?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Baloxavir marboxil (baloxavir) is a single-dose antiviral which was previously found to be a cost-effective alternative to laninamivir in otherwise healthy adults in Japan. This study aimed at investigating the cost-effectiveness of baloxavir versus laninamivir in patients with influenza at high risk for complications.
Methods
A decision tree was utilized to estimate costs and health gains associated with the use of antivirals. A lifetime horizon was applied to capture the long-term impact of influenza complications, and other events with associated costs and health outcomes were accounted for one influenza season. The study population was stratified into three categories: adolescents and non-elderly adults with high-risk conditions (HRC), elderly without other HRC, and elderly with other HRC. The cost-effectiveness was assessed from a public healthcare payer’s perspective. The duration of influenza symptoms, probabilities of complications and probabilities of adverse events were obtained from a clinical trial and network meta-analysis. The costs of influenza and adverse events management were derived from the JammNet claims database. Utility values were informed by the clinical trial data and literature. Sensitivity analyses were also performed.
Results
The baloxavir strategy was associated with higher costs (+¥144) and higher quality-adjusted life-years (QALYs) in adults with HRC, elderly without HRC and elderly with HRC (+0.00078, +0.00183 and +0.00350 respectively). The overall incremental cost/QALY for baloxavir versus laninamivir was ¥68,855, which was below the willingness-to-pay threshold of ¥5 million/QALY gained. Key drivers of the model results were the probability of pneumonia and bronchitis. The probability of baloxavir being cost-effective was 72%.
Conclusions
This study suggests that influenza treatment with baloxavir is cost-effective compared with laninamivir in the adult high-risk population in Japan.
Introduction
Influenza is an acute, contagious illness of the respiratory system caused by the influenza virus. Influenza infects about 10%–20% of the world’s populationCitation1, resulting in 291,000 to 646,000 respiratory deaths annuallyCitation2. Most severe cases of influenza occur in a high-risk population that includes elderly adults, pregnant women and patients with various comorbidities such as chronic obstructive pulmonary disease, congestive heart failure and diabetesCitation3.
The best routine practice for the management of influenza, as recommended by clinical guidelines, varies across countries. In the UK, for example, the use of antivirals is relatively low compared to Japan, where antivirals are routinely prescribedCitation4. Several options are available for the treatment of influenza, including over-the-counter medications for symptomatic relief and antiviral therapies. Three classes of antivirals are currently available for the treatment and/or prophylaxis of influenza infectionsCitation4–6: (1) the class of M2 inhibitors or adamantanes (e.g. amantadine) is the first generation of anti-influenza drugs that is not currently recommended for use due to widespread resistance and limited spectrum of activity; (2) neuraminidase inhibitors include zanamivir (RelenzaFootnotei), oseltamivir (TamifluFootnoteii), peramivir (RapiactaFootnoteiii) and laninamivir (InavirFootnoteiv) which are recognized as effective against influenza A and B; and (3) the cap-dependent endonuclease inhibitor baloxavir marboxil, hereafter referred as baloxavir (XofluzaFootnotev), active against influenza A and B. Baloxavir was first approved in Japan in 2018 for adults and children and also approved by the US Food and Drug Administration in 2018 for otherwise healthy adults, and in 2019 for high-risk population. Phase III trials in otherwise healthy (CAPSTONE-1, NCT02954354) and high-risk patients (CAPSTONE-2, NCT02949011) have proven safety and efficacy of this antiviral for both the normal- and high-risk infected populationCitation7–9. The study in a high-risk population has shown that a single dose of baloxavir significantly reduced the time to improvement of influenza symptoms and the risk for complications versus placebo. Compared to oseltamivir, which should be administered twice a day for 5 days, the clinical efficacy for the alleviation of influenza symptoms and reduction of complication rate was not significantly differentCitation8.
For the purpose of the cost-effectiveness analysis in the Japanese setting, the choice of the comparator for baloxavir was performed in line with the official guideline for economic evaluations in JapanCitation10,Citation11. It is recommended to select the comparator from technologies which are expected to be replaced by the evaluated technology. Among them, technologies which are widely used in clinical practice and which result in a better outcome should be selected. It is unclear, to date, which of the neuraminidase inhibitors performs better. However, according to reports of the Ministry of Health, Labour and Welfare of Japan (MHLW), laninamivir was the most often prescribed anti-influenza drug for adolescents and adults in Japan in the 2015/2016 to 2017/2018 influenza seasons (before the launch of baloxavir)Citation12,Citation13. Baloxavir became the most used antiviral in the following season (2018/2019), when laninamivir had the second largest market.Citation5 It should be also noted that laninamivir was considered as the price comparator for baloxavirCitation14. Therefore, laninamivir was chosen as an appropriate comparator for baloxavir.
The first cost-effectiveness study on baloxavir has shown that it is cost-effective compared to laninamivir in otherwise healthy adults in JapanCitation15. To date, however, the cost-effectiveness of baloxavir in patients with different health and age profiles, such as adults and the elderly with high-risk conditions (HRC), has not been reported. The objective of this study was to investigate the cost-effectiveness of baloxavir versus laninamivir in patients with influenza at high risk for complications in Japan.
Methods
Model overview
This study was performed using the adaptation of a previously developed modelCitation15. The course of influenza, and the associated costs and quality-adjusted life-years (QALYs) were described using a decision tree. The following events were considered in a patient pathway: confirmation of influenza using a rapid influenza diagnostic test (RIDT), development of drug-related adverse events (DRAEs), resistance to antiviral, occurrence of influenza-related complications, hospitalization following complications, and death due to complications or other reasons (). The model reflects one influenza season and considers the lifetime consequences of influenza complications. A public healthcare payer perspective was applied in line with the Japanese guideline for economic evaluationsCitation10,Citation11.
Figure 1. The model structure. The symbol [+] is used to simplify the scheme and to avoid repeating identical sections of the model. Pathways shown above [+] were also applied at the point indicated. Abbreviations. DRAE, Drug-related adverse event; RIDT, Rapid influenza diagnostic test.
![Figure 1. The model structure. The symbol [+] is used to simplify the scheme and to avoid repeating identical sections of the model. Pathways shown above [+] were also applied at the point indicated. Abbreviations. DRAE, Drug-related adverse event; RIDT, Rapid influenza diagnostic test.](/cms/asset/200ce8c4-c087-4ba0-975d-e9a7bfcafc84/icmo_a_1914942_f0001_c.jpg)
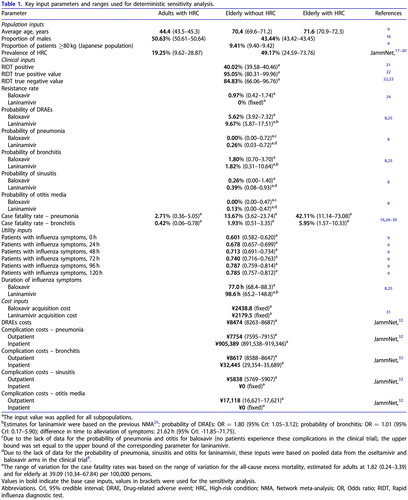
Key model parameters are presented in ; detailed information regarding inputs is provided in the .
Population characteristics
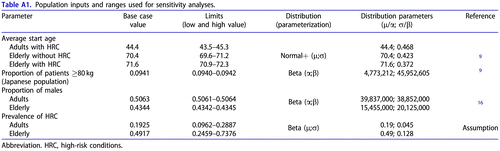
The model population consists of patients at high risk for influenza complications who are seeking medical attention for an influenza-like illness. The following subpopulations were analyzed in the model: (1) adolescents and adults (aged ≥12 and <65 years) with HRC, referred to as “adults with HRC” hereafter; (2) the elderly (aged ≥65 years) without other HRC; and (3) the elderly (aged ≥65 years) with other HRC. Patients older than 65 years are at higher risk for complications because of age. Therefore, the third subpopulation represents an overlap of two or more risk factors. The stratification of elderly in two subpopulations was considered important as influenza-associated mortality is highly heterogeneous in this age groupCitation29,Citation33.
The starting age in modelled subpopulations was based on a phase III clinical trial of baloxavir in patients at high risk for complicationsCitation9. The proportion of males was sourced from Japanese Government StatisticsCitation16, assuming that this parameter is not affected by the presence of HRC. Demographic characteristics from the phase III clinical trial were not used to avoid possible bias due to the low sample size.
The following list of HRC was considered in line with the Centers for Disease Control and Prevention’s definition and phase III clinical trial of baloxavirCitation3,Citation8:
Asthma or chronic lung disease.
Endocrine disorders.
Compromised immune system.
Neurological and neurodevelopmental disorders.
Heart disease (excluding hypertension).
Blood disorders.
Metabolic disorders.
Morbid obesity.
Women within 2 weeks postpartum – for patients aged <65 years only.
Residents of long-term care facilities – for patients aged ≥65 years only.
Comprehensive and consistent information on the prevalence of these HRC in adults and elderly was not identified, therefore it was estimated using several Japanese sources. The prevalence of HRC in adults was assumed to be 19% based on a real-world studyCitation34. Real-world evidence on the prevalence of HRC in the elderly was not available; for this reason, consistency between estimates was assured by applying the age-dependent increase of HRC prevalence based on a JammNet database analysis and the published literature. Additional sources were needed to retrieve data for two high-risk groups that were not covered in JammNet: women ≤2 weeks postpartum and residents of long-term care facilitiesCitation17–20. The ratio of the elderly with HRC to adults with HRC was 2.55, and the prevalence of HRC in the elderly was estimated at 49%. The obtained prevalence of HRC was applied to the general population estimatesCitation16, in order to assess the stratification of the high-risk population. Ultimately, the total high-risk population included 30% of adults with HRC, 36% of elderly without HRC and 34% of elderly with HRC.
Clinical and epidemiological data
Rapid influenza diagnostic test
The RIDT is systematically used in Japan to confirm the need for antivirals in patients with influenza-like symptomsCitation35,Citation36. The performance characteristics of the RIDT and the reported rate of positive results were applied in the model to estimate the probability of true influenza in patients with influenza-like illness and the proportion of patients treated with antiviralsCitation21–23. Although the RIDT is used for all patients in both treatment arms, it is represented in the pathway because the model is intended to be applied for several other countries, where routine testing is not recommended.
Resistance
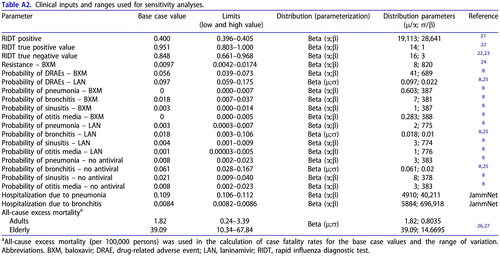
In case of infection with a resistant strain, the patient could experience DRAEs of an antiviral, but no efficacy in terms of alleviation of symptoms or avoided complications. Treatment-emergent resistance to antivirals was assumed to be captured in efficacy parameters obtained from the clinical trialCitation8. Additionally, the rate of resistance due to the circulation of mutant virus strains was explicitly considered in the model. Data regarding the baloxavir resistance observed in patients without baloxavir treatment (not captured in the trial) were sourced from the report of the National Institute of Infectious Diseases (NIID, Japan) during the 2018–2019 influenza seasonCitation24.
Drug-related adverse events
The total DRAEs were considered in the model. The phase III clinical trial provided the probability of DRAEs for baloxavirCitation9. An odds ratio obtained from the network meta-analysis (NMA) was used to calculate the probability of DRAEs for laninamivirCitation25.
Influenza-related complications
The following influenza-related complications were considered: pneumonia, bronchitis, sinusitis and otitis media; rates were retrieved from the phase III clinical trialCitation8. The probabilities of all complications for baloxavir were based on the data from the baloxavir arm of the trial. The probability of bronchitis for laninamivir was obtained from an NMACitation25. The probabilities of the other complications for laninamivir (pneumonia, sinusitis and otitis media) were based on pooled data from the oseltamivir and baloxavir armsCitation8, as no data for laninamivir were found. It was considered that patients with complications may be subsequently hospitalized. The probabilities of hospitalization due to pneumonia, bronchitis, sinusitis or otitis media were obtained from the JammNet database analysis. The same probability of hospitalization was considered for both antivirals.
Mortality
Background mortality and influenza-specific mortality were considered in the analysis. Background mortality was based on the Life Tables for JapanCitation37. The probability of death was estimated, considering the age and sex ratio in the modelled population. Further adjustment for the increase of the mortality risk due to HRC was performed using the available data regarding the most prevalent comorbidities (chronic obstructive pulmonary disease, asthma, diabetes, heart diseases)Citation38.
It was considered that influenza is not always reported as a cause of death in patients dying from complications following influenzaCitation29,Citation39. Therefore, all-cause excess mortality associated with influenza in a Japanese population was accounted for in the model to avoid underestimationCitation26,Citation27. It was assumed that influenza-associated deaths occur only in patients with complications, and among modelled influenza complications only pneumonia and bronchitis could result in death. The proportion of deaths due to pneumonia among all deaths due to respiratory complications was sourced from a study by Nunes et al.Citation28. Further, estimates were adjusted to account for the higher influenza-related mortality in a population with HRC, in line with Hoshi et al.Citation29. The prevalence of HRC in a population, influenza attack rateCitation30 and proportion of patients with complications were also taken into account. More details on calculations are provided in the Appendix.
Utilities
The Japanese studyCitation40 was used to obtain the age- and sex-specific baseline utility norms, representing quality of life in patients without influenza. Utility norms were decremented to account for the presence of HRC, duration of influenza symptoms, DRAEs and complications.
The median time to alleviation of influenza symptoms for baloxavir was 77.0 h (95% CI: 68.4–88.3 h), as observed in the clinical trialCitation8. The time to alleviation of symptoms for laninamivir was estimated at 98.6 h (95% CI: 65.2–148.8 h) in the NMACitation25.
The utility decrements for influenza-related complications and duration of complications were sourced from a health technology assessment (HTA) report by Burch et al.Citation6.
The utility decrement for DRAEs was derived from an HTA report by Tappenden et al.Citation41 The duration of DRAEs for baloxavir was based on the data from the baloxavir arm of the phase III clinical trialCitation9. The pooled data from the oseltamivir and baloxavir arms of the trial were applied for laninamivir, due to the lack of information for this antiviral.
Resource use and costs
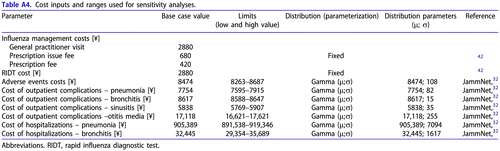
The unit costs of DRAEs management, influenza-related complications and hospitalizations were obtained from the JammNet database for the 2016–2017 influenza season. These costs were adjusted to 2020 price level with the revision rates for 2020Citation32.
The costs of the influenza diagnostics and management, as well as drug acquisition costs for baloxavir and laninamivir, were based on the relevant Japanese sourcesCitation31,Citation42. The dose of baloxavir was adjusted to the patient’s weight: for those with a weight of ≥80 kg, a single dose of 80 mg of baloxavir was used, while others received 40 mg. The proportion of patients with a weight of ≥80 kg was sourced from the phase III clinical trial of baloxavir (Japanese population)Citation9. The standard single dose was considered for laninamivir (40 mg).
Analysis
The cost-effectiveness in the total high-risk population was evaluated by aggregating the results obtained for subpopulations: adults with HRC, elderly without HRC and elderly with HRC.
QALYs were assessed over a lifetime horizon and discounted to present values; costs were assessed for one influenza season, and therefore not discounted. The willingness-to-pay threshold was considered at ¥5 million/QALY gainedCitation11.
Sensitivity analyses evaluated the impact of assumptions used in the model and the variability surrounding model inputs. For deterministic sensitivity analyses (DSA), one variable or assumption was changed at a time. One-way sensitivity analyses were conducted on all model parameters associated with uncertainty. Input values were successively replaced by the limits of the 95% confidence intervals around estimates used in the base case, or other values assumed to represent the plausible range of variation, and the results were summarized in a tornado chart. In order to explore the impact of joint uncertainty in all the model parameters, a probabilistic sensitivity analysis (PSA) was performed using the Monte-Carlo methodCitation43. Appropriate statistical distributions were assigned to model inputs. For each of the input values the chosen distribution was parametrized based on the available information (standard deviations, confidence intervals, etc.). When no data were available, the moments of a distribution were calibrated so that the ranges used in the PSA correspond to those used in the DSA. Values were drawn at random from statistical distributions for these variables and the process was iterated 2000 times to provide the statistical distributions of incremental costs, incremental QALYs, and the incremental cost-effectiveness ratio (ICER). The base case probabilistic results were presented at the incremental cost-effectiveness plane and the cost-effectiveness acceptability curve.
The base case analysis used incidence rates of complications from the phase III clinical trial and an NMA, which appeared to be relatively lowCitation8,Citation25, and were lower for baloxavir compared to laninamivir. The scenario analysis used higher rates of complications, based on the real-world data (JammNet claims database). Furthermore, it was designed to present a more conservative approach and to consider no benefits for baloxavir in terms of influenza complications, i.e. the same complication rates were applied for both treatments. It should be noted that changes in pneumonia and bronchitis rates also had an impact on the case fatality rates, as those were conditional on the occurrence of complications.
Input values applied in sensitivity analyses are provided in the and Appendix.
All analyses were conducted using Microsoft Excel 2017 (Microsoft Corp., Redmond, WA, USA).
Results
The base case
The results of the base case analysis for RIDT followed by baloxavir versus RIDT followed by laninamivir in the total high-risk population are shown in . Baloxavir treatment was associated with more favourable efficacy and safety profile, and therefore with a lower number of QALYs lost due to influenza symptoms, complications and DRAEs. The higher acquisition cost of baloxavir was partly offset by costs of avoided DRAEs and complications. Baloxavir showed a QALYs gain versus laninamivir (+0.00209 QALY per 1 patient), for an additional cost of ¥144. As a result, the ICER for baloxavir vs. laninamivir was estimated at ¥68,855 per QALY gained.
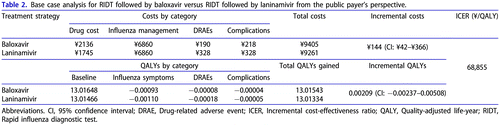
The analysis by subpopulation () showed that the baloxavir strategy was associated with higher QALYs vs. laninamivir in adults with HRC, elderly without HRC and elderly with HRC; ICERs were estimated at ¥185,709, ¥78,724 and ¥41,125 per QALY gained, respectively. The obtained results suggest that baloxavir was particularly beneficial for the elderly with HRC, mostly due to complications and complication-related mortality avoided.
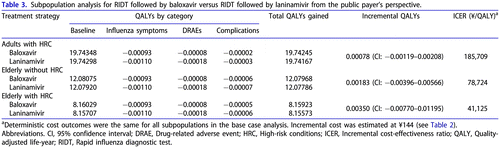
The cost outcomes were the same for all subpopulations and the total population. This analysis considered no difference between subpopulations in the uptake of antivirals, or probabilities of DRAEs, complications and hospitalizations, as no robust estimates for such differences were available from the main sources of data (the clinical trial, NMA and JammNet database analysis). Therefore, there were no subpopulation-specific parameters related to costs, and the differences in cost-effectiveness estimates were driven by QALY gains.
The obtained results suggest that baloxavir was likely cost-effective vs. laninamivir in all studied subpopulations and the total high-risk population, considering the willingness-to-pay threshold of ¥5 million/QALY gained.
Sensitivity analyses
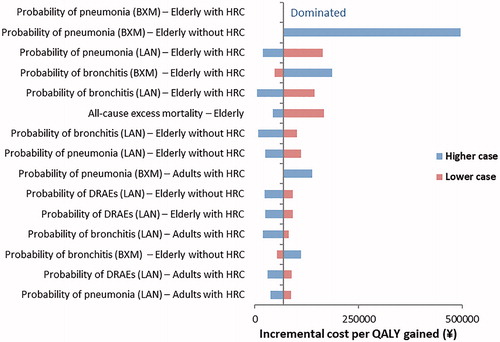
The results of the DSA are depicted in . It was shown that the main drivers of the model results were probability of complications and death for elderly subpopulations. The probability of DRAEs also had an impact on the model results.
Figure 2. The results of DSA for RIDT followed by baloxavir versus RIDT followed by laninamivir.
Abbreviations. BXM, Baloxavir marboxil; DRAE, Drug-related adverse event; DSA, Deterministic sensitivity analysis; HRC, High-risk condition; LAN, Laninamivir; QALY, Quality-adjusted life-year; RIDT, Rapid influenza diagnostic test.
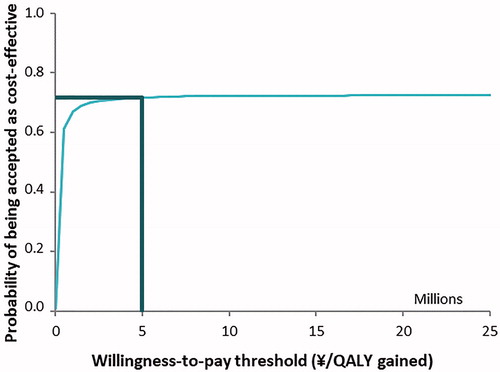
The results of the PSA are depicted in and . The incremental cost-effectiveness plane () presents the variability in incremental costs and QALYs for the base case comparison of baloxavir vs. laninamivir. There was a 95% chance of incremental costs to vary from ¥42 to ¥366 and for incremental QALYs to lie in a range between −0.00237 and 0.00508. The cost-effectiveness acceptability curve () shows baloxavir to be the cost-effective strategy in 72% of cases at the considered willingness-to-pay threshold in the total high-risk population. The analysis by subpopulation showed that the probability of being cost-effective for baloxavir vs. laninamivir was 72%, 67% and 66% in adults with HRC, elderly without HRC and elderly with HRC, respectively.
Figure 3. Incremental cost-effectiveness plane: RIDT followed by baloxavir versus RIDT followed by laninamivir.
Each dot represents the result of one model simulation, with input values taken from the statistical distribution around model parameters.
Abbreviations. CI, Confidence interval; QALY, Quality-adjusted life-year; RIDT, Rapid influenza diagnostic test; WTP, Willingness-to-pay threshold.
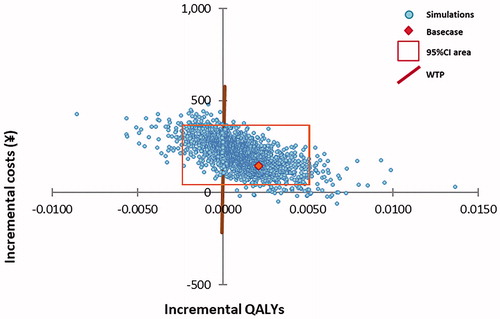
Figure 4. Incremental cost-effectiveness acceptability curve: RIDT followed by baloxavir versus RIDT followed by laninamivir.
Abbreviations. QALY, Quality-adjusted life year; RIDT, Rapid influenza diagnostic test.
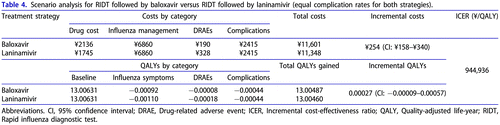
The results of the scenario analysis that explored the alternative assumptions and data source for complication-related inputs are shown in . This scenario was more conservative in comparison to the base case, as it considered no benefits for baloxavir in terms of complications and survival. Nevertheless, the baloxavir strategy remained favourable in terms of QALYs gained due to a shorter time to alleviation of symptoms and fewer DRAEs. Incremental QALYs were estimated at +0.00027 QALY per one patient. Incremental costs vs. laninamivir were higher than in the base case (+¥254), resulting in an ICER of ¥944,936/QALY, which was below the willingness-to-pay threshold. The probability of being cost-effective for baloxavir was estimated at 89%.
Discussion
This cost-effectiveness analysis suggests that baloxavir is cost-effective versus laninamivir from a public payer’s perspective when a willingness-to-pay threshold of ¥5 million/QALY gained is considered. The ICER was largely influenced by the probability of complications and complication-related mortality estimates, which had an impact on the value of treatment over a lifetime horizon. Nevertheless, the sensitivity analyses demonstrated that the cost-effectiveness analysis conclusions held when any model parameter was changed, apart from the probability of pneumonia for baloxavir.
The model was adapted from a previous model-based analysis, which showed that baloxavir is a cost-effective alternative to laninamivir in otherwise healthy adults in JapanCitation15. The costs and benefits for otherwise healthy adults were estimated over one influenza season, and the ICER was ¥2,231,260 per QALY gained. The model results were mainly driven by the lower probability of DRAEs and the shorter duration of influenza symptoms associated with baloxavir. This new model was intended to reflect the course of influenza in patients at high risk for complications, and the methodology was adjusted accordingly. In order to capture the outcomes which are relevant for the population of interest, the QALYs lost due to fatal complications were accounted over a lifetime horizon, which was in line with the HTA report by Burch et al.Citation6 The model also considered the heterogeneity of patient profiles, which allowed age- and HRC-related differences in quality of life and mortality due to influenza or other causes to be reflected. Therefore, the numeric results reported for the otherwise healthy and high-risk populations are not directly comparable, although similar conclusions were drawn in both studies.
The model focuses on the Japanese population and adheres to official guidelines for economic evaluation in Japan. A strength of the model is to rely on state-of-the-art clinical data, mainly based on a clinical trial and an NMA, and cost data reflecting the current clinical practice in Japan. In addition, the model has a comprehensive structure which includes all relevant influenza complications and adverse events of antivirals, utilizing a direct link between the type of complications and death. Furthermore, as reliable estimates for quality of life in influenza patients have been lacking in economic evaluations so farCitation44, this model applies utility data obtained from the EQ-5D instrument in the clinical trial of baloxavir. This is the most widely recommended approach for obtaining utility values for economic assessmentCitation10,Citation45,Citation46.
This study explicitly accounts for the current resistance to baloxavir. The treatment-emergent resistance was captured in the clinical trial results. The CAPSTONE-2 trial showed that mutant virus strains with reduced susceptibility to baloxavir were identified in about 5% of patients treated with baloxavir, and these patients were included in the efficacy analysesCitation8. The data reported by the NIID suggest that the rate of the resistant virus strains in circulation did not increase in the real world over the following influenza seasons after 2018/2019Citation24,Citation47. The resistance to antivirals due to circulation of mutant virus strains (patients without baloxavir treatment, not captured in the trial) was considered in a patient pathway, inputted in the model and explored in sensitivity analyses. The DSA and PSA showed that variation of applied resistance rate in the range of 0.42%–1.74% did not have a major impact on the study results: the ICER was ¥65,608 and ¥73,736 per QALY gained, at the lower and higher bound respectively. In line with the current evidence, the resistance to laninamivir was considered to be 0%Citation24, also representing a conservative approach. However, it should be kept in mind that resistance to both treatments may vary from year to year, and this could affect the treatment efficacy and cost-effectiveness estimates.
The model also has several limitations. First, the study presents only a comparison versus laninamivir. This choice was made in line with the official guideline for economic evaluation in Japan. It should also be noted that both medications require a single dose; however, laninamivir may be less appropriate for patients with chronic lung diseases as it should be administered by inhalation.
There were no patients with pneumonia and otitis media in the baloxavir arm of the CAPSTONE-2 trial. The base case reflected the clinical data and applied zero probability for these events. The impact of this assumption was tested in sensitivity analysis, which showed that the probability of pneumonia in the elderly is an important driver for the model results. The available evidence suggest that the incidence of this complication is low, although real-world studies would be needed to provide further information on the risk of pneumonia in patients taking baloxavir.
In addition, there is uncertainty around the relative effect of antiviral treatments, which is mainly due to the fact that trials were powered to detect differences vs. placebo, not between alternative antiviral productsCitation8,Citation25. It has been shown that baloxavir treatment was associated with a shorter time to alleviation of influenza symptoms, and lower incidence of bronchitis and DRAEs, when compared with laninamivir in the NMA. However, significant differences were found only for the incidence of DRAEs. Although the use of data regardless of the statistical significance could be criticizedCitation48, the methodological guidelines on the cost-effectiveness analyses recommend incorporating all available evidence and quantifying the associated limitations using sensitivity analysisCitation49,Citation50. In line with the current recommendations, we considered all available information, including the non-significant differences, and reported DSA and PSA, which fully accounted for the uncertainty surrounding the results of indirect treatment comparison for baloxavir and laninamivir.
Several assumptions were required to model influenza-related complications and mortality in a high-risk population. It was assumed that deaths occur only in patients with complications, which was further restricted to pneumonia and bronchitis. Due to the lack of Japanese data, the distribution of mortality between these types of complications was sourced from the Portuguese study by Nunes et al.Citation28. This distribution was then applied to the all-cause excess mortality associated with influenza in Japan, thus the model reflects total influenza-related deaths accurately.
A scenario analysis was performed to address the uncertainty around the relative effect of antiviral treatments on complications and mortality. First, this scenario applies higher complication rates, based on the alternative source of data (JammNet database). Second, the complication rates were equal for baloxavir and laninamivir, which represents a more conservative approach than the base case. Ultimately, it allowed concerns regarding the possible overestimation of case fatality rates to be ruled out, as no treatment-related differences were considered for mortality in this scenario. The obtained results showed that baloxavir remains likely cost-effective even when complication- and survival-related advantages are excluded.
The background mortality in the high-risk population was estimated using the shortlist of selected HRC due to the lack of data. The shortlist included chronic obstructive pulmonary disease, asthma, diabetes and heart diseases. These conditions are among the 16 main causes of death in JapanCitation38, and cover most of the patients in the target high-risk population according to JammNet. This approach assumes that mortality due to all HRC is equal to mortality due to the causes mentioned above. It should be also noted that due to the lack of reliable estimates for the adverse events of neuraminidase inhibitors, utility decrements were assessed among patients taking amantadineCitation41.
Finally, the current model is static, which ignores the contagious nature of influenza and the effect of antiviral treatment on reducing the number of new infections. As baloxavir reduces the viral load and shedding faster than other antiviralsCitation8,Citation25, it would keep patients infectious for a shorter period, possibly decreasing the risk of disease transmission. A dynamic model would be required to take into account the transmission aspect of the disease.
Conclusion
This study suggests that influenza treatment with baloxavir is cost-effective compared with laninamivir in the adult high-risk population in Japan.
Transparency
Declaration of funding
This study was funded by Shionogi & Co., Ltd, Japan.
Author contributions: M.D. programmed the model, performed statistical analyses, conducted the data collection, interpreted the results and prepared a draft of the manuscript. H.I. designed the study, conducted the data collection, interpreted the results and critically reviewed the manuscript. N.H., S.A., M.H. and A.I. designed the study, interpreted the results and critically reviewed the manuscript. A.A. programmed the model, performed statistical analyses, conducted the data collection and interpreted results. Y.O. and N.I. conducted the data collection and critically reviewed the manuscript. All authors reviewed the manuscript and approved the final version of the manuscript.
Declaration of financial/other relationships
H.I. and N.I. have disclosed that they are employees of Shionogi & Co., Ltd. M.H. has disclosed that he is an employee of Shionogi B.V. M.D., A.A., S.A. and Y.O. have disclosed that they are or were employed by Creativ-Ceutical, which had received research grants from Shionogi & Co., Ltd. N.H. has disclosed that he has financial relationships with Shionogi & Co., Ltd. for speaker honoraria, advisory board and funding for commissioned/joint research. A.I. has disclosed that he has received expenses for a scientific interview from Creativ-Ceutical.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Authors express their deep gratitude to Yuki Yoshida, Masakazu Fujiwara (Shionogi & Co., Ltd) and to Malgorzata Biernikiewicz (Creativ-Ceutical) for their support in this study.
Notes
i Relenza is a registered trade name of GlaxoSmithKline plc. London, UK.
ii Tamiflu is a registered trade name of Chugai Pharmaceutical Co., Ltd., Tokyo, Japan.
iii Rapiacta is a registered trade name of Shionogi & Co., Ltd., Osaka, Japan.
iv Inavir is a registered trade name of Daiichi Sankyo Company, Limited, Tokyo, Japan.
v Xofluza is a registered trade name of Shionogi & Co., Ltd., Osaka, Japan.
References
- Fischer WA 2nd, Chason KD, Brighton M, et al. Live attenuated influenza vaccine strains elicit a greater innate immune response than antigenically-matched seasonal influenza viruses during infection of human nasal epithelial cell cultures. Vaccine. 2014;32(15):1761–1767.
- Lee VJ, Ho ZJM, Goh EH, et al. Advances in measuring influenza burden of disease. Influenza Other Respir Viruses. 2018;12(1):3–9.
- People at High Risk of Developing Serious Flu–Related Complications: Centers for Disease Control and Prevention [Internet] [cited 2019 Mar 28]. Available from: https://www.cdc.gov/flu/about/disease/high_risk.htm
- Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Curr Treat Options Infect Dis. 2016;8(4):311–328.
- MHLW. Usage of anti-influenza virus drugs in the 2018/2019 season [Internet] [cited 2020 Sep 30]. Available from: https://www.mhlw.go.jp/content/11120000/000570536.pdf
- Burch J, Paulden M, Conti S, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technol Assess. 2009;13(58):1–265, iii-iv.
- Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913–923.
- Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204–1214.
- A phase 3, multicenter, randomized, doubleblind study of a single dose of S-033188 compared with placebo or oseltamivir 75 mg twice daily for 5 days in patients with influenza at high risk of influenza complications (ClinicalTrials.gov Identifier: NCT02949011). Shionogi; 2018.
- Shiroiwa T, Fukuda T, Ikeda S, et al. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health. 2017;20(3):372–378.
- Hasegawa M, Komoto S, Shiroiwa T, et al. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51.
- MHLW. Usage of anti-influenza virus drugs in the 2016/2017 season [cited 2020 May 6]. Available from: https://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000183976.pdf
- MHLW. Usage of anti-influenza virus drugs in the 2017/2018 season [cited 2020 May 19]. Available from: https://www.mhlw.go.jp/content/11121000/000377869.pdf
- MHLW. Price calculation for new drugs [Internet]. 2018 [cited 2020 July 7]. Available from: https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000196671.pdf
- Skrzeczek A, Ikeoka H, Hirotsu N, et al. Cost-effectiveness of baloxavir marboxil compared to laninamivir for the treatment of influenza in Japan. J Infect Chemother. 2020;27(2):296–305.
- Statistics Bureau of Japan. Population by Age, Sex and Sex ratio [Internet]. 2018 [cited 2021 Apr 17]. Available from: https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200524&tstat=000000090001&cycle=7&year=20180&month=0&tclass1=000001011679
- MHLW. Census [Internet]. 2018 [cited 2021 Apr 17]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/suikei18/dl/2018suikei.pdf
- Yamada T, Kawakami S, Yoshida Y, et al. Influenza 2014–2015 among pregnant Japanese women: primiparous vs multiparous women. Eur J Clin Microbiol Infect Dis. 2016;35(4):665–671.
- MHLW. Residents of nursing homes [Internet]. 2017 [cited 2021 Apr 17]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kaigo/service17/dl/kekka-gaiyou.pdf
- Deguchi Y, Takasugi Y, Nishimura K. Vaccine effectiveness for influenza in the elderly in welfare nursing homes during an influenza A (H3N2) epidemic. Epidemiol Infect. 2000;125(2):393–397.
- Rapid diagnostic kit measurement status in influenza – Kenkou data [Internet]. 2016/2017 [cited 2021 Apr 17]. Available from: http://www.kenkou.pref.mie.jp/topic/influ/kit/infkit1617.htm
- Akaishi Y, Matsumoto T, Harada Y, et al. Evaluation of the rapid influenza detection tests GOLD SIGN FLU and Quick Navi-Flu for the detection of influenza A and B virus antigens in adults during the influenza season. Int J Infect Dis. 2016;52:55–58.
- Tsushima Y, Uno N, Sasaki D, et al. Quantitative RT-PCR evaluation of a rapid influenza antigen test for efficient diagnosis of influenza virus infection. J Virol Methods. 2015;212:76–79.
- NIID. Detection of antiviral drug-resistant viruses in Japan during the 2018/2019 influenza season [Internet]. 2020 [cited 2020 June 12]. Available from: https://www.niid.go.jp/niid/images/flu/resistance/20191227/dr18-19e20191227-1.pdf
- Taieb V, Ikeoka H, Wojciechowski P, et al. Efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors in the treatment of influenza virus infection in high-risk and uncomplicated patients – a Bayesian network meta-analysis. Curr Med Res Opin. 2021;37(2):225–244.
- Takahashi M, Tango T. Estimation of excess mortality associated with influenza-epidemics by age and cause specific death in Japan, 1975–1999. Jpn J Hyg. 2002;57(3):571–584.
- Historical Statistics of Japan. Population by Five-year Age Groups and Sex (Population Estimates) (1920–2009) [Internet] [cited 2021 April 17]. Available from: http://www.stat.go.jp/english/data/chouki/02.html
- Nunes B, Viboud C, Machado A, et al. Excess mortality associated with influenza epidemics in Portugal, 1980 to 2004. PLoS One. 2011;6(6):e20661.
- Hoshi SL, Kondo M, Honda Y, et al. Cost-effectiveness analysis of influenza vaccination for people aged 65 and over in Japan. Vaccine. 2007;25(35):6511–6521.
- NIID. Influenza in 2018/2019 [Internet] [cited 2020 April 20]. Available from: https://www.niid.go.jp/niid/images/idsc/disease/influ/fludoco1819.pdf
- MHLW. National Health Insurance drug price list [Internet]. 2020 [cited 2020 May 7]. Available from: https://www.mhlw.go.jp/topics/2020/04/tp20200401-01.html
- MHLW. Revision of the medical fee index [Internet]. 2020 [cited 2020 May 12]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000106602.html
- Verhees RAF, Dondorp W, Thijs C, et al. Influenza vaccination in the elderly: is a trial on mortality ethically acceptable? Vaccine. 2018;36(21):2991–2997.
- Shibata N, Kimura S, Hoshino T, et al. Influenza vaccination effectiveness for people aged under 65 years in Japan, 2013/2014 season: application of a doubly robust method to a large-scale, real-world dataset. BMC Infect Dis. 2019;19(1):586.
- The Japanese Association for Infectious Diseases. New influenza treatment guideline, 1st version [Internet]. 2009 [cited 2020 May 19]. Available from: https://www.kansensho.or.jp/uploads/files/guidelines/influenza_guideline.pdf
- CDC. Rapid Influenza Diagnostic Tests [Internet] [cited 2020 May 6]. Available from: https://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm
- The 22nd Life Tables: MHLW [Internet]. 2015 [cited Mar 28 2019]. Available from: https://www.mhlw.go.jp/english/database/db-hw/lifetb22nd/index.html
- Statistics Bureau of Japan. Vital Statistics [Internet]. 2017 [cited 2021 April 17]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&stat_infid=000032020132
- Sellers SA, Hagan RS, Hayden FG, et al. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372–393.
- Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. 2016;25(3):707–719.
- Tappenden P, Jackson R, Cooper K, et al. Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation. Health Technol Assess. 2009;13(11):iii, ix-xii, 1-246.
- MHLW. Medical fee index (shinryo-hoshu, 2020 version) [Internet]. 2020 [cited 2020 May 7]. Available from: https://www.mhlw.go.jp/content/12400000/000603751.pdf
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Damm O, Scholz S, Ultsch B, et al. A systematic review of utility weights for influenza and influenza-like illness. Value Health. 2016;19:A419.
- McGhan WF, Al M, Doshi JA, et al. The ISPOR good practices for quality improvement of cost-effectiveness research task force report. Value Health. 2009;12(8):1086–1099.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- NIID. Detection of antiviral drug-resistant viruses in Japan during the 2019/2020 influenza season [Internet]. 2021 [cited 2021 Feb 16]. Available from: https://www.niid.go.jp/niid/images/flu/resistance/20200811/dr19-20e20200811-1.pdf
- Raftery J, Williams HC, Clarke A, et al. Not clinically effective but cost-effective – paradoxical conclusions in randomised controlled trials with ‘doubly null’ results: a cross-sectional study. BMJ Open. 2020;10(1):e029596.
- Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health. 2003;6(1):9–17.
- NICE Process and Methods Guides. Guide to the methods of technology appraisal. London: National Institute for Health and Care Excellence (NICE); 2013.
Appendix
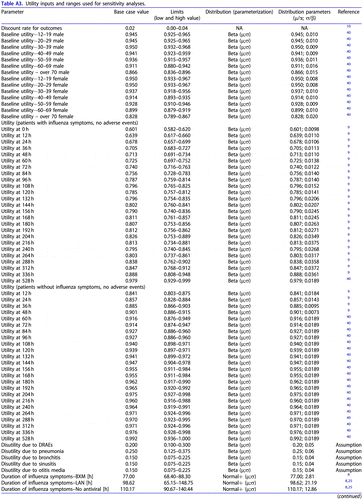
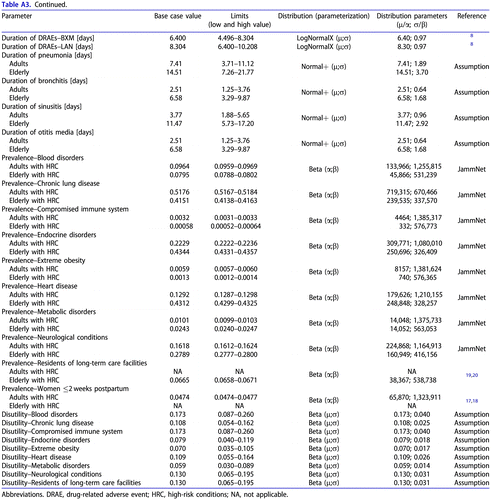
Table A3. Utility inputs and ranges used for sensitivity analyses.
Appendix A6. Estimation of influenza-related mortality
1. All-cause excess mortality (1990–1999) from Takahashi and TangoCitation26 and Historical Statistics of JapanCitation27 were used to estimate mortality rates for the whole population including normal-risk and high-risk individuals. Average excess mortality rates were equal to 1.82 and 39.09 per 100,000 persons for adults and elderly respectively.
2. Data regarding excess mortality rates due to pneumonia and all respiratory causes were obtained from the study by Nunes et al.Citation28 reporting statistics for Portugal. Deaths due to respiratory causes, not related to pneumonia, were considered to be associated with bronchitis. It was assumed that repartition of deaths by cause in Japan is similar to Portugal, but overall excess mortality differs. Therefore, average all-cause excess mortality in Japan was distributed per cause accordingly to Nunes et al.Citation28. Furtherly, Nunes et al.Citation28 reported data regarding excess mortality in two non-elderly cohorts – 5–54 years and 55–64 years. Thus, an estimate for adults was adjusted for respective population sizes in Japan, sourced from Government StatisticsCitation16. Excess mortality rate per cause was calculated as follows:
Where:
EM: Excess mortality rate
The obtained values are provided below:
EM pneumonia, Japan (adults) = 0.82 per 100,000 persons
EM bronchitis, Japan (adults) = 1.00 per 100,000 persons
EM pneumonia, Japan (elderly) = 18.47 per 100,000 persons
EM bronchitis, Japan (elderly) = 20.62 per 100,000 persons
3. The next step was to estimate the yearly probability of death among patients at high risk for complications. According to Hoshi et al.Citation29, the mortality rate for elderly people with risk conditions was assumed to be 3.08 times higher than for elderly patients in normal-risk population. The following formulas were used:
Where:
EMHR: Excess mortality rate in high-risk patients
EMNR: Excess mortality rate in normal-risk patients
PHR: Prevalence of HRC in population
PNR: Prevalence of patients without HRC; PNR = 1 - PHR
The obtained values are provided below:
EM pneumonia (adults with HRC) = 0.002%
EM pneumonia (elderly without HRC) = 0.009%
EM pneumonia (elderly with HRC) = 0.028%
EM bronchitis (adults with HRC) = 0.002%
EM bronchitis (elderly without HRC) = 0.010%
EM bronchitis (elderly with HRC) = 0.031%
4. Ultimately, the case fatal rate (CFR) was calculated to take into account the attack rate of influenza and probabilities of complications. The attack rate of influenza (8.59%) was sourced from the report of NIID regarding influenza incidence in 2016–2019Citation30. No antivirals were available in period reported by Takahashi and TangoCitation26, therefore for the base case the probabilities of pneumonia (0.008) and bronchitis (0.061) were sourced from a placebo arm of the clinical trial (Appendix Table A2)Citation8. The following formula was used:
Where:
AR: Attack rate of influenza
C: probability of complications among patients with HRC given influenza
The obtained values are provided below:
CFR pneumonia (adults with HRC) = 2.71%
CFR pneumonia (elderly without HRC) = 13.67%
CFR pneumonia (elderly with HRC) = 42.11%
CFR bronchitis (adults with HRC) = 0.42%
CFR bronchitis (elderly without HRC) = 1.93%
CFR bronchitis (elderly with HRC) = 5.95%
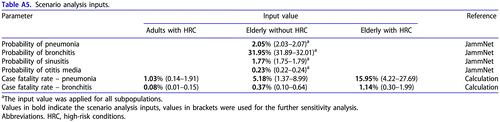
For the scenario analysis, the higher complication rates were applied for pneumonia (0.021) and for bronchitis (0.320), in line with the real-world data (JammNet database). As the complication rates appear in the denominator in the formula above, the calculation yielded lower CFRs.
The obtained values are provided below:
CFR pneumonia (adults with HRC) = 1.03%
CFR pneumonia (elderly without HRC) = 5.18%
CFR pneumonia (elderly with HRC) = 15.95%
CFR bronchitis (adults with HRC) = 0.08%
CFR bronchitis (elderly without HRC) = 0.37%
CFR bronchitis (elderly with HRC) = 1.14%