Abstract
Objective
To examine treatment patterns of real-world antifungal management of patients at high risk of invasive fungal infections (IFI) and evaluate healthcare resource utilization and costs associated with antifungal management of IFIs in Japan.
Methods
This retrospective, observational study extracted data from a hospital-based claims database for patients in Japan who either (a) underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT), or (b) were hospitalized with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) and received chemotherapy during the study period of January 2010 to January 2019.
Results
863 patients were included in the allo-HSCT cohort and 4498 patients were included in the AML/MDS cohort. In the allo-HSCT cohort, 91% received more than one antifungal drug during the index hospitalization. In the AML/MDS cohort, approximately 50% received more than one antifungal drug during the index hospitalization. For both the allo-HSCT and AML/MDS cohorts, about 90% of the total cost was attributed to inpatient costs. Of note, both the total cost (the total inpatient and outpatient cost) and the index hospitalization costs were higher in patients treated with multiple antifungal drugs than in those treated with a single antifungal drug during the index hospitalization. Despite being at high IFI risk, 12% of the patients in the AML/MDS cohort did not receive antifungal drugs during the index hospitalization.
Conclusions
Most patients with hematologic malignancy and high IFI risk underwent complicated antifungal management requiring use of multiple drugs, and accounted for high healthcare resource utilization and costs.
Introduction
Invasive fungal infections (IFIs) are common in high-risk patients with hematologic malignancies, such as acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), who receive aggressive immunosuppressive therapy such as induction chemotherapy or allogenic hematopoietic stem cell transplantationCitation1–10. The morbidity and mortality from IFIs in these high-risk patient populations is substantialCitation1,Citation3–8,Citation10,Citation11.
Treatment of IFIs is associated with significant healthcare resource utilization (HCRU) and costs, resulting in a high economic burdenCitation4,Citation7,Citation12–18; however, studies that have evaluated the economic impact of the index hospitalization are lacking.
A 2002 retrospective study among US inpatients and outpatients estimated that the total direct cost in 1998 was US$2.6 billion, with hospitalization costs (US1.4 billion) the largest driver of total costs. The average per-patient cost attributable to systemic fungal infections was US$31,200Citation18. A 2005 retrospective study among elderly hospitalized patients with cancer in the US found that among patients hospitalized for severe fungal infections, mean total hospitalization charges were US$110,767 for patients with AML and US$76,247 for those with squamous cell carcinoma of the head and neck; mean Medicare payments were US$34,268 for patients with AML and US$25,942 for those with squamous cell carcinoma of the head and neck. Patients with fungal infections had significantly greater hospitalization costs and Medicare payments compared to matched controlsCitation17. A 2009 retrospective study among patients hospitalized with IFI in the US reported that in 2004, these patients had mean costs of US$44,726; patients with IFI had significantly higher mean costs than those without IFI, and costs were highest for transplant recipients and those with cancerCitation14. A 2012 retrospective study among hospitalized patients in the US reported that the mean total hospital cost in 2004–2005 for patients with IFI was US$71,831, and were US$32,196 greater for patients with IFI compared to those without IFICitation13. A 2014 review of the economic burden of IFI in Europe estimated that the incremental cost burden attributable to IFI is between €10,530 and €51,033, depending on the certainty of the diagnosis and duration of follow-up; greater costs were consistently reported in patients with IFI compared to those without infection. Total hospital costs ranged from €26,596 to €83,300Citation15. A 2015 prospective study among patients in the UK found that mean cost of care per patient was £88,911 for those with proven/probable IFI, with mean cost attributable to IFI of £54,836; hospital inpatient stays accounted for nearly 74% of costsCitation7.
Patients who contract IFIs incur additional costs and experience longer hospitalizations than patients who do not contract IFIsCitation4,Citation7,Citation12–15. Large-scale database analysis has been previously used to evaluate the disease burden associated with Aspergillus infectionCitation19,Citation20, but using this approach to understand real-world treatment patterns, costs, and HCRU associated with antifungal management of IFIs in high-risk patients is lacking, both in Japan and internationally.
To address this question, we performed a retrospective, descriptive study using patient data (including hospital health claims and administrative data) from a commercially available database to examine treatment patterns of antifungal drug use for high-risk patients and to evaluate the HCRU and costs associated with antifungal management of IFIs in Japan.
Methods
Data source
This retrospective, observational study extracted data from a hospital-based administrative claims database provided by Medical Data Vision Co. Ltd. (MDV; Tokyo, Japan) for the study period of January 2010 to January 2019. The MDV database includes demographic information, inpatient and outpatient claims data, and cost data collected from approximately 26 million patients from approximately 382 hospitals, representing approximately 23% of all diagnosis procedure combination (DPC) hospitals. DPC is the claims-based payment system used by most hospitals in Japan.
Study population
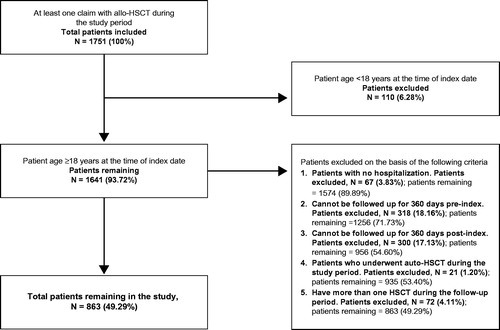
The study cohorts comprised adult patients (≥18 years) who either (a) underwent allo-HSCT, or (b) were hospitalized with a diagnosis of AML or MDS and received chemotherapy during the study period. For the allo-HSCT cohort, patients were included in the study if they underwent allo-HSCT during the study period and were aged ≥18 years on the index hospitalization date. Patients were excluded from the study if they had no record of hospitalization, could not be followed up for 360 days pre-index (the look-back period) and 360 days post-index (the follow-up period), underwent autologous transplant during the study period, or had more than one HSCT during the follow-up period.
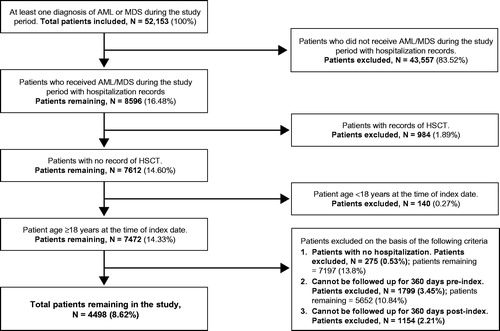
For the AML/MDS cohort, patients were included in the study if they had at least one diagnosis of AML or MDS during the study period, received chemotherapy during the study period, had no record of HSCT, and were aged ≥18 years on the index hospitalization date. Patients were excluded if they had no record of hospitalization or could not be followed up for 360 days pre-index and 360 days post-index.
The index date was defined as the date of a patient’s first hospital admission with an AML/MDS diagnosis or allo-HSCT treatment in the study period. HCRU and costs were evaluated for the index hospitalization and for 360 days afterward (the follow-up period).
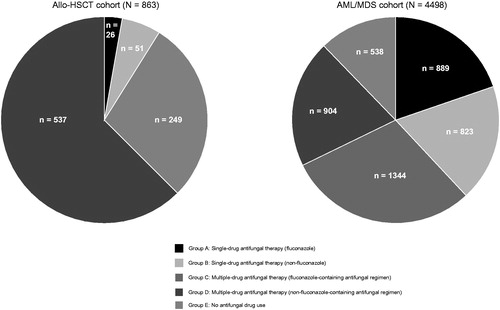
Each study cohort was then stratified into 5 treatment groups on the basis of antifungal drug utilization patterns during index hospitalization. Each cohort was first classified into use of single- or multiple-drug antifungal therapy, then further classified based on use of fluconazole or not. Other azole medications include itraconazole and voriconazole; and other antifungal drugs besides azole include micafungin, caspofungin and ambisome. Fluconazole is approved and widely used in Japan for managing patients at high IFI risk, but fluconazole has limited activity against most molds and there is little information regarding real-world clinical and economic outcomes associated with it.
Group A: Single antifungal therapy (fluconazole only).
Group B: Single antifungal therapy (non-fluconazole single antifungal only).
Group C: Multiple antifungal therapy (fluconazole-containing antifungal regimen).
Group D: Multiple antifungal therapy (non-fluconazole-containing antifungal regimen)
Group E: No antifungal drug use.
Outcomes
Demographic and clinical characteristics captured for each cohort included age at index date; gender; antifungal use during look-back period; indication for allo-HSCT; chemotherapy during index hospitalization (AML/MDS drugs of interest included cytarabine, enocitabine, daunorubicin, and idarubicin); mode of medication administration (oral or injection) during index hospitalization; stem cell source (e.g. bone marrow, peripheral blood, cord blood); hospital size (≤199, 200–499, or ≥500 total beds); year of admission; and Charlson comorbidity index (CCI) during the look-back period.
HCRU outcomes included length of the index hospitalization; length of total hospitalization; length of stay in the intensive care unit (ICU); total number and length of readmissions; number of outpatient visits; duration of antifungal treatments; duration of antibacterial treatments; imaging procedures (X-ray, computed tomography [CT] scan); and microbiologic studies ((1→3)-beta-D-glucan [BD glucan], Aspergillus antigen test [AG], culture).
Cost outcomes included total costs (the sum of all inpatient and outpatient costs); inpatient costs (including daily inpatient costs from the index date, costs during index hospitalization, and costs due to readmissions); outpatient costs (including all costs associated with outpatient care); drug costs for antifungal and antibacterial treatments; and examination costs (including those for imaging procedures and microbiologic studies). All costs were measured in Japanese yen and multiplied by a factor of 0.009 based on the exchange rate in January 2019 to estimate US dollar equivalents.
Statistical analyses
Analyses included all patients that met the inclusion/exclusion criteria. The study analyses are composed of a series of descriptive analyses. Categorical variables were described as the number and percentage of patients in each category. For continuous variables, mean, median, and standard deviation (SD) were calculated. For any statistical comparisons performed across groups, such as single antifungal therapy group A (fluconazole only) vs multiple antifungal therapy group C (fluconazole containing antifungal regimen) or single antifungal therapy group B (non-fluconazole single antifungal only) vs multiple antifungal therapy group D (non-fluconazole containing antifungal regimen), appropriate statistical methods were applied in consideration of characteristic of each endpoint (categorical variables: chi-square test; continuous variables: t-test [mean] and Wilcoxon rank sum test [median]) As this analysis was exploratory in nature, no adjustments for multiple comparisons were made for statistical comparisons performed across groups. A p value ≤.05 for between-group comparisons was considered statistically significant. A sensitivity analysis of cost/HCRU was conducted. The sensitivity analysis excluded patients who died within the follow-up period, because unequal numbers of inpatient deaths in each group would affect the final HCRU and cost outcomes.
Results
Study attrition
As shown in and , 1751 patients with at least one claim of allo-HSCT and 52,153 patients with at least one claim of AML or MDS were identified from the database. On the basis of the inclusion and exclusion criteria, 863 patients (49.3% of allo-HSCT claims) were included in the allo-HSCT cohort and 4498 patients (8.6% of AML/MDS claims) were included in the AML/MDS cohort.
Study population—demographic and clinical characteristics
Characteristics of the allo-HSCT cohort are shown in . Among 863 patients who met the inclusion and exclusion criteria, the mean age of the allo-HSCT cohort was 50 years old, and 57.5% were male. In terms of the indications for allo-HSCT, 48.1% received allo-HSCT for AML, 27.6% for MDS, and 16.7% for acute lymphoblastic leukemia (ALL). The proportion of patients who received bone marrow, peripheral blood, and cord blood as a stem cell source was 21.7%, 23.1%, and 55.3%, respectively. The mean CCI score was 4.3 for the overall population. A total of 60% of patients received antifungal drugs during the look-back period.
Table 1. Demographic and baseline characteristics of the allo-HSCT cohort.
Among patients in the allo-HSCT cohort, 95% received more than one antifungal drug during the index date. Of note, all patients in the allo-HSCT cohort received some antifungal drugs (i.e. no patients from this cohort in treatment group E; ).
Characteristics of the AML/MDS cohort are shown in . Among 4498 patients who met the inclusion and exclusion criteria, the mean age of the AML/MDS cohort was 65 years old, and 62.3% were male. A total of about 11% of patients received antifungal drugs during the look-back period. The mean CCI score was 3.4 for the overall population. About 87% of the AML/MDS cohort received cytarabine-based induction therapy. Unexpectedly, 12% of the patients in the AML/MDS cohort did not receive antifungal drugs during the index hospitalization (i.e. treatment group E) despite their risks for IFI.
Table 2. Demographic and baseline characteristics of the AML/MDS.
Antifungal drug use patterns
In the allo-HSCT cohort, the mean (SD) number of days after the index date of the first antifungal drug use was 4.8 (9.5). The mean (SD) duration of first antifungal drug use was 23.8 (32.0) days. Initial antifungal drug use was often started 8–14 days after the first allo-HSCT (n = 405, 46.9%) or 15 days after the first allo-HSCT (n = 324, 37.5%).
In the AML/MDS cohort, the mean (SD) number of days after the index date until the time of first antifungal drug use was 7.2 (12.8) days. The mean (SD) duration of first antifungal drug use after the index date was 26.7 (34.1) days.
The breakdown of antifungal drugs used during the index hospitalization is summarized in Supplemental Dataset 1.
Hcru
Hospitalizations, outpatient visits, and HCRU outcomes for the allo-HSCT and AML/MDS cohorts are summarized in and . Overall, imaging procedures and microbiologic examinations were more likely to have been conducted in patients who received multiple antifungal drugs compared to those who received a single antifungal drug. For the allo-HSCT cohort, the mean length of the index hospitalization was 112.5 days, and was significantly longer among patients who received multiple-drug antifungal therapy containing fluconazole than it was for those who received single-drug antifungal therapy with fluconazole (Group C: 122.9 days; Group A: 94.4 days; p = .0451). Mean length of index hospitalization was numerically longer among patients who received multiple-drug antifungal therapy not containing fluconazole than it was for those who received single-drug antifungal therapy not containing fluconazole (Group D: 110.3 days; Group B: 93.2 days, p = .1248). In the allo-HSCT cohort, the mean total inpatient days was 111.0 days and was significantly longer among patients who received multiple-drug antifungal therapy containing fluconazole compared to those who received single-drug antifungal therapy with fluconazole (Group C: 121.8 days; Group A: 94.4 days, p = .0379), and numerically longer among patients who received multiple-drug antifungal therapy not containing fluconazole compared to those who received single-drug antifungal therapy not containing fluconazole (Group D: 108.5 days; Group B: 93.2 days, p = .1170). The readmission rate of post allo-HSCT was low (total N = 2 (0.23%)), and there was almost no difference between mean length of index hospitalization (112.5 days) and mean length of total inpatient days (111.0 days).
Table 3. Hospitalization and HCRU during follow up period for the allo-HSCT cohort.
Table 4. Hospitalization and HCRU during follow up period for the AML/MDS cohort.
For the AML/MDS cohort, the mean length of the index hospitalization was 64.9 days, and was significantly longer among patients who received multiple-drug antifungal therapy containing fluconazole than it was for those who received single-drug antifungal therapy with fluconazole (Group C; 73.5 days; Group A; 52.5 days; p < .0001); and significantly longer among patients who received multiple-drug antifungal therapy not containing fluconazole compared to those who received single-drug antifungal therapy not containing fluconazole (Group D: 81.8 days; Group B: 60.6 days; p < .0001). Mean length of the index hospitalization was 42.1 days for patients who received no antifungal treatment (Group E).
In the AML/MDS cohort, the mean total inpatient days was 108.0 days and was significantly longer among patients who received multiple-drug antifungal therapy containing fluconazole compared to those who received single-drug antifungal therapy with fluconazole (Group C: 117.7 days; Group A: 107.7 days, p = .0007), and significantly longer among patients who received multiple-drug antifungal therapy not containing fluconazole (Group D; 118.8 days) compared to those who received single-drug antifungal therapy not containing fluconazole (Group D: 118.8 days; Group B: 101.9 days, p < .0001). The mean total inpatient days was 75.9 days for patients who received no antifungal treatment (Group E).
Costs
As summarized in , the mean total cost for the allo-HSCT cohort was equivalent to US$180,541. The mean index hospitalization cost was equivalent to US$148,213. Of note, about 90% of the cost was attributed to inpatient costs. Both the total cost (sum of total inpatient and outpatient costs) and the index hospitalization costs trended higher in patients treated with multiple antifungal agents during the index admission than they were in patients treated with single-agent antifungal therapy during the admission. The mean total cost of antifungal treatment was equivalent to US$18,175 and was highest among patients who received multiple-drug antifungal therapy not containing fluconazole (Group D; equivalent to US$191,336). As expected, antifungal treatment costs were higher in patients treated with multiple antifungal drugs (with or without fluconazole) compared to those who received single-drug antifungal treatment.
Table 5. Costs (in USD) for the allo-HSCT cohort during follow up period.
As summarized in , the mean total cost for the AML/MDS cohort was equivalent to US$105,929. The mean index hospitalization cost was equivalent to US$51,657. More than 90% of the cost was attributed to inpatient costs. Both the total cost (sum of total inpatient and outpatient costs) and the index hospitalization costs trended higher in patients treated with multiple antifungal drugs during the index admission than they were in patients treated with single-drug antifungal treatment during the admission. The mean total cost of antifungal treatment was equivalent to US$6,620 and was highest among patients who received multiple-drug antifungal therapy not containing fluconazole (Group D; equivalent to US$10,488). Antifungal treatment costs were higher in patients treated with multiple antifungal drugs (with or without fluconazole) compared to those who received single-drug antifungal treatment.
Table 6. Costs (in USD) for the AML/MDS cohort during follow up period.
Sensitivity analysis
The findings of the sensitivity analysis (data not shown) were consistent with those from the main analysis and demonstrated an increase in HCRU and costs with multiple-drug antifungal treatment compared to single-drug antifungal treatment.
Discussion
Diagnosing IFIs is challenging in clinical practice, and prophylactic antifungal therapy is recommended for patients at high risk of developing IFIsCitation21–25. There is limited evidence on patient characteristics and the economic burden of current antifungal management in Japan; therefore, the aim of this study was to understand aspects of real-world antifungal management in AML/MDS and allo-HSCT patients in Japan.
In our study, most patients with hematologic malignancy and high IFI risk underwent complicated antifungal management requiring use of multiple drugs, and accounted for high healthcare resource utilization and costs. Among 863 patients in the allo-HSCT cohort who met the inclusion and exclusion criteria, 91% received more than one antifungal drug during the index hospitalization. This suggests that the majority of allo-HSCT patients required IFI management beyond use of one antifungal drug during the index admission, possibly because the patient either experienced adverse events associated with the original antifungal drug or contracted an IFI. Among 4498 patients in the AML/MDS cohort, approximately 50% received more than one antifungal drug during the index hospitalization.
In our study, the mean duration of first antifungal drug use was 23.8 days for the allo-HSCT cohort and 26.7 days for the AML/MDS cohort. This duration in the AML/MDS cohort is consistent with the 24–29 days of antifungal prophylaxis reported in a clinical trial evaluating posaconazole, fluconazole, and itraconazole in patients with neutropeniaCitation26, but the durations in allo-HSCT was considerably shorter than the guideline-recommended usual duration of 90–100 days for primary antifungal prophylaxis in patients with hematologic malignanciesCitation27, suggesting that patients in our study may have received antifungal drugs as prophylaxis for shorter-than-recommended durations, or may have received those drugs not as prophylaxis, but rather to treat a confirmed IFI. For both the allo-HSCT and AML/MDS cohorts, about 90% of the total cost was attributed to inpatient cost. Both the total cost (the total inpatient and outpatient cost) and the index hospitalization costs trended higher in patients treated with multiple antifungal drugs during the index admission than they were in patients treated with a single antifungal drug during the admission. As expected, antifungal drug costs and imaging/examination costs were higher in patients treated with multiple antifungal drugs, with or without fluconazole, compared to those who received a single antifungal drug.
An unexpected finding in this study was that 12% of the patients with AML/MDS did not receive antifungal drugs during the index hospitalization (Group E) despite their risks for IFI, and despite the fact that antifungal prophylaxis is recommended for high-risk groups such as patients with AML/MDSCitation21–25.
Limitations of this retrospective database study include the fact that the MDV database only covers reimbursement claims processed within DPC hospitals, which are not specifically recorded for research purposes. Medical claims databases may not capture all patient services received because of out-of-pocket transactions and patient changes with respect to providers (e.g. if the patient transfers to another healthcare provider before or after the index date). The outcomes of laboratory examinations and imaging procedures were limited in the MDV database and thus not reflected in our study. The reasons for antifungal treatment received during the index hospitalization (e.g. prophylaxis, empirical treatment, targeted treatment) were not available; however, it is reasonable to assume that the majority of index hospitalization antifungal drug use was for prophylactic purposes. Additionally, this study was conducted prior to the approval of posaconazole in Japan in January 2020; posaconazole is recommended by international guidelines for prophylaxis of IFIs. Further studies are warranted to examine changes in IFI-related HCRU and costs over time, and that would reflect the recent introduction of using posaconazole to treat these patients at high risk of IFIs in Japan.
The MDV database is the largest available retrospective dataset, nationally representative of the Japanese population, and representative of the population affected by IFI. Thus, these study findings are generalizable to the larger Japanese population. To our knowledge, this is the first retrospective database study in Japan to use large-scale, real-world data to elucidate the antifungal management of patients at high risk for IFIs and the economic burden associated with IFI management; similar real-world database studies have evaluated these in other countriesCitation8,Citation19,Citation20,Citation28,Citation29. Our study demonstrates that antifungal management of patients at high risk of IFIs remains an unmet need. In this real-world database study of patients in Japan, most patients with hematologic malignancy and high IFI risk underwent complicated antifungal management requiring use of multiple drugs, and accounted for high healthcare resource utilization and costs.
Transparency
Declaration of funding
This study and preparation of this manuscript were supported by funding from MSD K.K., Tokyo, Japan.
Declaration of financial relationships
RU, SN, and GF are employees of MSD K.K., Tokyo, Japan. DA is an employee of IQVIA and has a research/consulting agreement with MSD K.K., Tokyo, Japan. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RU, SN, and GF contributed to the study design. DA contributed to the acquisition of study data. All authors contributed to the critical interpretation of data and drafting/revision of the manuscript content, have approved the final version of this manuscript, and take responsibility for the integrity of this research study.
Supplemental Dataset
Download MS Word (66.2 KB)Acknowledgements
The authors wish to thank Daniel J. Ruzicka and Itaru Hiraishi of MSD K.K., Tokyo, Japan for invaluable clinical insights to significantly enhance the manuscript; and Jeffrey Walter, Tracy-Ann Kernanet-Huggins, and Laura Huber of IQVIA for assistance with preparation of the manuscript.
Data availability statement
The data that support this study’s findings are available from MSD K.K., Tokyo, Japan upon request. Some restrictions apply to the availability of these data, which were used under license for this research study.
References
- Otto WR, Green AM. Fungal infections in children with haematologic malignancies and stem cell transplant recipients. Br J Haematol. 2020;189(4):607–624.
- Alfandari S, Berthon C, Coiteux V. Antifungal stewardship: implementation in a French teaching hospital. Med Mal Infect. 2014;44(4):154–158.
- Bays DJ, Thompson GR, 3rd. Fungal infections of the stem cell transplant recipient and hematologic malignancy patients. Infect Dis Clin North Am. 2019;33(2):545–566.
- Böhme A, Atta J, Mousset S, et al. Antifungal management and resource use in patients with acute myeloid leukaemia after chemotherapy—retrospective analysis of changes over 3 yr in a German hospital. Eur J Haematol. 2012;88(1):68–77.
- Cornely OA, Aversa F, Cook P, et al. Evaluating the role of prophylaxis in the management of invasive fungal infections in patients with hematologic malignancy. Eur J Haematol. 2011;87(4):289–301.
- Fu R, Gundrum J, Sung AH. Health-care utilization and outcomes of patients at high risk of invasive fungal infection. Clinicoecon Outcomes Res. 2018;10:371–387.
- Ceesay MM, Sadique Z, Harris R, et al. Prospective evaluation of the cost of diagnosis and treatment of invasive fungal disease in a cohort of adult haematology patients in the UK. J Antimicrob Chemother. 2015;70(4):1175–1181.
- Racil Z, Weinbergerova B, Kocmanova I, et al. Invasive aspergillosis in patients with hematological malignancies in the Czech and Slovak republics: Fungal InfectioN Database (FIND) analysis, 2005–2009. Int J Infect Dis. 2013;17(2):e101–e109.
- Tsutsumi I, Kunisawa S, Yoshida C, et al. Impact of oral voriconazole during chemotherapy for acute myeloid leukemia and myelodysplastic syndrome: a Japanese nationwide retrospective cohort study. Int J Clin Oncol. 2019;24(11):1449–1458.
- Zaragoza R, Pemán J, Salavert M, et al. Multidisciplinary approach to the treatment of invasive fungal infections in adult patients. Prophylaxis, empirical, preemptive or targeted therapy, which is the best in the different hosts? Ther Clin Risk Manag. 2008;4(6):1261–1280.
- Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006. Clin Infect Dis. 2010;50(8):1091–1100.
- Ananda-Rajah MR, Cheng A, Morrissey CO, et al. Attributable hospital cost and antifungal treatment of invasive fungal diseases in high-risk hematology patients: an economic modeling approach. Antimicrob Agents Chemother. 2011;55(5):1953–1960.
- Dodds Ashley E, Drew R, Johnson M, et al. Cost of invasive fungal infections in the era of new diagnostics and expanded treatment options. Pharmacotherapy. 2012;32(10):890–901.
- Menzin J, Meyers JL, Friedman M, et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm. 2009;66(19):1711–1717.
- Drgona L, Khachatryan A, Stephens J, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 2014;33(1):7–21.
- Cai J, Prehn A, Schlamm H, et al. Impact of invasive fungal infections on mortality, length of hospital stay, and costs in allogeneic hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2014;20(2):S216.
- Menzin J, Lang K, Friedman M, et al. Excess mortality, length of stay, and costs associated with serious fungal infections among elderly cancer patients: findings from linked SEER-Medicare data. Value Health. 2005;8(2):140–148.
- Wilson LS, Reyes CM, Stolpman M, et al. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5(1):26–34.
- Kim A, Nicolau DP, Kuti JL. Hospital costs and outcomes among intravenous antifungal therapies for patients with invasive aspergillosis in the United States. Mycoses. 2011;54(5):e301–312–e312.
- Dasbach EJ, Davies GM, Teutsch SM. Burden of aspergillosis-related hospitalizations in the United States. Clin Infect Dis. 2000;31(6):1524–1528.
- Kohno S, Tamura K, Niki Y, et al. Executive summary of Japanese domestic guidelines for management of deep-seated Mycosis 2014. Med Mycol J. 2016;57(4):e117–e163.
- Bassetti M, Righi E, Montravers P, et al. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J Antimicrob Chemother. 2018;73(suppl_1):i14–i25.
- Pound MW, Townsend ML, Dimondi V, et al. Overview of treatment options for invasive fungal infections. Med Mycol. 2011;49(6):1–580.
- Patterson TF, Thompson GR, 3rd, Denning DW, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):433–442.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant. 2009;44(8):453–558.
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–359.
- Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant. 2011;46(5):709–718.
- Kleinberg M. Aspergillosis in the CLEAR outcomes trial: working toward a real-world clinical perspective. Med Mycol. 2005;43(s1):289–S294.
- Kontoyiannis DP, Yang H, Song J, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis. 2016;16(1):730.