Abstract
Objective
Monitoring established Crohn’s disease (CD) through a “treat-to-target” strategy aims to reduce and prevent long-term bowel damage and disability. Despite the availability of different monitoring techniques, there is a current lack of integrated evidence to guide optimal monitoring in terms of appropriate tools and timing. Pan-intestinal video capsule endoscopy (PCE) enables non-invasive and direct visualization of the entire intestinal tract with proven safety and efficacy. This study aims to generate insights on the value of PCE for monitoring established CD from the physician’s perspective.
Methods
The Nominal Group Technique (NGT) was used to create discussion around pre-defined research questions aimed at identifying target patient populations for PCE, benefits of PCE in terms of improving disease management, comparative benefits of PCE over standard of care, research priorities to ratify the use of PCE, and hurdles to PCE utilization. A NGT panel was held in Brussels, Belgium in October 2018 with 9 gastroenterology experts. Data were collected from multiple rankings of statements to the research questions and analyzed descriptively.
Results
Consensus indicated that PCE is differentiated from other diagnostic tools, allowing for non-invasive and direct visualization of the luminal intestinal tract in one single procedure. Participants agreed that PCE is beneficial for mapping and grading established CD in all patients, enabling individual and tailored treatment decision-making. Time required to read PCE results was identified as the main utilization hurdle by participants. Well-designed studies are needed to confirm improved outcomes amongst patients with CD managed through a PCE-guided approach.
Conclusions
This study, using the NGT, generated expert opinion on the value of PCE for monitoring established CD in terms of target patient populations and benefits compared to other diagnostic modalities. Participants perceived PCE to facilitate a “treat-to-target” strategy for CD management. Further research is needed to support this value perception.
Introduction
Crohn’s disease (CD) is a debilitating chronic inflammatory disease that can affect the entire intestinal tract. Small bowel involvement is commonly observed (75%)Citation1,Citation2, where 30% of patients have exclusive small bowel diseaseCitation3. In Europe, approximately two million people are affected by CD and up to 70% require surgical intervention during their clinical historyCitation4,Citation5. Monitoring plays an important role in the management of established CD and preventing surgery through the identification of patients at risk of relapse and/or complications before the onset of clinical symptomsCitation6,Citation7 and the assessment of adequate response to treatmentCitation8. Follow up on these indicators furthermore guides treatment decision-makingCitation6,Citation9. Established CD is monitored by means of symptom assessment, clinical assessment, measurement of biomarkers, endoscopic assessment, and cross-sectional imaging. However, there is a current lack of integrated evidence to guide optimal monitoring in terms of appropriate tools and timingCitation10,Citation11.
The emergence of a “treat-to-target” strategy in CD managementCitation7,Citation8,Citation12–14 has had a significant impact on disease monitoringCitation15. Increasing emphasis has been placed on monitoring for endoscopic recurrence, known to precede and predict changes in inflammatory biomarkersCitation7,Citation12,Citation14,Citation16 and the development of clinical symptomsCitation17,Citation18. The gold standard for evaluating endoscopic remission is an ileocolonoscopyCitation19, an invasive technique restricted to the visualization of the colon and terminal ileum. The efficacy of measuring C-reactive protein (CRP) and fecal calprotectin (FCP), as surrogate markers of endoscopic remission, in monitoring established CD and guiding treatment has been demonstrated in the CALM studyCitation20. However, their efficacy to assess endoscopic remission is suboptimalCitation18. Approximately 30% of patients with CD do not present with elevated CRP levels during relapseCitation21,Citation22 and the correlation of FCP with active small bowel disease is poorCitation23–26.
Pan-intestinal video capsule endoscopy (PCE) offers a non-invasive method for the visualization of the entire intestinal tract, including the proximal small bowel. The adoption of PCE for monitoring patients with established CD is supported by its proven safety and efficacyCitation27, with a reported diagnostic yield as high as 85.7%Citation28. In addition, PCE can have a positive impact on disease management and clinical outcomes; change in therapy has been observed in 64% of patients at 18 month follow-up after PCECitation28. This finding was more recently confirmed in a prospective study amongst pediatric patients, where a PCE-based treat-to-target approach led to a change in therapy for 71% and 23% of patients at baseline and 24 weeks, respectively. Assessment with PCE at 24- and 52-week follow-up demonstrated a significant increase in the proportion of patients with endoscopic remission and deep remission compared to baseline (54% and 58% versus 21%, respectively)Citation29.
To date, evidence on PCE has mostly been generated using small bowel capsule endoscopy, and to a certain degree, colon capsule endoscopyCitation30,Citation31. However, the new PillCamFootnotei Crohn’s system offers a non-invasive option to visualize the small bowel and colonic mucosa in a single procedure. However, it is unclear how clinicians currently value the PillCam Crohn’s system relative to other techniques in the management of CD patients.
The aim of this study was to generate insights on the value of PCE for monitoring established CD from the physician’s perspective. Specifically, the Nominal Group Technique (NGT) was chosen as a small group consensus method to facilitate group discussion and prioritization of ideas with a panel of field expert participantsCitation32. Additional objectives were to identify target patient populations for PCE, benefits of PCE (in terms of improving disease management and in comparison to current standard of care), research priorities to ratify the use of PCE, and hurdles to PCE utilization, which can potentially be addressed by the PillCam Crohn’s system.
Methods
The NGT, developed by Delbecq and Van de Ven, is a consensus method that follows a systematic and democratic process that requires face-to-face group interactionCitation32. The methodology is flexible, whereby the process may vary due to time limitations or due to the level of clarification and/or consensus achievedCitation33. The NGT is recommended for groups of up to ten participantsCitation34, although participant numbers of up to fourteen have been reportedCitation33. Advantages of the NGT over other methods are that it facilitates equal participation and limits dominant participants from influencing the discussionCitation33. Furthermore, the NGT is a time-efficient research method, as results can be obtained through a single-occasion meetingCitation32,Citation33,Citation35. The NGT has been applied in numerous healthcare research settings for the prioritization of treatment outcomes and unmet needsCitation36–39 as well as the establishment of research prioritiesCitation40.
Participants
Participants for the NGT panel were clinicians identified through peer consultation and consideration of contributing authors and reviewers of the ECCO Guidelines on Crohn’s disease management. Identified clinicians were invited to participate in the panel through an introductory email detailing the study rationale and objectives. The research team shared full details of the study, including the proposed panel date, project goals, and research questions, to potential participants that expressed interest and answered any questions. Considering the consultation process involved clinicians, the study was not subject to review by an ethics committee. All participants provided consent for study participation.
Procedure
Prior to the panel, four research questions were defined based on established evidence on PCE in the context of monitoring established CD. On the day of the panel, the participants developed an additional research question to address strategic gaps for PCE adoption and utilization. An overview of the NGT panel research questions is presented in .
Table 1. Nominal group technique panel research questions.
The NGT panel was held in Brussels, Belgium in October 2018. The NGT panel consisted of a one-day meeting, organized into five rounds (one for each research question) and following the key stages of the NGT process. Prior to the panel, participants were asked to complete a questionnaire, consisting of 25 questions, to obtain information on participant characteristics as well as current practices and attitudes regarding monitoring of established CD. As a modification to the original NGT method, a re-ranking process was applied, allowing participants to revise their original ranking via a post-panel web survey.Citation33 In case of equal ranks, the statement with the highest frequency of votes was prioritized. An overview of the study procedure and key stages of the NGT process is shown in .
Results
Participants
Nine participants took part in the current study. provides an overview of participants’ characteristics, including country of representation, specialization, work setting, years of working experience, and number of patients treated.
Table 2. Participant characteristics.
Pre-panel questionnaire
Key takeaways
Monitoring of established Crohn’s disease, beyond just clinical symptoms, is perceived to be required in all post-surgical patients, in approximately 90% of symptomatic patients, and in approximately 65% of asymptomatic patients.
Video capsule endoscopy is considered most beneficial for monitoring post-surgical, mildly symptomatic, and asymptomatic patients.
Pre-panel questionnaire results
Eight out of the nine participants completed the pre-panel questionnaire on current practices and attitudes regarding monitoring of established CD beyond clinical symptoms. On average, respondents reported that 85% of patients with established CD require monitoring. More specifically, all respondents reported that monitoring is required in post-surgical patients, seven respondents reported that monitoring is required in severe symptomatic patients and mild symptomatic patients, and five respondents reported that monitoring is required in asymptomatic patients. Considering perceived benefit and weighted average scores for different monitoring techniques, including biomarkers measurement, endoscopic monitoring, monitoring with radiographic/ultrasound imaging techniques, and video capsule endoscopy (CE), the most beneficial monitoring technique for post-surgical, mild symptomatic, and asymptomatic patients was perceived to be CE (Small bowel CE or Pan-intestinal CE). For severe symptomatic patients, endoscopic monitoring and monitoring with radiographic/ultrasound imaging techniques was perceived to be more beneficial than CE. Levels of perceived benefit and weighted averages, by monitoring technique and patient sub-population, can be found in Supplementary Table 1. In total, six out of eight respondents stated that they recommend and/or utilize CE for monitoring established CD in their daily practice.
Nominal group technique panel results
Key takeaways
PCE is differentiated from other diagnostic tools as a non-invasive method allowing for direct visualization of the luminal intestinal tract in one single procedure.
PCE is considered beneficial for mapping and grading established Crohn’s disease in all patients without a suspected bowel stenosis, enabling individual and tailored treatment decision-making.
Well-designed studies are needed to confirm improved outcomes amongst patients with Crohn’s disease managed through a PCE-guided approach.
Figure 2. First ranked target patient populations for monitoring established Crohn’s disease using pan-intestinal video capsule endoscopy.

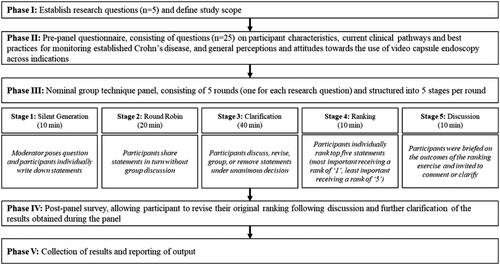
A total of 10 statements were generated for the second research question. presents an overview of statements that were ranked 1st (and number of votes per statement) by the participants on how monitoring of established CD using PCE can improve disease management.
Figure 3. First ranked statements on how monitoring of established Crohn’s disease using pan-intestinal video capsule endoscopy can improve disease management.
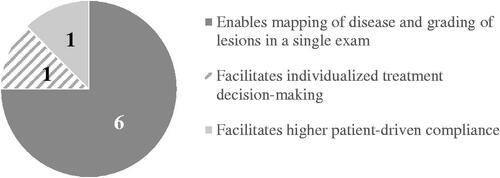
A total of 13 statements were generated for the third research question. provides an overview of statements that were ranked 1st (and number of votes per statement) by the participants on the benefit of using PCE over current monitoring practices/standard of care.
Figure 4. First ranked statements on the benefit of using pan-intestinal video capsule endoscopy over current monitoring practices/standard of care.
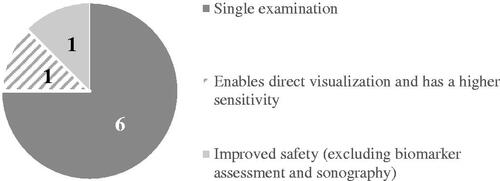
A total of 10 statements were generated for the fourth research question. provides an overview of statements that were ranked 1st (and number of votes per statement) by the participants on research priorities to ratify PCE for monitoring established CD.
Figure 5. First ranked statements on research priorities to ratify pan-intestinal video capsule endoscopy for monitoring established Crohn’s disease.
A total of 12 statements were generated for the fifth research question. provides an overview of statements that were ranked 1st (and number of votes per statement) by the participants on hurdles to use video capsule endoscopy for monitoring established CD.
Figure 6. First ranked statements on hurdles to use video capsule endoscopy for monitoring established Crohn’s disease.
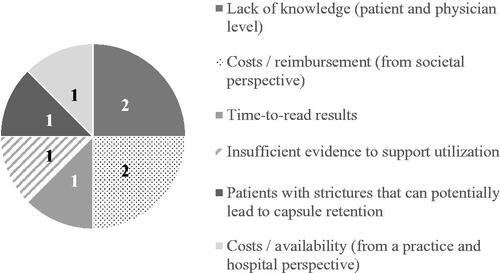
A complete overview of rankings and total scores, by research question, can be found in Supplementary Table 2–6. Selected top ranked statements (based on total scores) are discussed further below.
Comparison of results
Results from the pre-panel questionnaire and the NGT panel, although varying in objectives and scope, demonstrated that participants consistently perceived PCE as beneficial for post-surgical and asymptomatic patients. While perceived as the most beneficial monitoring technique for post-surgical and asymptomatic patients through the pre-panel questionnaire, participants further specified during the NGT panel that PCE justifies treatment change amongst these target populations. No inconsistencies were observed between the results.
Discussion
Undertaking research is important for understanding the potential clinical utility of innovative health technologies, identifying evidence gaps, and establishing research priorities. Through a NGT process, a group of clinicians explored the value of the PillCam Crohn’s system considering target patient populations for monitoring and the perceived benefits of PCE. In order to optimize the allocation of limited resources, research priorities for monitoring established CD with PCE were established and main hurdles for utilization identified.
Target patient populations
Through this study, clinicians agreed that patients with established CD and involvement of the colon and small bowel would benefit from monitoring with PCE considering that the disease can be mapped and monitored in a single examination with complete visualization of the entire luminal intestinal tract. The next group of patients that could benefit from monitoring with PCE would be any patient with established CD to justify treatment change or not, specifically for post-surgical and asymptomatic patients and following treatment induction or treatment de-escalation. The third most important target population for monitoring with PCE are pediatric patients considering that the procedure is non-invasive, has fewer complications, and does not require anesthesia.
Video capsule endoscopy and disease management
On the topic of monitoring established CD with PCE and its subsequent impact on disease management, clinicians reached relative consensus on the most important and second most important factor improving disease management, namely: ability of PCE to map disease and grade lesion severity in one single examination as the most important factor and the fact that PCE enables more frequent monitoring as the second most important factor.
However, it was more difficult for the clinicians to agree on the third most important advantage of PCE in disease management. They cited examples ranging from facilitation of patient driven compliance to the enablement of task delegation and decrease in inter-observer variability (due to storage of exams).
Comparative benefits of video capsule endoscopy
Video capsule endoscopy has the potential to address many limitations of other diagnostic modalities. Clinicians perceived the ability to perform PCE in a single procedure as the most important comparative benefit. The second most important comparative benefit of PCE was considered its ability to visualize the small bowel and colonic mucosa directly and with higher sensitivity. Compared to PCE, magnetic resonance enterography (MRE) presents with comparable diagnostic accuracy for small bowel disease and enables visualization of transmural and extramural abnormalitiesCitation41. However, MRE presents with a lower accuracy for mucosal inflammationCitation42, where its ability to monitor response to therapy has yet to be determinedCitation43. Finally, the available magnetic resonance index of activity (MaRIA) has only been validated on the terminal ileum and colonic segmentsCitation44.
Although biomarker measurement offers an initial evaluation of disease activity and may guide treatment decision-making independent of disease locationCitation20, its value as a surrogate maker for endoscopic remission is debatable. Various studies have demonstrated only a moderate correlation between FCP and a poor correlation between CRP and small bowel inflammationCitation18,Citation24,Citation25. One study demonstrated that both FCP and CRP had low negative predictive values for small bowel inflammation (LS ≥ 135) (24.1% and 20.5%, respectively), where PCE observed mucosal inflammation amongst patients with normal biomarker levelsCitation18. The third most important perceived comparative benefit was that PCE does not require anesthesia. Whereas the gold standard for evaluating endoscopic remission is ileocolonoscopy, the procedure is invasive in nature, requiring sedation or general anesthesia. Ileocolonoscopy as a monitoring technique is further limited by its maximal extent, inability to access the proximal small bowel, and association with perforation risk in patients with active disease.
Research priorities to ratify the use of video capsule endoscopy
More broadly, it would be beneficial to establish superiority of PCE over standard of care considering secondary endpoints such as health-related quality of life and cost-effectiveness. Superior tolerability of PCE over ileocolonoscopy has previously been demonstratedCitation45, suggesting improved treatment satisfaction and quality of life outcomes. In a more recent study, the cost and patient benefits of PCE compared to standard of care (ileocolonoscopy plus imaging) for monitoring established CD were assessed through a decision-analytic model from a US healthcare payer perspective. The results showed that compared to standard of care, monitoring established CD with PCE was associated with increased quality of life, with the highest gain observed amongst active symptomatic patients. Furthermore, PCE was considered cost-effective, with total savings over 5 years amounting to $36.5 millionCitation46. Similarly, CD monitoring with PCE was found to reduce costs and increase quality of life for patients from a UK payer perspective, and as such, the incremental cost effectiveness ratio was dominant over colonoscopyCitation47.
Hurdles to video capsule endoscopy utilization
Participants reported numerous hurdles to PCE utilization. Most importantly, time required to read PCE results was considered a major limitation. Reading PCE images reliably and quickly remains challenging, leading to missed lesions and interpersonal variability in the interpretation of results. Over the past years, various software applications have been developed with the aim to reduce reading time through automated selection and interpretation of images to diagnose CD (Quickview, top 100 images, Atlas). While these programs support the diagnostic ability of PCE, conventional reading is still requiredCitation48.
In addition, the use of artificial intelligence (AI) in medicine is rapidly progressing. As reported in a recent review of AI applications in gastroenterology, various models have been studied in the context of inflammatory lesions or gastrointestinal bleeding during wireless CE, demonstrating a high level of accuracy for disease detectionCitation49. Beyond the CD space, the recently introduced GI GeniusFootnoteii, intelligent endoscopy module (based on AI) offers real-time automatic detection of colorectal polyps of different shapes, sizes, and morphology with a noninvasive, patient-friendly deviceCitation50. This might represent a remarkable step forward in reducing the reading timeCitation51. The efficacy of such technologies in IBD remains to be proven.
Participants also noted that the required bowel preparation is a major hurdle for the adoption of PCE in terms of adverse events and uncertainties in bowel preparation optimization. Nonetheless, evidence shows that up to 80% achieve excellent or good bowel preparation with completed evaluation in 92% of patients within 12 hCitation52.
Lastly, risk of capsule retention in patients with strictures was noted as an important hurdle to PCE utilization. However, a recent meta-analysis on retention associated with PCE reported retention rates as low as 4.63% in established CD and 2.35% in suspected CD. For the overall CD cohort, retention rates of 3.49% and 1.64% have been reported for adult and pediatric patient populations, respectively. Furthermore, retention risk can be reduced through the use of the patency capsuleCitation53.
Limitations
A limitation of the panel was the relatively small group size of 9 experts, of which 6 participants indicated that they recommend and/or utilize CE for monitoring established CD in their daily practice. Therefore, the findings of this study cannot be generalized. In particular, participants may have been biased towards their own experience with PCE, personal agendas, and research priorities when ranking generated statements by their relative importance. As a result, commonly acknowledged barriers to PCE use such as lack of PCE training or experience in PCE, were not ranked of high importance. A larger group of participants, including experts that do not (regularly) utilize PCE, would have likely led to the generation of different statements and perceived importance of those. Nonetheless, perceptions on the value of PCE for monitoring established CD varied amongst the participants, which increased the quality of group discussions.
Second, our study focused solely on the use of PCE for monitoring established CD. As a result, benefits of other diagnostic tools in terms of disease diagnosis, such as the ability to perform a biopsy during ileocolonoscopy, remained out of scope.
In addition, insights were gathered from clinicians only and did not consider the perspectives of patients nor other relevant stakeholders including radiologists and pathologists. These limitations are particularly relevant with respect to establishing research priorities to support the use of PCE for monitoring established CD and to identifying strategies to address utilization hurdles considering the need for multi-level and multi-stakeholder engagement. Therefore, the results reported in this paper should be considered for further discussion and validation with other stakeholder groups. Despite these limitations, the NGT process is a valid method to systematically identify and prioritize ideas behind PCE for monitoring established CD.
Conclusions
In conclusion, participants had clear opinions on the value of PCE for monitoring established CD in terms of target patient populations and benefits compared to other diagnostic modalities. We believe the NGT is an efficient method to uncover the positioning of the PillCam Crohn’s system in the context of a “treat-to-target” strategy for CD management and to prioritize efforts in further research needs. Expert consensus indicated that more evidence should be generated to increase adoption and utilization of PCE for monitoring of established CD patients. Future studies should focus on comparing the PCE-guided approach to standard of care for all patients with established CD and involvement of both the colon and small bowel and should consider clinical, patient-reported, and economic outcomes. Future meetings with other experts might be considered after obtaining new evidence on this topic.
Transparency
Declaration of funding
The authors disclosed receipt of financial support from Medtronic to participate in the panel and surveys. The views expressed in this publication are those of the authors and not necessarily those of Medtronic.
Declaration of financial/other relationships
FC received honoraria from Amgen, BMS, Celltrion, Enterome, Ferring, Janssen, Medtronic, Pfizer, Pharmacosmos, Roche and Tillotts as well as lecture fees from Abbvie, Astra, BMS, Ferring, Janssen, MSD, Pfizer, Pileje, Takeda and Tillotts; CC reports consulting fees from Medtronic; MF reports financial support for research from Amgen, Biogen, Janssen, Pfizer, and Takeda, consulting fees from Abbvie, Boehringer-Ingelheim, Celltrion, Ferring, Janssen, Lilly, Medtronic, MSD, Pfizer, Sandoz, Takeda, and Thermo Fisher, and speaker fees from Abbvie, Amgen, Biogen, Boehringer-Ingelheim, Chiesi, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, Sandoz, Takeda, and Truvion Healthcare; AL reports consulting fees or speaker fees from Vifor Pharma, Shield Therapeutics, Abbvie, Janssen, Takeda, MSD, and Medtronic. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
NVL and AV contributed to the conception and design of the study and data analysis. CC and SO contributed to the interpretation of data and drafting the article. All authors contributed equally to article revision and final approval of the version to be published.
Supplemental Material
Download MS Word (57.2 KB)Acknowledgements
The research team would like to thank Monitor Deloitte Belgium, who helped to organize and run the panel, and all stakeholders who participated in the panel, including those who agreed to be named here: Franck Carbonnel, Marc Ferrante, Torben Knudsen, Alan J. Lobo, Lucian Negreanu, Salvatore Oliva, and Cristina Carretero.
Data availability statement
The data underlying this article are available in the article and in its supplementary material.
Notes
i PillCam is a trademark of Medtronic, Minneapolis, MN, USA.
ii GI Genius is a trademark of Medtronic, Minneapolis, MN, USA.
References
- Burisch J, Pedersen N, Čuković-Čavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63(4):588–597.
- Dubcenco E, Jeejeebhoy KN, Petroniene R, et al. Capsule endoscopy findings in patients with established and suspected small-bowel Crohn’s disease: correlation with radiologic, endoscopic, and histologic findings. Gastrointest Endosc. 2005;62(4):538–544.
- Cosnes J, Gower–Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794.e4.
- Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7(4):322–337.
- Lewis RT, Maron DJ. Efficacy and complications of surgery for Crohn’s disease. Gastroenterol Hepatol. 2010;6(9):587–596. http://www.ncbi.nlm.nih.gov/pubmed/21088749. Accessed December 20, 2018.
- Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11246–11259.
- Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s Disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3–25.
- Sauter B, Beglinger C, Girardin M, et al. Monitoring disease activity and progression in Crohn’s disease: a Swiss perspective on the IBD ahead ‘optimised monitoring’ recommendations. Digestion. 2014;89(4):299–309.
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434.
- Papay P, Ignjatovic A, Karmiris K, et al. Optimising monitoring in the management of Crohn’s disease: a physician’s perspective. J Crohns Colitis. 2013;7(8):653–669.
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–164.
- Orlando A, Guglielmi FW, Cottone M, et al. Clinical implications of mucosal healing in the management of patients with inflammatory bowel disease. Dig Liver Dis. 2013;45(12):986–991.
- Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47(4):352–386.
- Ooi CJ, Makharia GK, Hilmi I, et al. Asia pacific consensus statements on Crohn’s disease. Part 1: definition, diagnosis, and epidemiology: (Asia Pacific Crohn’s Disease Consensus-Part 1). J Gastroenterol Hepatol. 2016;31(1):45–55.
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338.
- Reinink AR, Lee TC, Higgins PDR. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta-analysis. Inflamm Bowel Dis. 2016;22(8):1859–1869.
- Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7(12):982–1018.
- Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small Bowel Crohn’s Disease using biomarkers, capsule endoscopy and imaging. Am J Gastroenterol. 2015;110(9):1316–1323.
- Sandborn WJ. The future of inflammatory bowel disease therapy: where do we go from here? Dig Dis. 2012;30(s3):140–144.
- Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–2789.
- Kopylov U, Rosenfeld G, Bressler B, et al. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(4):742–756.
- Burri E, Beglinger C, Lehmann FS. Monitoring of therapy for inflammatory Bowel Disease. Digestion. 2012;86(s1):1–5.
- Laharie D, Mesli S, El Hajbi F, et al. Prediction of Crohn’s disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. 2011;34(4):462–469.
- Koulaouzidis A, Douglas S, Rogers MA, et al. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011;46(5):561–566.
- Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54(3):364–368.
- Costa F, Mumolo MG, Bellini M, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35(9):642–647. http://www.ncbi.nlm.nih.gov/pubmed/14563186. Accessed December 20, 2018.
- Park S-K, Ye BD, Kim KO, et al. Guidelines for video capsule endoscopy: emphasis on Crohn’s disease. Clin Endosc. 2015;48(2):128–135.
- Lorenzo-Zúñiga V, de Vega VM, Domènech E, et al. Impact of capsule endoscopy findings in the management of Crohn’s Disease. Dig Dis Sci. 2010;55(2):411–414.
- Oliva S, Aloi M, Viola F, et al. A treat to target strategy using panenteric capsule endoscopy in pediatric patients with Crohn’s Disease. Clin Gastroenterol Hepatol. 2019;17(10):2060–2067.e1.
- Negreanu L, Smarandache G, Mateescu RB. Role of capsule endoscopy Pillcam COLON 2 in patients with known or suspected Crohn's disease who refused colonoscopy or underwent incomplete colonoscopic exam: a case series. Tech Coloproctol. 2014;18(3):277–283.
- Le Berre C, Trang-Poisson C, Bourreille A. Small bowel capsule endoscopy and treat-to-target in Crohn’s disease: a systematic review. World J Gastroenterol. 2019;25(31):4534–4554.
- Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38(3):655–662.
- Cantrill JA, Sibbald B, Buetow S. The Delphi and nominal group techniques in health services research. Int J Pharm Pract. 2011;4(2):67–74.
- Harvey N, Holmes CA. Nominal group technique: an effective method for obtaining group consensus. Int J Nurs Pract. 2012;18(2):188–194.
- Sanderson T, Hewlett S, Richards P, et al. Utilizing qualitative data from nominal groups: exploring the influences on treatment outcome prioritization with rheumatoid arthritis patients. J Health Psychol. 2012;17(1):132–142.
- Miller D, Shewchuk R, Elliot TR, et al. Nominal group technique: a process for identifying diabetes self-care issues among patients and caregivers. Diabetes Educ. 2000;26(2):305–314.
- Dewar A, White M, Posade ST, et al. Using nominal group technique to assess chronic pain, patients’ perceived challenges and needs in a community health region. Health Expect. 2003;6(1):44–52. http://www.ncbi.nlm.nih.gov/pubmed/12603627.
- Drennan V, Walters K, Lenihan P, et al. Priorities in identifying unmet need in older people attending general practice: a nominal group technique study. Fam Pract. 2007;24(5):454–460.
- Vella K, Goldfrad C, Rowan K, et al. Use of consensus development to establish national research priorities in critical care. BMJ. 2000;320(7240):976–980. http://www.ncbi.nlm.nih.gov/pubmed/10753149. Accessed December 4, 2018.
- Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105(6):1240–1248.
- Amitai MM, Ben-Horin S, Eliakim R, et al. Magnetic resonance enterography in Crohn’s disease: a guide to common imaging manifestations for the IBD physician. J Crohns Colitis. 2013;7(8):603–615.
- Rozendorn N, Amitai MM, Eliakim RA, et al. A review of magnetic resonance enterography-based indices for quantification of Crohn’s disease inflammation. Therap Adv Gastroenterol. 2018;11:1756284818765956.
- Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn’s. Disease. Gastroenterology. 2019;157(2):432–439.e1.
- Oliva S, Cucchiara S, Civitelli F, et al. Colon capsule endoscopy compared with other modalities in the evaluation of pediatric Crohn's disease of the small bowel and colon. Gastrointest Endosc. 2016;83(5):975–983.
- Saunders R, Torres RT, Konsinski L. Evaluating the clinical and economic consequences of using video capsule endoscopy to monitor Crohn’s disease. Clin Exp Gastroenterol. 2019;12:375–384.
- Lobo A, Torrejon Torres R, McAlindon M, et al. Economic analysis of the adoption of capsule endoscopy within the British NHS. Int J Qual Health Care. 2020;32(5):332–341.
- Kim SH, Yang DH, Kim JS. Current status of interpretation of small bowel capsule endoscopy. Clin Endosc. 2018;51(4):329–333.
- Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76–94.e2.
- Hassan C, Wallace MB, Sharma P, et al. New artificial intelligence system: first validation study versus experienced endoscopists for colorectal polyp detection. Gut. 2020;69(5):799–800.
- Klang E, Barash Y, Margalit RY, et al. Deep learning algorithms for automated detection of Crohn's disease ulcers by video capsule endoscopy. Gastrointest Endosc. 2020;91(3):606–613.e2.
- Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015;148(5):948–957.e2.
- Pasha SF, Pennazio M, Rondonotti E, et al. Capsule retention in Crohn’s Disease: a meta-analysis. Inflamm Bowel Dis. 2020;26(1):33–42.