choi EK, et al. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease. J Clin Med. 2019;8(10):1624.
- Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- AbuDagga A, Stephenson JJ, Fu AC, et al. Characteristics affecting oral anticoagulant therapy choice among patients with non-valvular atrial fibrillation: a retrospective claims analysis. BMC Health Serv Res. 2014;14(1):310.
- Lauffenburger JC, Farley JF, Gehi AK, et al. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol. 2015;115(8):1095–1101.
- Di Minno A, Frigerio B, Spadarella G, et al. Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev. 2017;31(4):193–203.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272.
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100.
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161.
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Statist Simulation Comput. 2009;38(6):1228–1234.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962.
- January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140:e125–e151.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214.
- Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66(12):1339–1347.