Abstract
Objective
Evidence is limited on the economic burden associated with treatment-resistant depression (TRD) among US veterans. We evaluated the economic burden among patients with major depressive disorder (MDD) with and without TRD, and those without MDD in the Veterans Health Administration (VHA).
Methods
Three cohorts were identified using VHA claims data (01APR2014-31MAR2018). Patients with MDD (aged ≥18) who failed ≥2 antidepressant treatments of adequate dose and duration were defined as having TRD; patients with MDD not meeting this criterion constituted the non-TRD MDD cohort (index: first antidepressant claim). The non-MDD cohort included those without MDD diagnosis (index: randomly assigned). Patients with psychosis, schizophrenia, manic/bipolar disorder, or dementia in the 6-month pre-index period were excluded. Patients with non-TRD MDD and non-MDD were matched 1:1 to patients with TRD based on demographic characteristics (age, gender, race, index year). Health care resource utilization (HRU) and costs were analyzed during the post-index period using a negative binomial model and ordinary least squares regression model, respectively.
Results
After 1:1 exact matching, 10,449 patients were included in each cohort (mean age: 48.9 years). Patients with TRD had higher per patient per year (PPPY) HRU than non-TRD MDD (all-cause inpatient visits: incidence rate ratio [IRR]: 1.70 [95% confidence interval: 1.57–1.83]) and non-MDD (IRR: 5.04 [95% confidence interval: 4.51–5.63]), and incurred higher total all-cause health care costs PPPY than non-TRD MDD (mean difference: $5,906) and non-MDD (mean difference: $11,873; all p<.0001).
Conclusion
Among US veterans, TRD poses a significant incremental economic burden relative to non-TRD MDD and non-MDD.
Introduction
Major depressive disorder (MDD) is recognized as one of the leading causes of disability in the United States (US) due to its high prevalence, clinically significant symptom distress, and impairment of social, occupational, or other important areas of functioningCitation1. This debilitating disorder was estimated to affect 7.1% of US adults ≥18 years of age in 2017 (17.3 million people)Citation2. and is characterized by severe depressive episodes lasting ≥2 weeks, which manifest as depressed mood or loss of interest or pleasureCitation3,Citation4. The burden of MDD in the US is growing, estimated at $83.1 billion in 2000, $173.2 billion in 2005, and $210.5 billion in 2010 (including direct and indirect costs)Citation4,Citation5.
A significant proportion of patients with MDD do not adequately respond to their first antidepressant (AD) therapy or subsequent lines of pharmacotherapy. Among those who do not respond to AD therapy, 10–30% exhibit treatment-resistant symptoms along with difficulties in social and occupational function, decline in physical health, suicidal thoughts, and increased health care resource utilization (HRU)Citation1. The Agency for Healthcare Research and QualityCitation6 provides a commonly used definition of treatment-resistant depression (TRD) found in the literature: patients with MDD who fail ≥2 ADs at adequate dose and duration. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that patients with MDD requiring a third step AD treatment or beyond had a substantially lower cumulative remission rate (approximately 14%) compared to patients requiring fewer AD treatment steps; approximately 86% of patients with MDD who required a third treatment step were unable to achieve remissionCitation7. Among those patients who did achieve remission, time to remission tended to be longer as treatment steps increasedCitation7. Given that remission remains an unmet need among this patient population and that TRD often presents with multiple comorbidities, the economic burden of TRD is significantly higher relative to MDD without treatment resistance (non-TRD MDD). Compared to patients with non-TRD MDD, patients with TRD are twice as likely to be hospitalized, have 6 times higher hospital-related expenditures, and pay more than twice the amount in total medical costsCitation8–10.
With most research efforts to date focusing on commercially-insured patients, there is a need to assess the burden of TRD among US veterans who are a unique patient population and may experience differences in the economic burden of illness compared with civilians. Veterans have additional risk factors for depression and mental illness, including exposure to combat and a history of traumaCitation11–13. Physical and psychological stressors experienced while deployed can lead to complications, such as post-traumatic stress disorder (PTSD), alcoholism, substance abuse, pain, and suicidal thoughtsCitation14–17.
Compared with the prevalence of US adults reporting ≥1 depressive episode (7.1%)Citation2, 13.5% of US veterans seen in the Veterans Health Administration’s (VHA’s) Patient Aligned Care Teams had a formal diagnosis of depressionCitation18. Although a community-based study of men aged ≥50 years found that veteran status was not associated with elevated rates of depressive symptoms (11.0% of veterans vs 12.8% of non-veterans), data from the Army Study to Assess Risk and Resilience in Service members (Army STARRS) revealed a 30-day prevalence of MDD in 4.8% of army personnel compared with 0.9% observed in the civilian populationCitation19. In another study of 1,522 veteran outpatients with non-psychotic MDD (Veteran’s Affairs [VA] Augmentation and Switching Treatments for Improving Depression Outcomes; VAST-D), 52% of those randomized had chronic depression, and the average duration of the current depressive episode was >7 yearsCitation20. Thus, it has been suggested that veterans with MDD may be at higher risk than those in the general population to develop TRDCitation20. The current study compares the economic burden of patients with TRD to those diagnosed with non-TRD MDD and with non-MDD in the VHA population.
Methods
Study design
This retrospective, matched-cohort study utilized health claims between 1 April 2014 and 31 March 2018 (the study period) from the US VHA Medical SAS Datasets. These datasets included national administrative data for VHA-provided health care (inpatient and outpatient medical and pharmacy claims) utilized primarily by veterans and some non-veterans (e.g. employees, family members). The data were de-identified as per the Health Insurance Portability and Accountability Act, and thus this analysis was not subject to institutional review board approval. The index date was designated as the date of the first AD prescription claim during the identification period (1 October 2014 to 30 September 2017) for patients with MDD (either TRD or non-TRD MDD cohorts); for patients with non-MDD, the index date was randomly assigned based on the index date distribution of patients included with MDD (Supplemental Figure 1 for study design details).
Patient population
Definition of study cohorts
The group of patients defined as having TRD were compared to 2 additional control cohorts: (1) patients with MDD without TRD (non-TRD MDD cohort); and (2) patients without MDD (non-MDD cohort). The 2 control cohorts were set up to better understand the incremental health and economic burden of TRD in the veteran population versus non-TRD MDD and non-MDD. TRD cohort: Patients with MDD who initiated a third AD after failing to respond to 2 AD treatment regimens, including augmentation therapy, of adequate dose and duration, as per the Agency for Healthcare Research and Quality criteriaCitation6. Failure of a treatment regimen was defined as a switch of an AD (i.e. ≤180 days after the end of the previous treatment), the addition of an AD, or the initiation of an augmentation therapy.
Non-TRD MDD cohort
Patients diagnosed with MDD who did not meet the TRD definition above within 3.5 years of the index date.
Non-MDD cohort
Randomly selected sample of US veterans without a MDD diagnosis and without other specific psychiatric comorbidities (schizophrenia, bipolar disorder/manic depression, and dementia); presence of other mental health–related conditions were not exclusion criteria for this cohort. Both non-TRD and non-MDD cohorts were matched to the TRD cohort based on age, gender, race, and index year.
Inclusion criteria
Inclusion criteria for the 3 cohorts are included in and described in the Supplemental Materials.
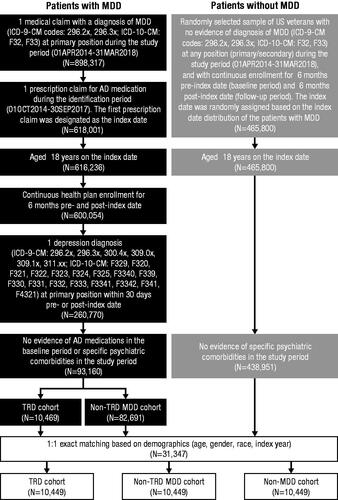
Figure 1. Patient selection criteria. Abbreviations. AD, antidepressant; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; MDD, major depressive disorder; TRD, treatment-resistant depression. Note: Psychiatric comorbidities that resulted in exclusion were schizophrenia (ICD-9-CM: 295.xx), bipolar disorder/manic depression (ICD-9-CM: 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.7x, 296.8x), and dementia (ICD-9-CM: 290.xx, 294.1x).
Outcome measures
Outcomes were assessed during the 6-month follow-up period, which began on or after the index date until the earliest of health plan disenrollment, end of study, or death. All-cause, mental health–related, MDD-specific, and suicide-related HRU, including inpatient length of stay and costs (inpatient, outpatient, pharmacy, facility use, and total costs), were assessed for all study cohorts. Treatment modalities, assessed during the follow-up period, included pharmacotherapy (ADs, antipsychotics, anticonvulsants, lithium, psychostimulants, and thyroid hormone medication) and non-pharmacologic therapy (electroconvulsive therapy, transcranial magnetic stimulation [TMS], and cognitive behavioral therapy). Time to partial remission, full remission, or relapse was calculated as number of days from index date to the first event during the follow-up period; see the Supplemental Materials for definitions of these terms. Patients with partial and full remission codes during the follow-up period were assigned a full remission code only.
Statistical analyses
Exact 1:1 matching based on demographic characteristics (age, gender, race, and index year) was conducted to create matched cohorts of patients with TRD, non-TRD MDD, and with non-MDD. The statistical methods used for cohort matching in this study were identical to previously published studies that compared the economic burden of TRD versus non-TRD MDD and non-MDDCitation21–26. Study variables were analyzed using descriptive statistics, including demographic and clinical characteristics, and outcome measures. Chi-square tests and student t tests were used to evaluate the statistical significance of differences for categorical variables and to assess differences in the means of continuous variables, respectively. Multivariate analysis was performed using a negative binomial model, estimating the rate ratios for HRU, and an ordinary least squares regression model, estimating the mean differences for costs during the follow-up period. Baseline Charlson Comorbidity Index (CCI) score and baseline total costs were adjusted in both models.
Results
Baseline period: clinical characteristics
A total of 898,317 members from the VHA database had ≥1 diagnosis of MDD, and 68.8% (618,001) had ≥1 claim for an AD medication; patient disposition is shown in . After application of other inclusion and exclusion criteria, 93,160 eligible patients with MDD (10,469 TRD and 82,691 non-TRD MDD) were identified. A total of 465,800 members with non-MDD were also screened, of whom 438,951 were eligible. After 1:1 exact matching of the TRD, non-TRD MDD, and non-MDD cohorts, a final total of 31,347 patients were included for analysis (10,449 patients in each cohort).
Mean patient age was 48.9 years, 81.9% of patients were male, and 68.3% were white (). Compared to patients with non-TRD MDD, a higher proportion of patients with TRD reported the presence of mental health–related comorbidities, including severe anxiety (47.2 vs. 42.5%), PTSD (25.6 vs. 22.2%), and suicidal ideation or attempt (6.5 vs. 4.6%), as well as non-mental health–related comorbidities, including chronic pain (39.2 vs. 34.3%) and lumbago (26.7 vs. 23.6%). Patients with TRD were more likely to be prescribed antipsychotics (4.5 vs. 2.0%) and anticonvulsants (18.0 vs. 14.1%) compared with patients with non-TRD MDD. Compared to patients with non-MDD, patients with TRD had a significantly higher average CCI score (0.5 vs. 0.3) and were more likely to report any investigated mental or non-mental health–related comorbidity. Patients with TRD had higher HRU and costs during the baseline period compared to both patients with non-TRD MDD and those with non-MDD (Supplemental Table 1).
Table 1. Baseline demographic and clinical characteristics by TRD, non-TRD MDD, and Non-MDD cohorts.
Follow-up period: adjusted HRU and costs
Patients with TRD had a higher rate of all-cause HRU per patient per year (PPPY) compared to patients with non-TRD MDD, including inpatient visits (incidence rate ratio [IRR]: 1.70 [95% confidence interval (CI): 1.57–1.83]) and outpatient visits (IRR: 1.45 [95% CI: 1.43–1.48]; ). This difference between cohorts was reflected in the higher rate of mental health–related HRU PPPY for patients with TRD, including both inpatient visits (IRR: 1.93 [95% CI: 1.77–2.11]) and outpatient visits (IRR: 1.74 [95% CI: 1.70–1.78]), a higher rate of MDD-specific HRU PPPY, including inpatient visits (IRR: 2.02 [95% CI: 1.83–2.23]) and outpatient visits (IRR: 1.74 [95% CI: 1.70–1.79]), and a higher rate of suicide-related HRU PPPY, including inpatient visits (IRR: 2.38 [95% CI: 2.00–2.84]) and outpatient visits (IRR: 2.34 [95% CI: 2.08–2.62]). Compared with patients with non-TRD MDD, patients with TRD also had higher all-cause health care costs PPPY (), including inpatient (adjusted cost difference: Δ$1963), outpatient (Δ$3626), pharmacy (Δ$316), and total costs ($19,268 vs. $12,919; Δ$5906; all p<.0001); higher mental health–related health care costs PPPY, including inpatient (Δ$1847), outpatient (Δ$2142), pharmacy (Δ$221), and total costs ($9406 vs. $4990; Δ$4210; all p<.0001); and higher MDD-specific health care costs PPPY, including inpatient (Δ$1358), outpatient (Δ$1478), pharmacy (Δ$170), and total costs ($6515 vs. $3390; Δ$3006; all p<.0001).
Figure 2. Adjusted HRU during the follow-up period: (A) patients with TRD versus patients with non-TRD MDD and (B) patients with TRD versus patients with non-MDD. Abbreviations. CCI, Charlson Comorbidity Index; CI, confidence interval; HRU, health care resource utilization; IRR, incidence rate ratio; LOS, length of stay; MDD, major depressive disorder; PPPY, per patient per year; TRD, treatment-resistant depression. Note: Adjusted HRU was estimated using a negative binomial regression model adjusted for baseline CCI score and baseline total costs.
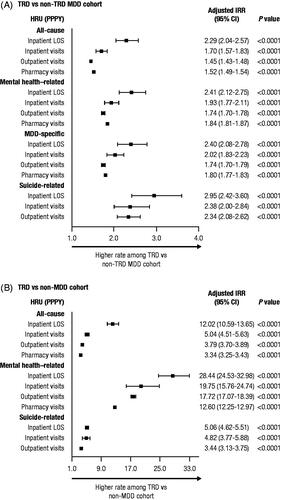
Figure 3. Adjusted health care cost difference among patients with TRD versus patients with non-TRD MDD, and patients with TRD versus patients with non-MDD during the follow-up period. Abbreviations. Adj., adjusted; CCI, Charlson Comorbidity Index; CI, confidence interval; MDD, major depressive disorder; PPPY, per patient per year; TRD, treatment-resistant depression; US, United States. Note: Adjusted cost differences were estimated using an ordinary least squares regression model adjusted for baseline CCI score and baseline total costs; 95% CIs were estimated using a non-parametric bootstrap procedure (N = 499).
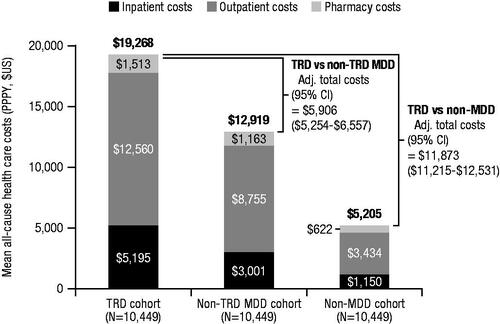
When compared to patients with non-MDD, patients with TRD also had a higher rate of all-cause HRU PPPY (), including inpatient visits (IRR: 5.04 [95% CI: 4.51–5.63]) and outpatient visits (IRR: 3.79 [95% CI: 3.70–3.89]), as well as a higher rate of mental health–related and suicide-related HRU PPPY. Compared with patients with non-MDD, patients with TRD also had higher all-cause health care costs PPPY (), including inpatient (Δ$2592), outpatient (Δ$8578), pharmacy (Δ$703), and total costs ($19,268 vs. $5205, Δ$11,873; all p<.0001), and higher mental health-related health care costs PPPY, including inpatient (Δ$3529), outpatient (Δ$4757), pharmacy (Δ$365), and total costs ($9406 vs. $489, Δ$8651; all p<.0001).
Follow-up period: treatment patterns
Treatment patterns during the follow-up period were compared among the 3 cohorts (). Patients with TRD used significantly more pharmacotherapy than patients with non-TRD MDD and patients with non-MDD. Patients with TRD, who, by definition, cycled through ≥2 ADs in the baseline period, continued to use multiple ADs during the follow-up period. Compared to patients with non-TRD MDD who used an additional 1 or 2 ADs during the follow-up period, patients with TRD used significantly more ADs at adequate dose and duration (3 or 4 ADs). The 10 most prevalent ADs used by patients with TRD during the follow-up period are shown in Supplemental Figure 2. Small proportions of patients with non-MDD were also prescribed ADs (), possibly due to off-label use or non-MDD related conditions (e.g. sleep disorders, pain)Citation27. With regards to non-pharmacologic therapy use, TMS use was significantly greater among patients with TRD compared with both patients with non-TRD MDD and with non-MDD ().
Table 2. Unadjusted treatment patterns during the follow-up period for pharmacotherapy and non-pharmacologic therapy by TRD, non-TRD MDD, and non-MDD cohorts.
Follow-up period: disease outcomes
Remission, relapse, and suicidal ideation or attempt were examined among patients with TRD and those with non-TRD MDD. More patients with TRD experienced partial remission compared with patients with non-TRD MDD (11.9 vs. 8.2%; p<.0001), and fewer patients with TRD experienced full remission (5.7 vs. 6.5%; p=.0108). Time to both partial (335 vs. 304 days; p=.0117) and full remission (416 vs. 358 days; p=.0005) was longer for patients with TRD versus non-TRD MDD, respectively. Furthermore, patients with TRD experienced more relapses than those with non-TRD MDD (0.20 vs. 0.08; p<.0001), although the time to relapse was not significantly different.
Discussion
To date, there has been limited evidence on the economic burden of TRD among US veterans, despite this population having a higher disposition towards depression relative to the civilian populationCitation19. This retrospective VHA claims analysis demonstrated that patients with TRD had a higher incremental health care cost burden than patients with non-TRD MDD and non-MDD. Among the 30% of patients who were classified as having severe MDD, 50% were considered to have TRD. Patients with TRD had significantly higher rates of mental health – and non-mental health–related comorbidities compared to either the non-TRD MDD or non-MDD cohorts, including substance abuse, severe anxiety, PTSD, suicidal ideation or attempt, insomnia, and chronic pain. These comorbidities are often related to physical and psychological stressors experienced by veterans, such as exposure to combat and a history of trauma, that pose additional risk for depression and mental illnessCitation11–16. Additionally, the higher prevalence of comorbidities among those with TRD indicates that veterans with these conditions should be more closely monitored as they are likely at an increased risk for developing TRD.
Increases in all-cause, mental health–related, MDD-specific, and suicide-related HRU and costs were also observed among patients with TRD compared to the control cohorts. This may be due to patients with TRD being more likely to have a higher comorbidity burden, which can not only complicate MDD treatment, but also lead to an increase in health care system interaction. Similar differences in HRU and costs between matched cohorts of patients with TRD, non-TRD MDD, and non-MDD have been reported in previous studies using commercial and Medicaid health care claims databasesCitation21,Citation23. Patients with TRD incurred larger pharmacy costs, which was likely attributable to requiring multiple steps of AD therapy and other drug costs related to non-depression comorbidities. Lower remission rates and longer times to remission among this patient population with TRD have been reported in the STAR*D study to be associated with severe depression, comorbid general medical and psychiatric conditions, male gender, poverty, and a history of traumaCitation28, which are common characteristics found among the US veteran population studied in this analysisCitation29.
Of the 93,160 patients with MDD included in the current analysis, 10,469 (11%) met the criteria for TRD. This is consistent with the range of prevalence (9–16%) observed in previous analyses of MedicareCitation22,Citation24, integrated delivery networksCitation25, and commercial health care databasesCitation21,Citation26. Likewise, in the STAR*D clinical study, 11% of enrolled participants with MDD entered a third antidepressant therapy step following failure or intolerability of two previous steps (similar to the definition of TRD used in the current analysis)Citation7. It would be beyond the scope of the current analysis to make any comparisons regarding the burden of TRD in the veteran population with those in the general population taken from other databases; such a comparison would require matching of patients from the VHA database with non-veterans from a commercial database.
Obtaining satisfactory treatment for depression and mental health disorders can be challenging for veteransCitation30. Previous studies have shown that less than one-half of service members and veterans with serious mental health conditions receive necessary care, and more than half of those who begin mental health treatment for depression or PTSD withdraw from treatment before being adequately treatedCitation30. Contributing barriers include stigma associated with seeking help for mental health conditions (particularly among men), avoidance of PTSD and depression symptoms, belief in self-sufficiency, lack of trust in government agencies, and negative perceptions that mental health professionals will be able to helpCitation30. In the VAST-D study, 24% of screened participants were not eligible for randomization because their medication dose had not reached predetermined minimum levelsCitation20. Many patients had been on the same treatment without augmentation or combination treatments for months to years, representing distinctly suboptimal treatment for such a debilitating conditionCitation20. Thus, increased economic costs of mental illness among veterans with TRD, as shown in this study, exist concurrently with substantial under treatment among this population. To improve detection and treatment of TRD in veterans it is necessary to address the specific barriers to mental health care in this population and offer support to overcome them. Primary care clinicians should collaborate with mental health and social service providers in order to supply patient-centered and integrated approaches to treatmentCitation15. It has also been recommended that the number of appropriately trained mental health providers within the VA should be increased and that new models of care should continue to be developed and tested as new interventions become availableCitation31.
This study should be interpreted within the context of certain limitations. First, data were obtained from an administrative claims database; therefore, the findings are subject to potential miscoding, and some diagnoses may have been entered for administrative processing rather than for clinical completeness (e.g. reluctance on the part of physicians to use remission as a diagnostic code to avoid complications with third-party payers). Second, the treatment failure algorithm used to identify patients with TRD may not be representative of all patients with TRD as only patients with an adequate dose and duration of AD were selected. It is unknown whether a switch of an AD was due to lack of tolerability rather than lack of efficacy. Furthermore, it is possible that a patient identified as having non-TRD within the period of the current study could experience failure of their current therapy at a later date. However, as specified in the Patient population section of the Methods, inclusion in the non-TRD cohort required that patients not meet the TRD criteria within 3.5 years of the index date (i.e. prescription of their first antidepressant). Based on these criteria, for the period of the retrospective follow up, the TRD and non-TRD groups were considered to be sufficiently distinct in terms of their response to antidepressant treatments. Third, although 1:1 matching on demographic variables was conducted to minimize confounding between the TRD, non-TRD MDD, and non-MDD cohorts, there may be residual confounding due to unmeasured confounders. Fourth, if patients sought treatment outside the VA system, either before or during the time period assessed in the current analysis, those data were not captured. Although recent changes in federal law now allow for greater choice of community providers for veteransCitation32, the previous Veterans Choice program also allowed VA participants to seek care outside the VA systemCitation33. Reimbursed claims for care outside the VA system may or may not have been captured in this study. In addition, TRD and clinical outcomes were defined using pharmacy claims and excluded other clinical considerations that may influence treatment response, remission, or failure, such as persistence of symptoms despite continuation of therapy appearing to be treatment “success” or lack of adherence contributing to treatment “failure”. Lastly, the study follow-up period was limited to 6 months and generalizability of the findings to the broader US population is not applicable; however, this study design was purposely chosen to evaluate HRU and economic burden of TRD in the VHA population. Ultimately, our findings are observational, and future controlled studies are needed to evaluate treatment strategies for TRD and their impact on health care costs in the veteran population.
Conclusion
In conclusion, US veterans with TRD pose a significant incremental economic burden for the VHA, relative to veterans with non-TRD MDD and non-MDD. Future analyses of effect size to measure the magnitude of the difference between the burden of TRD and non-TRD diagnoses may prove valuable.
Transparency
Declaration of funding
This research was supported by Janssen Scientific Affairs, LLC (Titusville, NJ, USA). The sponsor had no role in the design, analysis, interpretation, or publication of this study.
Declaration of financial/other relationships
H. Szukis, K. Joshi, T.B. Amos, and C.J. Benson are employees of Janssen Scientific Affairs, LLC, and stockholders in Johnson & Johnson. A. Huang and L. Wang are employees of STATinMED Research and are contracted to provide services for Janssen Scientific Affairs, LLC. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
CJB was involved with the conception of the study; HS, AH, TBA, LW, and CJB were involved in the design of the study; AH and LW were involved in the analysis of the data; and HS, KJ, TBA, and CJB were involved in the interpretation of the data. All authors contributed to drafting and critical review of the manuscript, approved the final version, and agree to be accountable for all aspects of the work.
JCH-HEC-69741_VA_burden_of_TRD_Suppl.docx
Download MS Word (147.8 KB)Acknowledgements
Writing and editorial support was provided by Courtney St. Amour, PhD (MedErgy, Yardley, PA) and was funded by Janssen Scientific Affairs, LLC. Content from this manuscript was previously presented, in part, in a poster at the 31st Annual US Psychiatric & Mental Health Congress (Orlando, Florida; October 25-28, 2018).
Data availability statement
The US VHA Medical SAS Datasets were made available to the author by the US Veterans Health Administration. More information about accessing the US VHA Medical SAS Datasets can be found at: https://www.va.gov/health/access-audit.asp.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013.
- National Institute of Mental Health. Major depression [Internet]. Bethesda (MD): National Institute of Mental Health; 2019.
- National Institute of Mental Health. Major depression among adults [Internet]. Bethesda (MD): National Institute of Mental Health; 2017.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162.
- Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–1475.
- Gaynes BN, Asher G, Gartlehner G, et al. Definition of treatment-resistant depression in the Medicare population. Technology assessment program. Project ID: PSYT0816. (Prepared by RTI–UNC Evidence-Based Practice Center under Contract No. HSA290201500011I_HHSA29032006T). Rockville (MD): Agency for Healthcare Research and Quality; 2018 February 9.
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. AJP. 2006;163(11):1905–1917.
- Kubitz N, Mehra M, Potluri RC, et al. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial claims database. PLOS One. 2013;8(10):e76882.
- Corey-Lisle PK, Birnbaum HG, Greenberg PE, et al. Identification of a claims data “signature” and economic consequences for treatment-resistant depression. J Clin. Psychiatry. 2002;63(8):717–726.
- Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26(10):2475–2484.
- Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care.N Engl J Med. 2004;351(1):13–22.
- Blore JD, Sim MR, Forbes AB, et al. Depression in Gulf War veterans: a systematic review and meta-analysis. Psychol Med. 2015;45(8):1565–1580.
- Bonde JP, Utzon-Frank N, Bertelsen M, et al. Risk of depressive disorder following disasters and military deployment: systematic review with meta-analysis. Br J Psychiatry. 2016;208(4):330–336.
- Seal KH, Metzler TJ, Gima KS, et al. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. Am J Public Health. 2009;99(9):1651–1658.
- Spelman JF, Hunt SC, Seal KH, et al. Post deployment care for returning combat veterans. J Gen Intern Med. 2012;27(9):1200–1209.
- Pietrzak RH, Goldstein MB, Malley JC, et al. Risk and protective factors associated with suicidal ideation in veterans of operations enduring freedom and Iraqi Freedom. J Affect Disord. 2010;123(1–3):102–107.
- Li G, Fife D, Wang G, et al. All-cause mortality in patients with treatment-resistant depression: a cohort study in the US population. Ann Gen Psychiatry. 2019;18(1):23.
- Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105(12):2564–2569.
- Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry. 2014;71(5):504–513.
- Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require “next-step” treatments: a VAST-D report. J Affect Disord. 2016;206:232–240.
- Amos TB, Tandon N, Lefebvre P, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a U.S. commercial claims database. J Clin Psychiatry. 2018;79(2):11725.
- Benson C, Szukis H, Sheehan JJ, et al. An evaluation of the clinical and economic burden among older adult medicare-covered beneficiaries with treatment-resistant depression. Am J Geriatr Psychiatry. 2020;28(3):350–362.
- Pilon D, Sheehan JJ, Szukis H, et al. Medicaid spending burden among beneficiaries with treatment-resistant depression. J Comp Eff Res. 2019;8(6):381–392.
- Pilon D, Joshi K, Sheehan JJ, et al. Burden of treatment-resistant depression in Medicare: a retrospective claims database analysis. PLOS One. 2019;14(10):e0223255.
- Pilon D, Szukis H, Joshi K, et al. US integrated delivery networks perspective on economic burden of patients with treatment-resistant depression: a retrospective matched-cohort study. Pharmacoecon Open. 2020;4(1):119–131.
- Zhdanava M, Kuvadia H, Joshi K, et al. Economic burden of treatment-resistant depression in privately insured U.S. patients with physical conditions. J Manag Care Spec Pharm. 2020;26(8):996–1007.
- Mercier A, Auger-Aubin I, Lebeau JP, et al. Evidence of prescription of antidepressants for non-psychiatric conditions in primary care: an analysis of guidelines and systematic reviews. BMC Fam Pract. 2013;14:55.
- Trivedi MH, Rush AJ, Wisniewski SR, et al., STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. AJP. 2006;163(1):28–40.
- Rosenheck R. Mental health and substance abuse services for veterans: experience with mental health performance evaluation in the Department of Veterans Affairs. Improving the quality of health care for mental and substance-use conditions: quality chasm series. Washington (DC): Institute of Medicine Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders; 2004. p. 423–482.
- Hunt SC, Hoge CW. Health care for military veterans. In: Jameson JL, Fauci AS, Kasper DL, et al., editors. Harrison’s principles of internal medicine. 20th ed. New York (NY): McGraw-Hill Education; 2018.
- RAND Research. Improving the quality of mental health care for veterans: lessons from RAND Research (document number RB-10087); 2019.
- US Department of Veterans Affairs. Veterans community care program. 38 CFR part 17. RIN 2900-AQ46. Federal Register; 2019.
- US Department of Veterans Affairs. Veterans choice program (VCP) [Internet]. Washington (DC): US Department of Veterans Affairs; 2019 June 6.
