Abstract
Objective
This pooled claims database study evaluated the risk of recurrent Venous Thromboembolism (VTE) and major bleeding (MB) among patients with VTE and active cancer prescribed apixaban, low-molecular weight heparin (LMWH), or warfarin stratified by high-risk subgroups.
Methods
Patients diagnosed with VTE in the setting of active cancer who initiated apixaban, LMWH, or warfarin were identified using four US commercial claims databases from 01SEP2014 to the end of the study period (MarketScan: 01MAR2014-30JUNE2017; Optum and Humana: 01MAR2014-31DEC2017; PharMetrics: 01MAR2014-31MAR2018). Stabilized inverse probability treatment weighting (IPTW) was used to balance treatment cohorts. Cox proportional hazard models were used to evaluate the risk of recurrent VTE and MB for each subgroup stratification: VTE risk level based on cancer types, metastatic diagnosis, cancer treatment, chemotherapy, gastrointestinal cancer, and index VTE event type (PE vs. DVT). Statistical significance (p < .10) of the interaction between treatment effects and subgroups was evaluated.
Results
Eligible subjects included 3393 apixaban, 6108 LMWH, and 4585 warfarin patients. After IPTW, all patient characteristics were balanced. Analyses stratified by the VTE risk level, metastatic diagnosis, cancer treatment, chemotherapy, gastrointestinal cancer and index VTE event type showed generally consistent results according to the respective subgroup (most of the p values for interaction >0.10). Two significant interactions were observed between apixaban vs. LMWH and VTE risk level (interaction p = .051) and metastatic diagnosis (interaction p < .001) for recurrent VTE; one significant interactions were observed between apixaban vs. LMWH and cancer treatment for MB (interaction p = .074). Additionally, for warfarin vs. LMWH, two significant interactions were observed between treatment and VTE risk level (interaction p = .005) and metastatic diagnosis (interaction p = .002) for recurrent VTE.
Conclusions
Across these high-risk subgroups of VTE cancer patients, treatment outcomes associated with apixaban were generally positive compared to LMWH and warfarin.
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication in patients with cancerCitation1,Citation2. Although direct oral anticoagulants (DOACs) are recommended for the treatment of VTE, the higher risk of recurrent VTE and major bleeding associated with cancer makes anticoagulant treatment challenging in these patientsCitation3. A number of clinical trials and real-world evidence studies have evaluated the use of DOACs vs. low-molecular-weight heparin (LMWH) for the treatment of cancer-associated VTECitation4–11. Based on clinical trial evidence, clinical practice guidelines have recommended DOACs be included among the treatment options for cancer-associated VTECitation9,Citation11.
A variety of clinical and patient-related factors have been associated with the risk of cancer-associated VTE including age, race, cancer treatment, primary site of cancer, time since diagnosis, and extension of diseaseCitation12–14. There has been limited evidence about the effectiveness and safety of DOAC use among cancer patients with a high risk for VTE recurrence. The CARAVAGGIO randomized trial found that apixaban was noninferior to dalteparin for the treatment of cancer-associated VTE without an increased risk of major bleeding (MB). The analysis of subgroups – such as index VTE event type, metastatic diagnosis at randomization, cancer treatment, and receiving chemotherapy – demonstrated consistent findings with the study’s overall results and did not show any significant interactionsCitation15. The effectiveness and safety of apixaban in routine clinical practice across high-risk VTE cancer subgroups remained unexplored. A large real-world study of data pooled from several health claims databases of VTE patients with active cancer showed that apixaban was associated with a lower risk of recurrent VTE and MB compared to LMWH patients and lower risk of recurrent VTE compared to warfarin patientsCitation8. The current study further assessed the study population to evaluate the effectiveness and safety of apixaban, warfarin, and LMWH among VTE patients with active cancer stratified by high-risk subgroups – VTE risk level (based on cancer type), index VTE event type, metastatic diagnosis, cancer treatment, chemotherapy, and upper and lower gastrointestinal (GI) cancer.
Methods
Data source and patient selection
This retrospective claims database study pooled data from four US commercial claims databases – IBM MarketScan Commercial Claims and Encounter and Medicare Supplemental and Coordination of Benefits Database (MarketScanFootnotei), IQVIA PharMetrics Plus (PharMetricsFootnoteii), Optum Clinformatics Data Mart (OptumFootnoteiii), and the Humana Research Database (HumanaFootnoteiv).
Patients were included if they had ≥1 medical claim for VTE (index VTE event) in any position in inpatient or outpatient setting (including emergency department) from 01SEP2014 to the end of the study period (MarketScan: 01MAR2014-30JUNE2017; Optum and Humana: 01MAR2014-31DEC2017; PharMetrics: 01MAR2014-31MAR2018) and an active cancer diagnosis (defined as having ≥2 medical claims for cancer diagnosis [excluding non-melanoma skin cancer] or 1 claim for cancer diagnosis plus ≥1 claim for cancer treatment within 6 months before or until 30 days after the VTE event). Adult patients (aged ≥18 years) with ≥1 pharmacy claim for apixaban, LMWH, or warfarin (index date) within 30 days following the index VTE were selected. Patients were excluded if they had two anticoagulants (OAC or parental anticoagulant [PAC]) on the index date except if warfarin was bridged with a PAC. Patients were followed from the day after the index date through the earliest of the following: 6-month period, health plan disenrollment, death, index therapy discontinuation, switch to a non-index OAC/PAC, or study endCitation16. Additional details about the study population selection and results can be found in a prior publicationCitation8.
After applying all selection criteria, the following six subgroups were identified: VTE risk level (very high risk vs. high risk vs. other cancers), cancer metastasis (yes vs. no metastasis), cancer-related treatment (yes vs. no cancer-related treatment), chemotherapy (yes vs. no chemotherapy), index VTE diagnosis (DVT only vs. PE with/without DVT), GI cancer (yes vs. no GI cancer). VTE risk level (based on ICD codes only) was defined in the following manner: very-high-risk (brain, stomach, or pancreas cancer), high-risk (lung, lymphoma, gynecologic, bladder, testicular, renal cell carcinoma cancer), or other cancersCitation3,Citation14,Citation17–20.
Clinical outcomes
Primary outcome measures were recurrent VTE and MB, whereas clinically relevant non-major (CRNM) bleeding was a secondary outcome (). Definitions for recurrent VTE, MB, and CRNM bleeding were previously reported in the publication of the overall primary analysisCitation8. Briefly, recurrent VTE and MB events were identified based on inpatient claims with VTE or MB as the first-listed diagnosis. A CRNM bleeding event was identified as an inpatient admission with a secondary diagnosis for “non-critical site” bleeding or an outpatient encounter with a diagnosis code for non-critical sites of bleeding. CRNM bleeding events that followed a MB event were not included in the analysis of CRNM bleeding.
Patient demographics, clinical characteristics, and index-VTE-event-related variables were measured during the 6 months prior to and on the index date (baseline period). Cancer-related variables such as cancer metastasis, VTE risk level, type of cancer, and cancer-related treatment were measured 6 months prior to the index date until 30 days after the index date.
Statistical methods
Treatment cohorts were balanced using stabilized inverse probability treatment weighting (IPTW) to retain all eligible subjectsCitation21. IPTW uses propensity scores to obtain estimates of the average treatment effect. The propensity score was calculated using a multinomial logistic model with three treatment cohorts (apixaban, warfarin, and LMWH) included in the model, using LMWH patients as the reference (i.e. control cohort). Covariates including demographics, type of VTE diagnosis, modified comorbidity index (CCI) score (did not include cancer)Citation22, comorbidities, medication use, cancer metastases, VTE risk level, and cancer-related treatment were used to define the probability of a patient receiving a certain treatment. After IPTW, all the baseline characteristics were well-balanced, and patients were pooled from the four databases for further analysis.
The post-IPTW cohorts were stratified by the VTE risk level, metastatic diagnosis, cancer treatment, chemotherapy, index VTE event type, and GI cancer, respectively. Cox proportional hazards models were used to estimate hazard ratios (HRs) of recurrent VTE, MB, and CRNM bleeding for each subgroup stratification and test a statistical significance (p < .10) of the interaction between treatments and each of the subgroups on recurrent VTE, MB and CRNM bleeding. After stratification if a baseline variable (included in IPTW) was unbalanced, it was added as a covariate in the Cox model that was used to test the interaction between treatment and the subgroup. Additional Cox models were developed to evaluate the interaction between treatment and GI cancer status on the components of MB–gastrointestinal bleeding, intracranial hemorrhage (ICH) bleeding, and bleeding at other sites (genitourinary bleeding, respiratory tract bleeding, ocular bleeding, joint bleeding/hemarthrosis, transfusion of blood and blood components, other bleeding, or no bleeding site specified).
Institutional review board statement
This retrospective database analysis did not involve the collection, use, or transmittal of individual identifiable data. As such, Institutional Review Board approval to conduct this study was not required and considered exempt according to 45CFR46.101(b)(4): Existing Data and Specimens – No Identifiers. Both the data set itself and the security of the offices where the data are housed meet the requirements of the Health Insurance Portability and Accountability Act of 1996.
Data sharing statement
The datasets generated during and/or analyzed during the current study are not publicly available due to a data licensing agreement with the data vendors.
Results
A total of 14,086 VTE patients with active cancer were included in the analysis where 6108 (43.4%) were prescribed LMWH, 4585 (32.6%) were prescribed warfarin, and 3393 (24.1%) were prescribed apixaban (). Post-IPTW, all patient characteristics were well balancedCitation8. shows the subgroups being evaluated in this study. About 15–16% of the patients had upper or lower GI cancer. Most patients (57–59%) had DVT only as their index VTE event. The majority of patients had cancer-related treatment (75–77%) with chemotherapy being used in 63–64% of patients. With respect to VTE risk category, around 15% of patients had cancers associated with a very high risk of VTE and around 40% of patients had cancers associated with a high risk of VTE. More than 50% of patients had a diagnosis of metastatic cancer. Baseline characteristics for the subgroups are listed in .
Table 1. Post-IPTW baseline characteristics among VTE patients with active cancer.
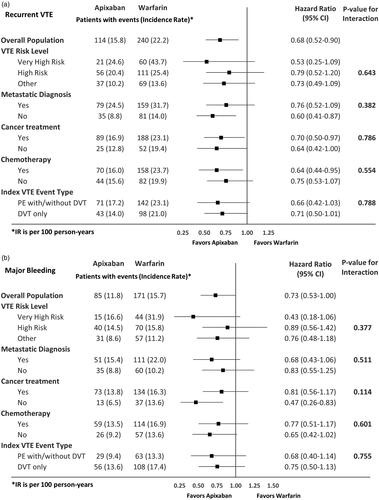
presents the HRs of recurrent VTE and MB among VTE cancer patients prescribed apixaban vs. LMWH in the overall population and stratified by high-risk subgroups. Apixaban had a lower risk of recurrent VTE and MB compared to LMWH in the overall populationCitation8 and generally consistent findings were observed across the subgroups. No significant interactions were found between the effects of apixaban vs. LMWH and the following subgroups on recurrent VTE: cancer treatment, chemotherapy, or index VTE event type. Significant interactions were evident between the effects of apixaban vs. LMWH and the following two subgroups (VTE risk levels and metastatic status) for recurrent VTE. Apixaban trended towards a lower risk of recurrent VTE compared to LMWH across all three cancer-associated VTE risk levels, but the magnitude of the difference was larger in the “other” cancer group vs. the very-high-risk and high-risk cancer groups (p = .051). Apixaban had a lower risk of recurrent VTE vs. LMWH in patients without a metastatic diagnosis, whereas apixaban had a similar risk of recurrent VTE vs. LMWH in patients with a metastatic diagnosis (p < .001). For MB, no significant interactions were found between the effects of apixaban vs. LMWH and the following subgroups: VTE risk level, metastatic diagnosis, chemotherapy, or index VTE event type. Although a significant interaction was observed for the effects of apixaban vs. LMWH according to cancer treatment status on MB (yes vs. no, p = .074) with apixaban having a lower risk of major bleeding in those not on cancer treatment, apixaban trended to have a lower risk of MB vs. LMWH regardless of cancer treatment.
shows the HRs of recurrent VTE and MB among VTE cancer patients prescribed warfarin vs. LMWH in the overall population and stratified by high-risk subgroups. No significant interactions were observed for the effects of warfarin vs. LMWH with any of the subgroups on MB. While most of the subgroups showed consistent effects of warfarin vs. LMWH on recurrent VTE, two significant interactions were observed – the effects of warfarin vs. LMWH on recurrent VTE had different trends with different VTE risk levels (p = .005; ) with very high risk cancers favoring LMWH. Additionally, warfarin patients had a lower risk of recurrent VTE vs. LMWH in patients without a metastatic diagnosis but had a similar risk of recurrent VTE in patients with a metastatic diagnosis (p = .002; ). summarizes the HRs of recurrent VTE and MB among VTE cancer patients prescribed apixaban vs. warfarin in the overall population and stratified by high-risk subgroups. No significant interactions were found for the effects of apixaban vs. warfarin with any of the subgroups for both recurrent VTE and MB ().
Similarly, findings on CRNM bleeding according to the subgroups were generally consistent with the overall population. Few significant interactions were observed between the treatment effects and the subgroups for CRNM bleeding ().
Figure 1. Hazard ratio of recurrent VTE and major bleeding among VTE cancer patients that initiated apixaban vs LMWH stratified by high-risk subgroups. (a) Recurrent VTE. (b) Major bleeding. Abbreviations. CI, Confidence interval; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; IPTW, inverse probability treatment weighting; PE, pulmonary embolism; VTE, venous thromboembolism; IR, Incidence Rate.
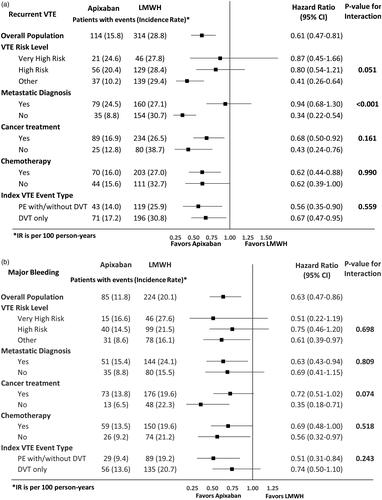
Figure 2. Hazard ratio of recurrent VTE and major bleeding among VTE cancer patients that initiated warfarin vs LMWH stratified by high-risk subgroups. (a) Recurrent VTE. (b) Major bleeding. Abbreviations. CI, Confidence Interval; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; IPTW, inverse probability treatment weighting; PE, pulmonary embolism; VTE, venous thromboembolism.
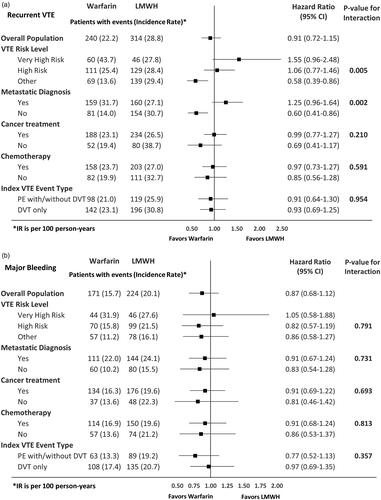
Figure 3. Hazard ratio of recurrent VTE and major bleeding among VTE cancer patients that initiated apixaban vs warfarin stratified by high-risk subgroups. (a) Recurrent VTE. (b) Major bleeding. Abbreviations. CI, Confidence interval; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; IPTW, inverse probability treatment weighting; PE, pulmonary embolism; VTE, venous thromboembolism.
GI cancer
shows HRs of recurrent VTE, MB and components of MB among VTE patients with GI cancer vs. other cancer types. No significant interactions were found between the treatment effects and GI cancer status for recurrent VTE and MB (). Additional analyses on components of MB including GI MB showed consistent results regardless of GI cancer status for all the comparisons. Findings on CRNM bleeding stratified by GI cancer status can be found in .
Table 2. Hazard ratio for recurrent VTE and MB among VTE patients with and without GI cancer.
Discussion
This retrospective study used four large US datasets to compare the risks of recurrent VTE and MB among VTE patients with active cancer who initiated treatment with apixaban, LMWH, or warfarin stratified by high-risk subgroups. The high-risk subgroups included VTE risk level, metastasis status, cancer treatment, chemotherapy, index VTE event type, and GI cancer status. Analyses stratified by these subgroups showed generally consistent results across the subgroups and with the overall population. Across the subgroups, apixaban was generally associated with lower risks of recurrent VTE and MB compared with LMWH. Additionally, apixaban was associated with a lower risk of recurrent VTE and similar risk of MB compared to warfarin. Finally, warfarin was generally associated with similar risks of recurrent VTE and MB compared to LMWH patients stratified by subgroupsCitation8.
Recently, guidelines have recommended the use of DOACs for the treatment of VTE in active cancer patientsCitation9; however, limited research has been conducted across high-risk subgroups in VTE patients with active cancer. In the CARAVAGGIO trial, apixaban was noninferior to dalteparin for the treatment of cancer-associated VTE without an increased risk of MBCitation15. Consistent findings were observed across some high-risk subgroups as no significant interactions were observed for recurrent VTE between treatment effects and index VTE diagnosis (p = .3337), metastatic diagnosis at randomization (p = .9026), or receiving cancer treatment (p = .6656)Citation15. Similarly, there was no significant interaction between treatment and index VTE diagnosis (p = .5640), or receiving cancer treatment (p = .2837) for MBCitation15. The current study expanded on the CARAVAGGIOCitation15 and Cohen et al.Citation8 studies and assessed different high-risk subgroups including VTE risk level, metastasis status, cancer treatment, chemotherapy, index VTE event type, and GI cancer. The results of the current study were generally consistent with CARVAGGIO trial, although the current study did find a significant interaction for treatment effects according to VTE risk level and metastatic diagnosis for recurrent VTE – apixaban trended towards a lower risk of recurrent VTE compared to LMWH across all three VTE risk levels (very high vs. high vs. other), but the magnitude of the difference was larger in the other cancer group vs. the very-high-risk and high-risk groups. Additionally, apixaban patients also had a lower risk of recurrent VTE in patients without a metastatic diagnosis and a similar risk in patients with a metastatic diagnosis compared to LMWH. The differences in the treatment effects could be due to different patient characteristics which may result in different responses to the treatments.
Safety and effectiveness of anticoagulation treatment depend on the risk stratification for VTE and the presence of metastasis at the time of cancer diagnosisCitation23,Citation24. Therefore, it is important to identify the risk-benefit ratio of anticoagulant treatments in high-risk VTE patients with active cancer. Few retrospective observational studies comparing LMWH to oral anticoagulants have been conductedCitation4,Citation25–27; however, there are limited analyses of apixaban among high-risk subgroups of VTE cancer patients. One real-world study reported that DOACs were superior in effectiveness, with a lower risk of bleeding compared to warfarin among VTE patients with active cancerCitation28. No significant interaction was found between treatment effects and cancer-related VTE risk category (very high risk vs. high risk vs. low risk for VTE) for recurrent VTE or between treatment and GI cancer status (GI cancer vs. non-GI cancer) for MBCitation26.
The recent NCCN guidelines recommend the use of DOACs in VTE patients with cancer. However, due to the increased risk of GI/genitourinary bleeding associated with some DOACs, LMWH is still recommended for VTE patients with GI cancers and DOACs are recommended to be used with caution among these patientsCitation9. There are no head-to-head clinical trials that have compared DOACs vs. LMWH in GI cancer patients. A post hoc analysis of the HOKUSAI VTE trial reported an increased risk of MB in resected or unresected GI cancers for edoxaban vs. dalteparinCitation29. The SELECT-D trial also showed that patients with esophageal or gastroesophageal cancer tended to have more MB with rivaroxaban than with dalteparin.Citation27 A recent meta-analysis of randomized control trials reported that the risk of MB was higher for DOACs (rivaroxaban, edoxaban) vs. LMWH for patients with GI cancer (relative risk: 2.30; 95% CI: 1.08–4.88) whereas it was similar in non-GI cancer patients (relative risk: 1.22; 95% CI: 0.60–2.48)Citation30. Although the CARAVAGGIO trial has not published any post hoc analysis on GI cancer to date, the trial did include one-third of patients with cancer at GI sites and has inferred that VTE patients with GI cancer may be eligible for treatment with apixabanCitation15. A recently published sub analysis of the CARAVAGGIO trial reported that the number of GI bleeds was comparable between patients who received apixaban compared to dalteparin in the treatment of cancer associated VTECitation31. Additionally, GI bleeding was also similar across the two treatments among patients with GI cancerCitation28. The current study conducted an interaction analysis between the treatment effects and GI cancer and did not find any significant interaction for the treatment effects according to GI cancer status for recurrent VTE or MB, including GI MB. Further studies are needed to assess the effectiveness and safety of oral anticoagulants among VTE patients with GI cancer and other high-risk subgroups of VTE cancer patients. As the current analysis restricted the maximum follow-up to 6 months, additional studies should be conducted to evaluate the effects of the three treatments beyond 6 months.
Several limitations of this analysis should be noted. First, diagnoses for DVT and PE were identified using ICD-9/10-CM codes. Therefore, the presence or absence of a diagnostic code may not indicate a positive presence of disease, as they may be incorrectly coded or included as rule-out criteria than actual disease. Second, duplicates were not excluded from the pooled dataset. However, prior literature reported only 0.5% of patients were duplicates between two databasesCitation32. Third, laboratory parameters such as hemoglobin values and cancer stage information were not available; therefore, MB may have been underestimated. Fourth, complete death information for the patients was unavailable in the databases. Consequently, we could not evaluate mortality and fatal recurrent VTE among VTE patients with active cancer, and mortality may be a competing risk in this patient population. Fifth, the majority of LMWH patients in the current study used enoxaparin as their index drug whereas dalteparin is the most commonly used LMWH in clinical trials. The current study results may not be comparable to the trial findings. Sixth, we could not assess whether patients were dosed according to the product label for each of the treatments due to lack of dosing frequency information available in the databases to make the assessment. Finally, the results may not be generalizable to the entire US VTE cancer population: uninsured patients or patients with governmental insurances such as Medicare, Medicaid, and Veterans Affairs were not evaluated.
Conclusions
This was one of the largest observational studies of VTE patients with active cancer that compared effectiveness and safety of anticoagulants stratified by high-risk subgroups – VTE risk level, metastatic diagnosis, cancer treatment, chemotherapy, index VTE event type, and GI cancer. Across the subgroups, apixaban was generally associated with lower risks of recurrent VTE and MB compared to LMWH and lower risks of recurrent VTE vs. warfarin. Warfarin patients generally had similar risks of recurrent VTE and MB compared to LMWH patients across the subgroups. These results provide additional evidence to help clinicians evaluate and select anticoagulation treatment options for high-risk subgroups of VTE patients with active cancer.
Transparency
Declaration of funding
This study was funded by Pfizer Inc. and Bristol Myers Squibb Company.
Declaration of financial/other relationships
AC received research support from Pfizer Inc. and Bristol Myers Squibb Company. AK and JS are employees of STATinMED Research, a paid consultant to the study sponsors – Pfizer Inc. and Bristol Myers Squibb Company in connection with the development of the manuscript. TL, PH, and XL are employees of Pfizer, Inc., one of the study sponsors. LR is an employee of Bristol Myers Squibb Company, one of the study sponsors. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
AC was involved in the conception and design as well as revising the paper critically for intellectual content. AK and JS were involved in the conception and design, analysis and interpretation of the data, the drafting of the paper, and revising it critically for intellectual content. TL, PH, XL, and LR were involved in the conception and design as well as revising the paper critically for intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgements
This study was supported by research funding from the study sponsors – Pfizer Inc. and Bristol Myers Squibb Company.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/03007995.2023.2268985)
Notes
i MarketScan, IBM Watson Health, Armonk, NY, USA.
ii PharMetrics, IQVIA Inc., Danbury, CT, USA.
iii Optum, Eden Prairie, MN, USA.
iv Humana, Humana Inc., Louisville, KY, USA.
References
- Lee AYY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17–I21.
- Blom JW, Doggen CJM, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722.
- Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624.
- Win KZ, Wilson N, Stenehjem DD, et al. Effectiveness and safety of rivaroxaban in treatment of venous thromboembolism in cancer patients. Blood. 2015;126(23):2319–2319.
- Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1891–1894.
- McBane RD, 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411–421.
- Cohen AT, Keshishian A, Lee T, et al. Effectiveness and safety of apixaban, low-molecular-weight heparin, and warfarin among venous thromboembolism patients with active cancer: a US claims data analysis. Thromb Hemost. 2021;121(3):383–395.
- Streiff MB, Holmstrom B, Angelini D, et al. NCCN guidelines insights: cancer-associated venous thromboembolic disease, version 1. 2020. [Internet]. Plymouth Meeting (PA): National Comprehensive Cancer Network; c1993. 2020. [cited 2020 Aug 17]; [133 screens]. Available from: http://medi-guide.meditool.cn/ymtpdf/9E219C23-6627-2971-9CC9-2BB8D86873AA.pdf.
- Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566–e581.
- Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496–520.
- Khorana AA, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119(3):648–655.
- Martín-Martos F, Trujillo-Santos J, Del Toro J, et al. Gender differences in patients with venous thromboembolism and five common sites of cancer. Thromb Res. 2017;151(Suppl 1):S16–S20.
- Khorana AA, McCrae KR. Risk stratification strategies for cancer-associated thrombosis: an update. Thromb Res. 2014;133(Supply 2):S35–S38.
- Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607.
- Teutsch C, Huisman MV, Lip GYH, et al. Persistence with dabigatran therapy for stroke prevention in patients with non-valvular atrial fibrillation: the Gloria-AF Registry. Blood. 2016;128(22):2616–2616.
- Chee CE, Ashrani AA, Marks RS, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood, J Am Soc Hematol. 2014;123(25):3972–3978.
- Trujillo-Santos J, Ruiz-Gamietea A, Luque JM, et al. RIETE Investigators. Predicting recurrences or major bleeding in women with cancer and venous thromboembolism. Findings from the RIETE Registry. Thromb Res. 2009;123(Suppl 2):S10–S15.
- Louzada ML, Majeed H, Dao V, et al. Risk of recurrent venous thromboembolism according to malignancy characteristics in patients with cancer-associated thrombosis: a systematic review of observational and intervention studies. Blood Coagul Fibrinolysis. 2011;22(2):86–91.
- Insin P, Vitoopinyoparb K, Thadanipon K, et al. Prevention of venous thromboembolism in gynecological cancer patients undergoing major abdominopelvic surgery: a systematic review and network meta-analysis. Gynecol Oncol. 2021;161(1):304–313.
- Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655.
- NCI Comorbidity Index Overview [Internet]. Bethesda (MD): National Cancer Institute; c1937. 2019. May 23 [cited 2020 Aug 17]; [about 6 screens]. https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html.
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839–4847.
- Ay C, Dunkler C, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382.
- Ross JA, Miller MM, Hernandez CMR. Comparative effectiveness and safety of direct oral anticoagulants (DOACs) versus conventional anticoagulation for the treatment of cancer-related venous thromboembolism: a retrospective analysis. Thromb Res. 2017;150:86–89.
- Wysokinski WE, Houghton DE, Casanegra AI, et al. Comparison of apixaban to rivaroxaban and enoxaparin in acute cancer-associated venous thromboembolism. Am J Hematol. 2019;94(11):1185–1192.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–2023.
- Dawwas GK, Dietrich E, Smith SM, et al. Comparative effectiveness and safety of direct-acting oral anticoagulants and warfarin in patients with venous thromboembolism and active cancer: an observational analysis. Clin Ther. 2020;S0149-2918(20):30332–30335.
- Kraaijpoel N, Di Nisio M, Mulder FI, et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: results from the Hokusai VTE cancer study. Thromb Haemost. 2018;118(8):1439–1449.
- Moik F, Posch F, Zielinski C, et al. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: updated systematic review and meta-analysis of randomized controlled trials. Res Pract Thromb Haemost. 2020;4(4):550–561.
- Ageno W, Vedovati MC, Cohen A, et al. Bleeding with apixaban and dalteparin in patients with cancer-associated venous thromboembolism: results from the Caravaggio study. Thromb Haemost. 2021;121(5):616–624.
- Broder MS, Neary MP, Chang E, et al. Treatments, complications, and healthcare utilization associated with acromegaly: a study in two large United States databases. Pituitary. 2014;17(4):333–341.
Appendices
Appendix Figure 1. Hazard ratio for CRNM bleeding among VTE cancer patients that initiated apixaban, LMWH, or warfarin stratified by high-risk subgroups. Abbreviations. CI, Confidence Interval; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; IPTW, inverse probability treatment weighting; PE, pulmonary embolism; VTE, venous thromboembolism.
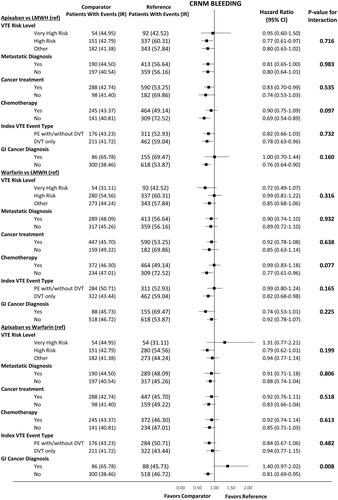
Appendix Table 1. Outcome definitions and codes.
Appendix Table 2. Pre-IPTW baseline characteristics of VTE cancer patients prescribed apixaban, LMWH, or warfarin.
Appendix Table 3. Post-IPTW baseline characteristics of VTE cancer patients prescribed apixaban, LMWH, or warfarin stratified by VTE risk level.
Appendix Table 4. Post-IPTW baseline characteristics of VTE cancer patients prescribed apixaban, LMWH, or warfarin stratified by cancer metastasis.
Appendix Table 5. Post-IPTW baseline characteristics of VTE cancer patients prescribed apixaban, LMWH, or warfarin stratified by cancer related treatment.
Appendix Table 6. Post-IPTW baseline characteristics of VTE cancer patients prescribed apixaban, LMWH, or warfarin stratified by chemotherapy (Yes/No).
Appendix Table 7. Post-IPTW baseline characteristics of VTE cancer patients prescribed apixaban, LMWH, or warfarin stratified by index DVT/PE diagnosis.
Appendix Table 8. Post-IPTW baseline characteristics of VTE cancer patients prescribed apixaban, LMWH, or warfarin stratified by GI Cancer/No GI Cancer.
