Abstract
Objectives
To evaluate geographic variation in the prevalence of autosomal dominant polycystic kidney disease (ADPKD) in the US, including ADPKD at risk of rapid progression.
Methods
Claims data from the IBM MarketScan Commercial and Medicare Supplemental databases (01/16/2016–12/31/2017) were used to estimate the 2017 annual and 2016–2017 two-year prevalence of diagnosed ADPKD and ADPKD at risk of rapid progression in the US overall, and stratified by census regions and states. Risk of rapid progression was identified based on either: hypertension <35 years, hematuria <30 years, albuminuria, stage 2 chronic kidney disease (CKD) <30 years, stage 3 CKD <50 years, and stage 4/5 CKD or kidney transplant <55 years.
Results
Annual prevalence was estimated at 2.34 and two-year prevalence at 3.61 per 10,000 in the US. Across census regions, two-year prevalence per 10,000 was highest in the Northeast (4.14) and lowest in the West (3.35). Prevalence was significantly correlated with the proportion of individuals in urban areas (r = .34, one-sided p = .026). In 2017, 37.5% of patients were identified as being at risk for rapid progression, and this proportion was larger among patients in the South (42.1%, p < .001).
Conclusion
This estimate for ADPKD prevalence is consistent with previously reported national estimates, with regional variation suggesting that ADPKD might be under-diagnosed in rural areas with more limited access to care. More than one-third of ADPKD patients presented risk factors associated with rapid progression, highlighting the need for timely identification, as disease-modifying therapy may delay progression to end-stage renal disease.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited disease characterized by the gradual growth of fluid-filled cysts in kidneys, leading to urinary concentrating defects, nephrolithiasis, hematuria, infections, and progressive loss of kidney function [Citation1,Citation2]. The gradual loss of renal function resulting from the disease often leads to end-stage renal disease (ESRD), which is typically diagnosed between age 55 and 75 [Citation3]. Overall, ADPKD is the 4th leading cause of ESRD in the United States (US) behind diabetes, hypertension, and glomerulonephritis [Citation4].
Patients with ADPKD progress to ESRD at varying rates. Previous studies found several genetic (i.e. PDK1 versus PDK2 mutations), clinical (i.e. gross hematuria, albuminuria, hypertension, total kidney volume), and demographic (i.e. age, gender) risk factors associated with rapid progression to ESRD [Citation2,Citation5–9], which can be used to identify a subset of patients at risk of progressing or experiencing complications more rapidly [Citation10,Citation11]. However, the prevalence of ADPKD at risk of rapid progression remains poorly documented at the national and regional level in the US.
Comprehensive epidemiologic data are instrumental in understanding the lifetime clinical course of ADPKD and for planning interventions to delay or prevent ESRD. While the prevalence of ADPKD has been evaluated in several European studies [Citation12–15], epidemiologic studies in the US are scarce [Citation16]. In particular, little is known about regional differences in the prevalence of diagnosed ADPKD in the US. Many chronic conditions show regional variation in diagnosed prevalence based on a number of factors, including population characteristics, environmental differences in rural versus urban areas, and availability of healthcare services [Citation17–19]. We hypothesize that the prevalence of ADPKD would also show regional variation. An in-depth assessment of the prevalence of ADPKD at the regional level in the US is warranted to assess this hypothesis.
This retrospective claims-based analysis was conducted to evaluate geographic variation in the prevalence of diagnosed ADPKD, including ADPKD at risk of rapid progression, in the US.
Methods
Data source
This analysis was conducted using data from the IBM MarketScan Commercial and Medicare Supplemental databases (MarketScan database) from 01/01/2016 to 12/31/2017. The MarketScan database is a large, private-sector health data resource that reflects the healthcare experience of employees and their dependents, as well as Medicare-eligible retirees with employer-provided Medicare Supplemental plans. All US census regions are represented, with a larger proportion of individuals from the South compared to the US population. The database includes records of patient demographic variables (e.g. age, gender, region of residence), medical service use, prescription drug claims, and monthly eligibility of covered individuals. Data are de-identified and comply with the requirements of the Health Insurance Portability and Accountability Act (HIPAA); therefore, no institutional review board approval was required for this analysis.
Study design and sample selection
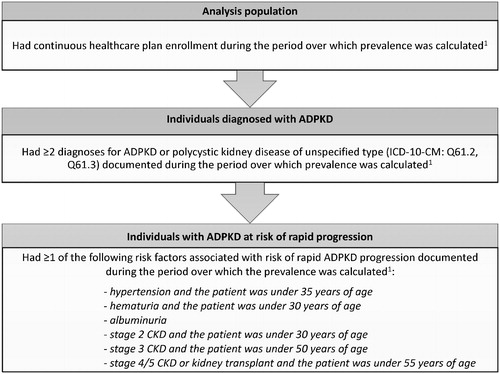
An annual prevalence of ADPKD and ADPKD at risk of rapid progression was estimated for the most recent year of data availability (i.e. 2017). A two-year prevalence of ADPKD and ADPKD at risk of rapid progression was also estimated for the two most recent years of data availability (i.e. 2016–2017). The total analysis population comprised all individuals who were continuously enrolled in their healthcare plan during the period over which prevalence was calculated (i.e. from 01/01/2017 to 12/31/2017 for the annual prevalence, and from 01/01/2016 to 12/31/2017 for the two-year prevalence; ). To decrease the likelihood of including individuals for whom an ADPKD diagnosis may have been incorrectly recorded or used as a rule-out diagnosis, individuals were required to have ≥2 diagnoses to be considered “diagnosed with ADPKD” during the period over which the prevalence was calculated. Individuals diagnosed with ADPKD were further categorized as being at risk of rapid progression based on risk factors available in claims data. Specifically, individuals were considered at risk of rapid progression if they had ≥1 of the following risk factors during the period over which the prevalence was calculated: (1) hypertension and the patient was under 35 years of age; (2) hematuria and the patient was under 30 years of age; (3) albuminuria; (4) stage 2 chronic kidney disease (CKD) and the patient was under 30 years of age; (5) stage 3 CKD and the patient was under 50 years of age; or (6) stage 4/5 CKD or kidney transplant and the patient was under 55 years of age [Citation10,Citation20].
Figure 1. Sample selection criteria. Abbreviations. ADPKD, autosomal dominant polycystic kidney disease; CKD, chronic kidney disease; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification. Note: 1. The period over which prevalence was calculated spanned from 01/01/2017 to 12/31/2017 for the annual prevalence, and from 01/01/2016 to 12/31/2017 for the two-year prevalence.
Outcomes and statistical analysis
Demographic and clinical characteristics of individuals with ADPKD in 2017 were summarized and stratified by census region. Demographic characteristics were measured on 12/31/2017 and included age, gender, insurance plan type, census region, and state of residence. Clinical characteristics were measured from 01/01/2017 to 12/31/2017 and included the most severe CKD stage recorded during that period, dialysis, kidney transplant or evidence of prior kidney transplant, comorbidities, and risk of rapid progression (as defined earlier) [Citation10,Citation20]. Specialist visits (i.e. nephrologist, urologist) and renal imaging procedures (i.e. computed tomography scan, ultrasound, magnetic resonance imaging) were also measured from 01/01/2017 to 12/31/2017. Descriptive statistics are presented, including means, standard deviations, and medians for continuous variables and frequency counts and percentages for categorical variables. Statistical comparisons of demographic characteristics and comorbidities across regions were conducted with Kruskal-Wallis tests for continuous variables and Chi-square tests for categorical variables.
Prevalence estimates were calculated as the number of individuals with diagnosed ADPKD (or the subgroup at risk of rapid progression) divided by the number of individuals with continuous healthcare plan enrollment during the period over which prevalence was calculated. Annual and two-year prevalence of diagnosed ADPKD were estimated overall and stratified by US census region and state. In addition, annual and two-year prevalence of ADPKD at risk of rapid progression were estimated overall and stratified by US census region. Binomial proportion confidence intervals (CIs) were constructed for all prevalence estimates at a 95% CI using the Wilson score interval [Citation21]. All analyses were conducted using SAS Enterprise Guide, Version 7.1 (SAS, Cary, North Carolina, USA). As an exploratory analysis, a one-sided spearman correlation test was used to assess the relationship between the proportions of individuals residing in urban areas in each state versus the two-year prevalence of ADPKD. The proportion of individuals residing in urban areas was determined based on US Census Bureau [Citation22], and included individuals residing in urbanized areas (50,000 or more people) and urban clusters (between 2500 and 49,999 people).
Results
Characteristics of individuals with diagnosed ADPKD in 2017
Among 19,377,241 individuals continuously enrolled in the database in 2017, 4536 had diagnosed ADPKD. Among individuals with diagnosed ADPKD, mean age ranged from 47.2 years in the South to 49.6 years in the Northeast (p = .0013), and the proportion of females ranged from 49.2% in the Northeast to 57.1% in the South (p = .0010; ).
Table 1. Characteristics of individuals diagnosed with ADPKD.
The most common comorbidities associated with ADPKD were hypertension, hyperlipidemia, and abdominal, flank, or low back pain, and comorbidity rates varied across census regions (all p < .025; ). The proportion of individuals with hypertension was the highest in the Northeast (80.1%) and South (81.7%), and the lowest in the West (73.6%). The proportion of individuals with hyperlipidemia was also the lowest in the West (37.8%; other regions: 42.6%–45.5%), while the proportion of individuals with abdominal, flank, or low back pain was the lowest in the Northeast (30.8%; other regions: 36.0%–36.9%). The proportion of individuals with a recorded diagnosis of obesity ranged from 10.6% in the West to 15.0% in the South.
Stage 3 was the most prevalent CKD stage (ranging from 18.5% in the West to 22.3% in the Northeast), followed by CKD stage 5 (ranging from 16.2% in the West to 19.0% in the South). The proportions of individuals with CKD stages 1, 2, and 4 ranged between 5.2% and 8.4% in all four census regions. Yet, 39.8% to 44.6% of individuals with ADPKD had an unknown CKD stage across census regions (all p > .05; ). The proportion of individuals with diagnosed ADPKD undergoing dialysis ranged from 7.1% in the West to 11.0% in the South (p = .0106), and the proportion of individuals with a kidney transplant or evidence of prior kidney transplant ranged from 13.5% in the West to 16.7% in both the Northeast and Midwest (p = .2815; ).
The overall proportion of individuals at risk of rapid progression was 37.5% in the study sample; this figure ranged from 31.6% in the West to 42.1% in the South (p < .0001; ). The proportion of individuals with stage 4/5 CKD or kidney transplant by age 55 years was the highest in the South (18.2%; other regions: 13.1%–13.5%; p = .0001). The proportion of individuals who were seen by a nephrologist varied widely across census regions (56.8% in the Midwest to 72.9% in the South, p < .0001; ).
Annual and two-year prevalence of diagnosed ADPKD and ADPKD at risk of rapid progression, stratified by census region
The estimated annual prevalence of diagnosed ADPKD was 2.34 per 10,000 individuals in 2017. Annual prevalence estimates ranged from 2.19 per 10,000 in the West to 2.72 per 10,000 in the Northeast (). The annual prevalence of ADPKD at risk of rapid progression was 0.88 per 10,000, with estimates ranging between 0.69 per 10,000 in the West and 0.94 per 10,000 in both the Northeast and South ().
Figure 2. Annual prevalence and two-year prevalence of ADPKD and ADPKD at risk of rapid progression by census region. Abbreviations. ADPKD, autosomal dominant polycystic kidney disease.
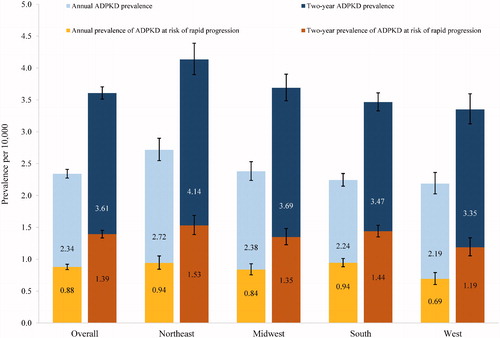
Out of 14,892,914 individuals continuously enrolled in the database in 2016–2017, 5373 were diagnosed with ADPKD, leading to an estimated two-year prevalence of 3.61 per 10,000. Two-year prevalence estimates ranged from 3.35 per 10,000 in the West to 4.14 per 10,000 in the Northeast (). Among individuals diagnosed with ADPKD, 2074 (38.6%) were identified as being at risk of rapid progression in 2016–2017, leading to an estimated two-year prevalence of 1.39 per 10,000. Two-year prevalence estimates of ADPKD at risk of rapid progression ranged from 1.19 per 10,000 in the West to 1.53 per 10,000 in the Northeast ().
Annual and two-year prevalence of diagnosed ADPKD, stratified by state
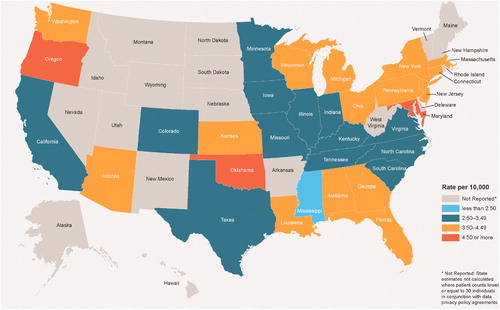
The annual prevalence of ADPKD varied by state, with estimates ranging from 1.57 per 10,000 in Kentucky to 3.30 per 10,000 in Maryland (data not shown). Similarly, the two-year prevalence of ADPKD ranged between 2.11 per 10,000 in Mississippi and 4.79 per 10,000 in Maryland (). The variability observed between states may have been driven by the small sample sizes in certain states.
Discussion
In this large, retrospective claims-based analysis of commercially insured (including Medicare-supplemented) individuals in the US, the annual estimated prevalence of ADPKD was 2.34 per 10,000, and two-year prevalence was 3.61 per 10,000. Prevalence estimates varied across states and regions, with the lowest figures observed in the West census region and the highest observed in the Northeast census region. The annual and two-year prevalence estimates of ADPKD at risk of rapid progression were 0.88 and 1.39 per 10,000, respectively. These figures also varied across regions, with the lowest estimates observed in the West and the highest estimates observed in the Northeast and South. Among patients with ADPKD, the proportion of individuals at risk of rapid progression was highest in the South.
Regional and state-level variation in the prevalence of ADPKD suggests the existence of geographic factors influencing its diagnosis or development. In an exploratory analysis, a positive and significant correlation was observed between the two-year state-specific prevalence of diagnosed ADPKD and the proportion of individuals living in urban areas (r = .34, one-sided p = .026; Supplemental Figure 1), suggesting that prevalence is higher in urban than rural areas. Of note, this observation may not be specific to ADPKD given that a similar correlation was found between the two-year state-specific CKD prevalence and the proportion of individuals living in urban areas (r = .25, one-sided p = .037). This correlation may be explained by better access to healthcare services in urban areas than in rural areas – and in particular, access to specialists with adequate training to diagnose ADPKD, which could lead to higher diagnosis rates. Indeed, a report published by the Rural Health Reform Policy Research Center (RHRPRC) on national population demographics and healthcare coverage in the US showed that urban areas had a larger supply of physicians, especially specialists, who typically require a larger population to build a sustainable practice [Citation23]. In this report, the proportion of residents living in large metropolitan urban areas was highest in the Northeast, which was also the region with the highest number of physicians per 100,000 inhabitants in 2010 [Citation23]. Conversely, the size of the urban population was lowest in the West, which was also the region with the lowest number of physicians per 100,000 inhabitants [Citation23]. In the current study, the prevalence of ADPKD was highest in the Northeast and lowest in the South, which is consistent with the aforementioned regional disparities in healthcare access. Interestingly, a separate study conducted in Japan found that higher population density was correlated with higher prescribing rates of tolvaptan, a newly approved treatment for ADPKD, further suggesting disparities in urban and rural settings in ADPKD [Citation24]. However, the generalizability of these findings to the US population is unknown.
Estimating the prevalence of ADPKD is particularly challenging, since early-stage disease can remain asymptomatic and undiagnosed for years. The natural progression of ADPKD varies across patients, and kidney function may not decline until the third or fourth decades of life [Citation2,Citation11]. Studies of other progressive conditions found that limited accessibility to physicians and specialists is associated with a delay in diagnosis in rural areas [Citation25,Citation26]. Thus, delayed diagnosis of ADPKD, especially in rural areas with a lack of specialists who may recognize the early signs of ADPKD, may have contributed to the observed regional variation in prevalence. Indeed, the relatively low proportion of ADPKD patients with CKD stages 1 and 2 also suggests that delayed diagnosis may be an important contributing factor, with some individuals receiving a diagnosis at later CKD stages.
Our finding that the proportion of ADPKD patients with risk factors for rapid progression (such as hypertension and early-onset CKD) varies regionally is consistent with regional patterns of chronic disease in the US. The 2016 Global Burden of Disease study found that CKD burden (based on death rates and disability-adjusted life-years) was more than twice as high in southern US states than in other areas [Citation27]. In addition, self-reported prevalence of hypertension and use of antihypertensive medication is highest in southern states and lowest in the West [Citation28]. Furthermore, risk factors for hypertension, such as obesity, were higher in the southern US in 2018, according to the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System [Citation29]. Similarly, in our sample of ADPKD patients, obesity, which conveys additional risk for hypertension [Citation30], was more common in ADPKD patients in the South. This pattern of regional variation in risk factors that affect ADPKD progression suggests that environmental context may be a disease modifier for some ADPKD patients, making disease management even more complex and challenging. These challenges underscore the need for early diagnosis, assessment, and treatment, as well as future longitudinal research on regional differences in ADPKD progression.
Our ADPKD prevalence estimates are consistent with those reported in a US study conducted by Willey et al. [Citation16]. Similar to the present study, Willey et al. used data from the IBM MarketScan database and estimated the average annual prevalence of diagnosed ADPKD at 2.06 per 10,000 among commercially insured (including Medicare-supplemented) individuals for years 2013 to 2015. This figure is consistent with the present annual prevalence estimate of 2.34 per 10,000 in 2017. In addition to using administrative health insurance claims, Willey et al. also used data from the National Ambulatory Medical Care Survey (NAMCS) to estimate the prevalence of ADPKD, independent of insurance coverage. The NAMCS prevalence estimate was 4.30 per 10,000, similar to our two-year prevalence estimate of 3.61 per 10,000.
Prior European studies are also consistent with our results. In a meta-analysis of eight European population-based studies, Solazzo et al. estimated an average ADPKD prevalence of 2.7 per 10,000 [Citation14]. Another study by Willey et al. used renal registry data and estimated the minimum prevalence of ADPKD in the European Union at 3.3 per 10,000 individuals, which could reach up to 4.0 per 10,000 with enhanced screening and surveillance [Citation15]. Variations in prevalence estimates across studies can be explained by differences in study design, including the use of registry-based data and varying time periods to calculate prevalence, as well as inherent geographical differences in populations and healthcare systems between countries.
The current analysis provides an example of how the use of varying time periods to calculate prevalence may lead to different estimates. Given that some individuals with ADPKD may not have ≥2 ADPKD diagnoses recorded in a given year, the annual ADPKD prevalence estimated in the current analysis provides a lower bound estimate of prevalence. Allowing for a longer, two-year period to observe ≥2 diagnoses for ADPKD resulted in the identification of more individuals with ADPKD (n = 5373 in 2016–2017 vs n = 4536 in 2017). By providing both annual and two-year prevalence estimates, the current analysis contributes important and comprehensive insights on the prevalence of diagnosed ADPKD in the US amidst the sparse epidemiologic literature. Further studies are warranted to confirm these findings and the observed regional variability.
Finally, findings from this analysis show that more than one-third of individuals with ADPKD present risk factors associated with a higher risk of rapid progression to ESRD. The recent Food and Drug Administration (FDA) approval of disease-modifying therapy for ADPKD patients at risk of rapid progression will help support the complex management of these high-risk patients [Citation31]. Timely identification of these patients is of utmost importance as initiating treatment has been shown to slow the rate of decline in kidney function [Citation32], which may help to reduce the substantial burden associated with ADPKD [Citation33].
Limitations
Despite including a large sample size, which led to precise prevalence estimates, this analysis was subject to some limitations. First, the analysis population was limited to commercially insured and Medicare-supplemented individuals and their dependents; therefore, findings may not be generalizable to the overall US population. Second, the database did not include details regarding the patients’ race, which may have provided additional insight since health disparities are often observed across races, including certain risk factors for CKD, like hypertension [Citation28]. The high prevalence of ADPKD and risk factors for rapid progression in the South may thus be partly driven by racial and socioeconomic determinants of health given the high proportion of minorities in this region [Citation34]. In particular, non-Hispanic black individuals with ADPKD were found to progress to ESRD more rapidly than non-Hispanic whites [Citation35], and a similar phenomenon is well documented for CKD in general [Citation36]. Third, the definition of ADPKD at risk of rapid progression has not been established by regulatory agencies, and current definitions vary by country [Citation37,Citation38]. As such, the factors associated with risk of rapid ADPKD progression in the current analysis remain to be clinically validated, but have been used to define this subpopulation in previous studies [Citation10,Citation20]. Fourth, the prevalence of diagnosed ADPKD, as reported in this study, is likely an underestimation of the true prevalence of ADPKD since subjects can remain asymptomatic and undiagnosed for years. While genetic screening may provide more reliable estimates, it is not widely used due to its high cost [Citation2] and the rarity of ADPKD [Citation15,Citation16], and the corresponding results would not be accessible in the database. Moreover, given that the prevalence was estimated based on recorded ADPKD diagnoses within health insurance claims, even individuals with diagnosed ADPKD may not have been identified if ADPKD was not systematically recorded, or if certain individuals did not require medical services in the period over which the prevalence was calculated.
Conclusion
Annual and two-year prevalence estimates of ADPKD were consistent with previously reported national estimates, with regional variation suggesting possible under-diagnosis in rural areas with more limited access to care. More than one-third of individuals with ADPKD presented risk factors associated with rapid progression, highlighting the need for timely identification of these patients, as disease-modifying therapy may delay progression to ESRD. The proportion of ADPKD patients with risk factors for rapid progression was greater in the South, which suggests additional challenges for clinical management in areas where underlying risk for hypertension and other factors associated with ADPKD progression is higher. These prevalence estimates provide new insights into the descriptive epidemiology of ADPKD and a national and regional public health perspective on needs for enhanced ADPKD diagnosis, treatment, and awareness.
Transparency
Declaration of funding
Financial support for this research was provided by Otsuka Pharmaceutical Development & Commercialization, Inc. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Declaration of financial/other relationships
CW has served as an epidemiologic consultant to Otsuka Pharmaceutical Development & Commercialization, Inc. MGL, MC, SS, and JM are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Otsuka Pharmaceutical Development & Commercialization, Inc., which funded the development and conduct of this study and manuscript. RS is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. MSA was an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. at the time the study was conducted. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MGL, MC, SS, and JM contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. CW, RS, and MSA contributed to study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Statement of ethics
Data used in this study were de-identified and complied with the requirements of the HIPAA; therefore, no institutional review board approval was required for this analysis.
Supplemental Material
Download MS Word (19 KB)Acknowledgements
Medical writing support was provided by professional medical writers, Samuel Rochette and Christine Tam, who are employees of Analysis Group, Inc.
References
- Lanktree MB, Chapman AB. Autosomal dominant polycystic kidney disease. CMAJ. 2017;189(45):E1396.
- Ong AC, Devuyst O, Knebelmann B, et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet (London, England). 2015;385(9981):1993–2002.
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364(16):1533–1543.
- United States Renal Data System. Chapter 1: Incidence, Prevalence, Patient Characteristics, and Treatment Modalities 2018 [cited 2020 April 6]. Available from: https://www.usrds.org/2018/download/2018_Volume_2_ESRD_in_the_US.pdf.
- Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354(20):2122–2130.
- Cornec-Le Gall E, Audrezet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006–1013.
- Schrier RW, Brosnahan G, Cadnapaphornchai MA, et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014;25(11):2399–2418.
- Cornec-Le Gall E, Audrezet MP, Rousseau A, et al. The PROPKD Score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(3):942–951.
- Kistler AD, Poster D, Krauer F, et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009;75(2):235–241.
- Blanchette CM, Liang C, Lubeck DP, et al. Progression of autosomal dominant kidney disease: measurement of the stage transitions of chronic kidney disease. Drugs Context. 2015;4:212275.
- McEwan P, Bennett Wilton H, Ong ACM, et al. A model to predict disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD): the ADPKD Outcomes Model. BMC Nephrol. 2018;19(1):37.
- McGovern AP, Jones S, van Vlymen J, et al. Identification of people with autosomal dominant polycystic kidney disease using routine data: a cross sectional study. BMC Nephrol. 2014;15:182.
- Neumann HP, Jilg C, Bacher J, Else-Kroener-Fresenius-ADPKD-Registry, et al. Epidemiology of autosomal-dominant polycystic kidney disease: an in-depth clinical study for south-western Germany. Nephrol Dial Transplant. 2013;28(6):1472–1487.
- Solazzo A, Testa F, Giovanella S, et al. The prevalence of autosomal dominant polycystic kidney disease (ADPKD): a meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLoS One. 2018;13(1):e0190430.
- Willey CJ, Blais JD, Hall AK, et al. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. 2017;32(8):1356–1363.
- Willey CJ, Kamat S, Stellhorn R, et al. Analysis of nationwide data to determine the incidence and diagnosed prevalence of autosomal dominant polycystic kidney disease in the USA: 2013–2015. Kidney Dis (Basel). 2019;5(2):107–117.
- Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease – United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(7):205–211.
- Mantri S, Fullard ME, Beck J, et al. State-level prevalence, health service use, and spending vary widely among Medicare beneficiaries with Parkinson disease. NPJ Parkinsons Dis. 2019;5:1.
- Ward BW, Black LI. State and regional prevalence of diagnosed multiple chronic conditions among adults aged ≥18 years – United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(29):735–738.
- Knight T, Schaefer C, Krasa H, et al. Medical resource utilization and costs associated with autosomal dominant polycystic kidney disease in the USA: a retrospective matched cohort analysis of private insurer data. Clinicecon Outcome Res. 2015;7:123–132.
- Brown L, Cai T, DasGupta A. Interval estimation for a binomial proportion. Statist Sci. 2001;16(2):101–117.
- United States Census Bureau. 2010. Census 2010. [Accessed 2020 January 17]. Available from: https://www.census.gov/.
- Meit M, Knudson A, Gilbert T, et al. The 2014 update of the rural-urban chartbook. Rural Health Reform Policy Research Center. 2014;1–153.
- Inoue R, Nishi H, Inoue D, et al. Regional variance of the early use of tolvaptan for autosomal dominant polycystic kidney disease. Kidney360. 2020;1(8):740–745.
- Blankart CR. Does healthcare infrastructure have an impact on delay in diagnosis and survival? Health Policy. 2012;105(2–3):128–137.
- Zahnd WE, Fogleman AJ, Jenkins WD. Rural-urban disparities in stage of diagnosis among cancers with preventive opportunities. Am J Prev Med. 2018;54(5):688–698.
- Bowe B, Xie Y, Li T, et al. Changes in the US burden of chronic kidney disease from 2002 to 2016: an analysis of the Global Burden of Disease Study. JAMA Netw Open. 2018;1(7):e184412.
- Samanic CM, Barbour KE, Liu Y, et al. Prevalence of self-reported hypertension and antihypertensive medication use among adults - United States, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(14):393–398.
- Centre for Disease Control and Prevention. Adult Obesity Prevalence Maps 2019. [cited 2020 July 3]. Available from: https://www.cdc.gov/obesity/data/prevalence-maps.html.
- Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006.
- Otsuka. Otsuka’s JYNARQUE™ (tolvaptan) Approved by U.S. FDA as the First Treatment to Slow Kidney Function Decline in Adults at Risk of Rapidly Progressing Autosomal Dominant Polycystic Kidney Disease (ADPKD) 2018. [cited 2019 November 11]. Available from: https://www.otsuka-us.com/discover/articles-1188.
- Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418.
- Cloutier M, Manceur AM, Guerin A, et al. The societal economic burden of autosomal dominant polycystic kidney disease in the United States. BMC Health Serv Res. 2020;20(1):126.
- United States Census Bureau. Overview of Race and Hispanic Origin: 2010. 2011. [cited 2020 October 15]. Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf.
- Murphy EL, Dai F, Blount KL, et al. Revisiting racial differences in ESRD due to ADPKD in the United States. BMC Nephrol. 2019;20(1):55.
- Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19(7):1261–1270.
- Chebib FT, Perrone RD, Chapman AB, et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018;29(10):2458–2470.
- Gansevoort RT, Arici M, Benzing T, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016;31(3):337–348.