Abstract
Objective
In CAPTAIN, a double-blind, parallel-group, Phase IIIA study, fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) improved lung function, symptoms and asthma control versus FF/VI in patients with inadequately controlled asthma. Here, we report efficacy and safety from a Japanese cohort in CAPTAIN.
Methods
Adults with inadequately controlled asthma despite inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) were randomized (1:1:1:1:1:1) to once-daily FF/VI (100/25 mcg or 200/25 mcg) or FF/UMEC/VI (100/31.25/25 mcg, 100/62.5/25 mcg, 200/31.25/25 mcg, or 200/62.5/25 mcg) for ≥24 weeks. Endpoints included change from baseline in clinic trough FEV1 (primary), annualized rate of moderate/severe asthma exacerbations (key secondary), clinic FEV1 3 h post-dose, and Asthma Control Questionnaire (ACQ)-7, St George’s Respiratory Questionnaire (SGRQ) (all Week 24), Evaluating Respiratory Symptoms (E-RS): Asthma total scores (Weeks 21–24) (all secondary). Adverse events and adverse events of special interest were monitored. Clinical trials.gov registry no: NCT02924688.
Results
Overall, 229 of 2436 patients in the intention-to-treat (ITT) population were from Japan. In this cohort, change from baseline in trough FEV1 for FF/UMEC/VI 100/62.5/25 mcg versus FF/VI 100/25 mcg was 105 mL (95% confidence interval −5, 216) and 69 mL (–42, 179) for 200/62.5/25 mcg versus 200/25 mcg. These observations were supported by clinic FEV1 at 3 h post-dose. Moderate/severe exacerbation incidence was low and similar across pooled treatment groups (FF/VI, FF/UMEC 31.25 mcg/VI, FF/UMEC 62.5 mcg/VI). All pooled groups demonstrated clinically important improvements from baseline in ACQ-7, SGRQ and E-RS: Asthma total scores. Safety profiles were consistent with the overall ITT population, with no new safety concerns.
Conclusion
FF/UMEC/VI is an effective option with a favorable risk-benefit profile in Japanese patients with uncontrolled moderate or severe asthma on ICS/LABA.
Introduction
Asthma is a chronic respiratory condition, characterized by reversible airflow limitation, airway hyperresponsiveness, and airway inflammationCitation1. It is estimated that 358 million individuals worldwide are affected by asthmaCitation2, with up to 18% of the global population having the conditionCitation1. In Japan, the prevalence of asthma in adults is reported at between 6% and 10%Citation3.
The Global Initiative for Asthma (GINA) report recommends a step-wise approach to asthma management, with inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA) combination therapy the preferred treatment for patients with asthma who are inadequately controlled with ICS aloneCitation1. However, approximately 30–50% of patients with moderate/severe asthma remain uncontrolled, despite adherence to ICS/LABA therapyCitation4–7. In Japan, an observational study of patients with asthma managed in specialist settings showed that 65% of patients had either partly controlled or uncontrolled asthmaCitation8. Indeed, patients with severe, uncontrolled asthma in Japan more frequently require oral corticosteroids and have higher rates of hospitalization and medical costs than those with severe controlled asthma or mild-to-moderate asthma, highlighting a clear need for more effective treatmentsCitation9.
The addition of tiotropium (a long-acting muscarinic antagonist; LAMA) benefits patients whose asthma remains uncontrolled on ICS/LABA therapyCitation10,Citation11, and is recommended as an add-on therapy by both GINA and the Japanese Society of AllergologyCitation1,Citation3. The efficacy and safety of the once-daily single inhaler ICS/LAMA/LABA combination of fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) in comparison with FF/VI in patients with asthma inadequately controlled on ICS/LABA was evaluated in the recently published Phase IIIA Clinical Study in Asthma Patients Receiving Triple Therapy in a Single Inhaler (CAPTAIN)Citation12. Results in the overall study population showed improved lung function, improved symptoms and asthma control, and numerical reductions in the annualized rate of moderate/severe exacerbations with FF/UMEC/VI versus FF/VI, with no new or unexpected safety findingsCitation12. CAPTAIN was a multinational, multicenter study, allowing for important evaluations into the consistency of outcomes in distinct ethnically diverse populations, an important consideration given the potential for altered pharmacokinetics in patients of differing ethnicitiesCitation13. Here, we report the efficacy and safety of FF/UMEC/VI versus FF/VI in the Japanese cohort of patients included in the CAPTAIN study.
Methods
Study design
CAPTAIN was a Phase IIIA, multinational, multicenter, randomized, double-blind, active-controlled, 24–52-week, parallel-group study conducted in 322 centers across 15 countries between October 2016 and February 2019 (GSK ID: 205715; clinical trials.gov registry number: NCT02924688). Sixty-three Japanese centers participated in the study. The study design has been described in detail previouslyCitation12. In brief, the study consisted of a 3-week run-in period, during which pre-study ICS/LABA therapy was replaced with open-label fluticasone propionate (FP)/salmeterol 250/50 mcg combination therapy administered via the DISKUS dry-powder inhaler (DPI) (GSK, Research Triangle NC, USA) twice daily, followed by a 2-week stabilization period during which run-in treatment was replaced with open-label FF/VI 100/25 mcg administered via the ELLIPTA DPI (Glaxo Operations UK, Hertfordshire, UK) once daily in the morning (Supplementary Figure 1). Patients were then randomized (1:1:1:1:1:1) to receive double-blind treatment with FF/VI 100/25 mcg, FF/UMEC/VI 100/31.25/25 mcg, FF/UMEC/VI 100/62.5/25 mcg, FF/VI 200/25 mcg, FF/UMEC/VI 200/31.25/25 mcg, or FF/UMEC/VI 200/62.5/25 mcg via the ELLIPTA DPI once daily in the morning. Randomization was stratified across each of the six treatment groups by pre-study ICS dose at study entry (medium [>250–500 mcg FP daily or equivalent] or high [>500 mcg FP daily or equivalent]). The study had a variable treatment period, with all patients completing a minimum of 24 weeks of treatment after randomization, and patients enrolled earlier during the recruitment period continuing to 36 weeks or a maximum of 52 weeks of treatment. Patients who prematurely discontinued study treatment were encouraged to continue to participate in the study and attend all remaining clinic visits. The data for the remaining visits were recorded as post-treatment.
The study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice guidelines and applicable country-specific regulatory requirements. The protocol was reviewed and approved by an internal GSK review board and received approval from central or local institutional review boards or independent ethics committees. All patients provided written informed consent and patient anonymity was preserved using methods approved by the institutional review board.
Patients
Eligibility criteria have been described in detail previouslyCitation12. In summary, patients were ≥18 years of age with symptomatic, inadequately controlled asthma despite maintenance therapy with daily ICS/LABA for ≥12 consecutive weeks prior to screening, with no changes to therapy in the 6 weeks immediately prior to pre-screening, and documented healthcare contact or temporary changes in asthma therapy for acute asthma symptoms within 1 year prior to screening. Patients were required to have an Asthma Control Questionnaire (ACQ)-6 total score ≥1.5 (at both screening and enrollment), pre-bronchodilator morning forced expiratory volume in 1 s (FEV1) ≥30–<85% of the predicted normal value at screening, and an increase in FEV1 of ≥12% and ≥200mL 20–60 min following 4 salbutamol/albuterol inhalations at screening. Patients with chronic obstructive pulmonary disease or other respiratory disorders, including pneumonia risk factors, were excluded, as were current and former smokers with a smoking history of ≥10 pack years.
The intention-to-treat (ITT) population comprised all randomized patients except those randomized in error. The Japanese cohort was derived from the overall ITT population and included only patients of Asian-Japanese heritage or those of mixed race that included Asian-Japanese heritage who were resident in Japan at the time of the study.
Endpoints
Study endpoints have been described in detail previouslyCitation12. Change from baseline in clinic trough FEV1 at Week 24 was the primary endpoint. The annualized rate of moderate/severe asthma exacerbations throughout the study (Weeks 1–52) was assessed as the key secondary endpoint. Proportions of patients with moderate/severe asthma exacerbations are also reported. Moderate asthma exacerbations were defined as deteriorations in either asthma symptoms or lung function, or increased rescue bronchodilator use, requiring a physician-directed temporary change in maintenance treatment in order to prevent progression to a severe exacerbationCitation14,Citation15. Severe exacerbations were defined as an asthma deterioration requiring systemic corticosteroid use (or doubling of the current maintenance systemic corticosteroid dose) for ≥3 days, or an emergency department visit or hospitalization due to asthma requiring systemic corticosteroids. Exacerbations that occurred <7 days from the last exacerbation were treated as a continuation of the same exacerbation. Additional secondary endpoints included changes from baseline in clinic FEV1 at 3 h post-dose at Week 24, ACQ-7 total score at Week 24, St George’s Respiratory Questionnaire (SGRQ) total score at Week 24, and Evaluating Respiratory Symptoms (E-RS): Asthma total score over Weeks 21–24 (all secondary endpoints)Citation12. An analysis of the proportion of ACQ-7 total score responders, defined as a ≥ 0.5-point improvement (decrease) from baseline at Week 24, was also conducted (pre-specified for the overall ITT population, post hoc for the Japanese cohort). The minimal clinically important difference (MCID) was defined as 0.5 for ACQ-7 total score, 4.0 for SGRQ total score, and 2.0 for E-RS: Asthma total scoreCitation16–18.
For lung function endpoints, the two FF/VI dose groups were each compared with FF/UMEC/VI at two different UMEC doses (31.25 and 62.5 mcg) in order to measure the additional effect of UMEC: FF/UMEC/VI 100/31.25/25 mcg or 100/62.5/25 mcg versus FF/VI 100/25 mcg, and FF/UMEC/VI 200/31.25/25 mcg or 200/62.5/25 mcg versus FF/VI 200/25 mcg. For evaluation of non-lung function endpoints, it was pre-specified that data from the two FF/UMEC/VI arms for each fixed UMEC dose were pooled and compared with pooled data from the two FF/VI arms: FF/UMEC 31.25 mcg/VI versus FF/VI, and FF/UMEC 62.5 mcg/VI versus FF/VI.
Safety endpoints included the incidence of adverse events (AEs), serious AEs (SAEs) including deaths, and AEs of special interest (AESI).
Statistical analyses
Details of sample size calculations for the overall ITT population have been described previouslyCitation12. The primary endpoint (change from baseline in clinic trough FEV1 at Week 24) was analyzed using all on- and post-treatment data using mixed-model repeated measures with covariates of treatment group, sex, region (for the overall ITT population), visit, pre-study ICS dosage at screening, age, and baseline value, and interactions of baseline value by visit and treatment group by visit. The annualized rate of moderate/severe exacerbations was analyzed using all on- and post-treatment data by a generalized linear model assuming a negative binomial probability distribution with covariates of treatment group, sex, region (for the overall ITT population), pre-study ICS dosage at screening, age, and number of severe exacerbations in the previous year (0, 1, ≥2), and with logarithm of time (year) on study as an offset variable. Change from baseline in clinic FEV1 at 3 h post-dose at Week 24 was analyzed by analysis of covariance using on-treatment data only. Changes from baseline in ACQ-7, SGRQ and E-RS: Asthma total scores were analyzed in a similar manner to the primary endpoint, using all on- and post-treatment data. The ACQ-7 responder analysis was pre-specified for the overall ITT population and post hoc for the Japanese cohort, and was performed using all on- and post-treatment data using a generalized linear mixed model with a logit link function with covariates of treatment, age, sex, visit, pre-study ICS dosage at screening and baseline value, and interactions of baseline value by visit and treatment group by visit.
For the overall ITT population, a step-down closed-testing hierarchy was used to account for multiplicity across UMEC doses and efficacy endpoints. The hierarchy was broken as the key secondary endpoint was not met; all subsequent analyses were not adjusted for multiplicityCitation12. The Japanese cohort was not sufficiently powered to demonstrate statistical significance for any endpoint and no multiplicity adjustments were applied in this cohort.
Results
Study population
A total of 2436 patients were included in the ITT population, including 229 patients from Japanese centers (Supplementary Figure 2). Baseline demographics and clinical characteristics were generally similar between the Japanese cohort () and the overall ITT population (Supplementary Table 1), with some exceptions. The Japanese cohort included higher proportions of males and former smokers, with a lower average body mass index (BMI) and lower prevalence of cardiovascular risk factors. Additionally, proportionally more patients in the Japanese cohort had experienced ≥2 exacerbations in the prior 12 months and were receiving 3 or ≥4 maintenance therapies at study entry.
Table 1. Baseline demographics and clinical characteristics in the Japanese cohort, by treatment group.
Lung function
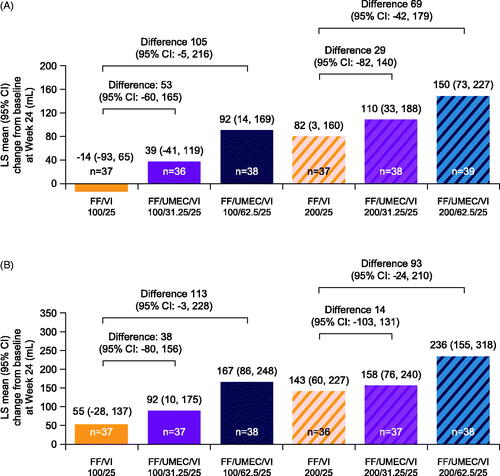
The addition of UMEC 31.25 mcg or 62.5 mcg to FF/VI resulted in greater improvements from baseline in clinic trough FEV1 at Week 24 versus FF/VI 100/25 mcg or FF/VI 200/25 mcg in both the Japanese cohort () and the overall ITT population (Supplementary Figure 3(A)). Similarly, improvements in clinic FEV1 at 3 h post-dose at Week 24 were greater in patients receiving FF/UMEC/VI 100/31.25/25 mcg or 100/62.5/25 mcg compared with those receiving FF/VI 100/25 mcg in both the Japanese cohort () and the overall population (Supplementary Figure 3(B)), with similar patterns seen when FF was given at a dose of 200 mcg. For both lung function endpoints, improvements associated with the addition of UMEC to FF/VI were dose related, with greater improvements seen with the 62.5 mcg dose than with the 31.25 mcg dose both in the Japanese cohort and in the ITT population. However, the magnitude of this difference was greater in the Japanese cohort compared with the ITT population.
Figure 1. LS mean change from baseline in clinic trough FEV1 (A) (on- and post-treatment) and clinic FEV1 at 3 h post-dose (B) (on-treatment) at Week 24 in the Japanese cohort (ITT population). All doses are mcg; baseline values were last value prior to randomization; n = patients with analyzable data at Week 24. Abbreviations. CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, fluticasone furoate; ITT, intention-to-treat; LS, least squares; UMEC, umeclidinium; VI, vilanterol.
Moderate/severe exacerbations
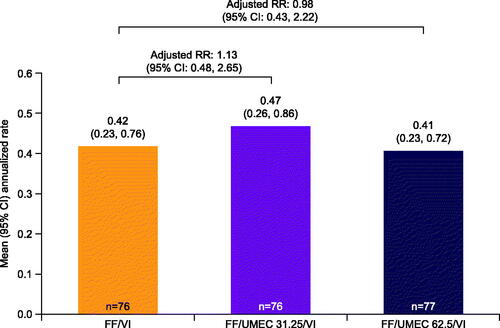
The overall incidence of moderate/severe exacerbations was low, and lower in the Japanese cohort (47 [21%] patients) than the overall population (688 [28%] patients). There were no clear differences in the annualized rate of moderate/severe exacerbations across the three pooled treatment groups (FF/VI, FF/UMEC 31.25 mcg/VI, and FF/UMEC 62.5 mcg/VI) in the Japanese cohort (), whereas the addition of UMEC 62.5 mcg led to a numerical decrease in the rate of moderate/severe exacerbations versus FF/VI in the pooled analysis of the overall ITT population (Supplementary Figure 4). As the step-down closed-testing hierarchy was broken here for the overall ITT population, all subsequent analyses of the ITT population were considered descriptive and were not adjusted for multiplicityCitation12. The median duration of moderate/severe exacerbations across treatment groups in the Japanese cohort (6–11 days) was generally similar to that of the overall population (9–10 days).
Figure 2. Annualized rate of moderate/severe exacerbations in the Japanese cohort ITT population (on- and post-treatment, Weeks 1–52; pooled analysis). All doses are mcg. n = patients with analyzable data. Abbreviations. CI, confidence interval; FF, fluticasone furoate; ITT, intention-to-treat; RR, rate ratio; UMEC, umeclidinium; VI, vilanterol.
Patient-reported outcomes
In the Japanese cohort, all three pooled treatment groups showed clinically meaningful improvements exceeding the MCID (0.5 points) in ACQ-7 total score at Week 24 compared with baseline (), in line with observations seen in the overall ITT population (Supplementary Figure 5(A)). The addition of UMEC 62.5 mcg to FF/VI was associated with numerical improvements from baseline in ACQ-7 total score versus FF/VI at Week 24 in the Japanese cohort (); however, in contrast to the ITT population (Supplementary Figure 5(A)), there was no improvement over FF/VI in ACQ-7 total score following the addition of UMEC 31.25 mcg in Japanese patients (Figure 5(A)).
Figure 3. LS mean change from baseline in ACQ-7 total score (A) and post hoc analysis of proportion of ACQ-7 responders (B) at Week 24 in the Japanese cohort (ITT population, on- and post-treatment, pooled analysis). All doses are mcg; n=patients with analyzable data at Week 24; athe MCID (-0.5 units) is indicated by the dashed horizontal lineCitation17; bresponder threshold is defined as an improvement (decrease) of ≥0.5 points from baseline; baseline values were taken at randomization. Abbreviations. ACQ, Asthma Control Questionnaire; CI, confidence interval; FF, fluticasone furoate; ITT, intention-to-treat; LS, least squares; MCID, minimal clinically important difference; UMEC, umeclidinium; VI, vilanterol.
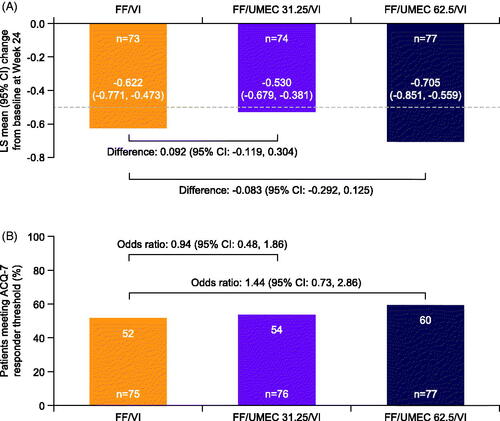
In both the Japanese cohort (post hoc analysis) and the overall ITT population (pre-specified analysis), addition of UMEC 62.5 mcg to FF/VI was associated with a numerical increase in the rate of ACQ-7 responders (defined as a change greater than or equal to 0.5, equivalent to the MCID) versus FF/VI (; Supplementary Figure 5(B)). However, consistent with the change from baseline in ACQ-7 total score, there was no clear change in the rate of ACQ-7 responders in the Japanese cohort following the addition of UMEC 31.25 mcg to FF/VI.
In the Japanese cohort, all study treatment groups achieved clinically meaningful improvements (≥4-point improvement, equivalent to the MCID) from baseline in SGRQ total score at Week 24, but with no clear differences between treatment groups, consistent with the overall ITT population (; Supplementary Figure 6(A)).
Figure 4. LS mean change from baseline in SGRQ total score at Week 24 (A) and E-RS: Asthma total score at Weeks 21–24 (B) in the Japanese cohort (ITT population, on- and post-treatment, pooled analysis). All doses are mcg; for SGRQ total score, baseline values were taken at randomization; for E-RS: Asthma total score, baseline was defined as the mean of E-RS: Asthma total scores over the last 14 days prior to the randomized treatment start; n = patients with analyzable data at Week 24 (SGRQ)/Weeks 21–24 (E-RS: Asthma); the MCID (–4.0 units for SGRQ total score, –2.0 units for E-RS: Asthma total score) is indicated by the dashed horizontal lineCitation16,Citation18. Abbreviations. CI, confidence interval; E-RS, Evaluating Respiratory Symptoms; FF, fluticasone furoate; ITT, intention-to-treat; LS, least squares; MCID, minimal clinically important difference; SGRQ, St George’s Respiratory Questionnaire; UMEC, umeclidinium; VI, vilanterol.
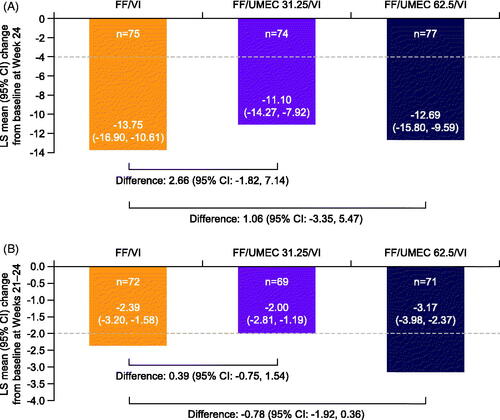
Improvements in E-RS: Asthma total score over Weeks 21–24 that were equal to or greater than the MCID (2.0 points) were observed in all pooled treatment groups in both populations (; Supplementary Figure 6(B)). In the Japanese cohort, improvements in E-RS: Asthma total score following addition of UMEC 62.5 mcg, but not UMEC 31.25 mcg, were numerically greater than those with FF/VI (). Results from the overall ITT population showed improvements over FF/VI with addition of both UMEC doses, which were less pronounced with UMEC 31.25 mcg than with UMEC 62.5 mcg (Supplementary Figure 6(B)).
Safety
The safety profile of FF/UMEC/VI in the Japanese cohort was generally consistent with that seen in the overall ITT population, with no new safety concerns (; Supplementary Table 2)Citation12. Across the six treatment groups in the Japanese cohort, 42–66% of patients experienced AEs. Only one AE leading to treatment discontinuation was reported (constipation and pancreatic carcinoma in the FF/VI 100/25 mcg group). Dry mouth/drying of the airway secretions (broad focus) was the most commonly reported AESI group in all six treatment groups (range: 26–42%; ), driven by the number of patients with nasopharyngitis (range: 18–34%). Dry mouth/drying of the airway secretions (narrow focus [defined using seven pre-specified preferred terms, excluding nasopharyngitis]) was not reported. The incidence of SAEs was 0–3% (n = 0–1) across the treatment groups, with the exception of FF/UMEC/VI 100/31.25/25 mcg (8% [n = 3]). There were no major adverse cardiovascular events and no deaths in the Japanese cohort.
Table 2. On-treatment AEs and AESIs in the Japanese cohort.
Discussion
FF/UMEC/VI was approved in the United States and Japan in 2020 as a once-daily treatment for asthmaCitation19, based on the findings of the Phase IIIA CAPTAIN study which showed that FF/UMEC/VI is an effective treatment for patients with moderate or severe uncontrolled asthma on ICS/LABACitation12. FF/UMEC/VI is also widely approved as a once-daily treatment for chronic obstructive pulmonary disease (COPD)Citation19. In this analysis of Japanese patients included in the CAPTAIN study, FF/UMEC/VI improved lung function compared with FF/VI, in line with the effects seen in the overall ITT population. Improvements in asthma control and symptoms were also seen when UMEC was added to FF/VI, although the overall rate of exacerbations was so low that differences between treatment groups in the annualized rate of moderate/severe exacerbations were difficult to detect. In general, these findings were consistent with those from the ITT analysis of the overall CAPTAIN study and provide valuable insight into the effects of triple therapy in a Japanese asthma population who are uncontrolled on dual therapy.
In the current analysis, addition of UMEC 62.5 mcg to FF/VI 100/25 mcg or 200/25 mcg led to similar improvements in lung function to those seen in the overall ITT population, with effects seen as early as 3 h after administration as shown by the analysis of clinic FEV1 at 3 h post-dose at Week 24. For both lung function endpoints, greater improvements were seen with UMEC 62.5 mcg compared with UMEC 31.25 mcg, both in the Japanese cohort and the overall ITT population. However, the magnitude of this difference was greater in the Japanese cohort compared with the ITT populationCitation12. Lung function benefits of UMEC 62.5 mcg-containing triple therapies versus dual therapy are consistent with those seen in previous studies of other ICS/LAMA/LABA combination therapiesCitation10,Citation20,Citation21 and support the benefits of triple therapy in Japanese asthma populations.
The incidence of moderate/severe exacerbations was low overall, and lower in the Japanese cohort versus the overall population, limiting the power to identify differences between treatment groups. As noted in the original study, moderate exacerbations required a physician to assess the patient and determine that additional therapy was warranted, as per the American Thoracic Society-European Respiratory Society joint statementCitation14 and were therefore considered to be clinically meaningful. Where this requirement was not applied in previous studies, the rate of exacerbations has been higherCitation21. While there were no clear differences in the rate of moderate/severe exacerbations across the three pooled treatment groups in the Japanese population, in the pooled analysis of the overall ITT population the mean annualized rate of moderate/severe exacerbations in patients receiving FF/UMEC 62.5 mcg/VI was 0.61 compared with 0.70 in patients receiving FF/VI. This finding is in line with previous studies of ICS/LAMA/LABA versus ICS/LABA that have shown reductions in the rate of moderate and/or severe exacerbations with the addition of LAMACitation10,Citation20,Citation21. Furthermore, real-world evidence from Japan has shown lower numbers of exacerbations in patients with asthma in the year after triple therapy initiation compared with the year priorCitation22.
As in the overall ITT analysis, all three pooled treatment groups in the Japanese cohort showed clinically meaningful improvements in asthma control, symptoms and health-related quality of life (HRQoL), as assessed by ACQ-7, E-RS: Asthma, and SGRQ total scores. Also consistent with results from the ITT population, addition of UMEC 62.5 mcg to FF/VI resulted in additional improvements in asthma control and a reduction in symptoms in the Japanese cohort. However, HRQoL was not improved by UMEC beyond the already considerable improvement with FF/VI, as in the overall populationCitation12. In the overall ITT population, small numerical improvements were observed in both asthma control and symptoms following the addition of UMEC 31.25 mcg to FF/VI. In contrast, no improvements in symptoms or asthma control were observed in the Japanese population following the addition of UMEC 31.25 mcg, with only the addition of UMEC 62.5 mcg leading to an improvement in these outcomes. In addition, the ACQ-7 responder analysis should be interpreted with caution due to the smaller sample size of the Japanese cohort and variability in the proportions of responders/non-responders across covariates. Clinically meaningful improvements in the changes from baseline in ACQ-7 and E-RS: Asthma total scores were observed for all treatment groups however not when FF/UMEC/VI was compared with FF/VI, in line with many asthma studiesCitation23. However, the higher proportion of ACQ-7 responders with FF/UMEC 62.5 mcg/VI versus FF/VI combined with the UMEC dose response on these outcomes support the conclusion that, as in the overall population, Japanese patients experience benefits from triple therapy beyond lung function, including improvements in important patient-centric measures of symptoms and disease control.
Differences in demographic characteristics between the Japanese cohort and the overall ITT population (including higher proportions of males, former smokers, greater previous use of asthma maintenance therapies, and more exacerbations) may have contributed to the generally smaller magnitude of effect on efficacy endpoints with UMEC 31.25 mcg in the Japanese cohort versus the overall CAPTAIN population. Furthermore, there was greater variability across the six treatment groups in the Japanese cohort compared with the overall ITT population, reflecting the smaller sample size of the Japanese cohort.
Although UMEC 62.5 mcg consistently showed greater effects on clinical outcomes than UMEC 31.25 mcg, safety profiles between the two doses were similar. As such, the safety profile for FF/UMEC/VI in the Japanese cohort was generally consistent with that of the overall population, with no new safety concerns. This finding is further supported by a dedicated long-term safety study of FF/UMEC/VI in Japanese patients with asthma, which found that no new safety concerns were associated with long-term treatment with once-daily FF/UMEC/VI 100/62.5/25 mcg or 200/62.5/25 mcgCitation24.
This study has a number of notable strengths. First, the inclusion of Japanese participants in the global study allowed assessment of the consistency of effects in the Japanese cohort with those in the overall ITT population within the same study. Additionally, the study included a robust 5-week pre-randomization period where standardized medication was provided, and enabled the measurement of the effect of UMEC in a controlled way on top of the improvements gained in the pre-randomization phases, as both FF/VI and FF/UMEC/VI were administered by the ELLIPTA DPI. Furthermore, we were able to directly compare all treatment options in a single study. The study included a broad range of endpoints, including spirometry and patient-reported outcomes to facilitate a broad assessment of the effects of triple versus dual therapy. However, there are also several limitations to consider. First, analysis of the Japanese cohort was not sufficiently powered to detect statistical differences between groups, although consistency with the overall ITT population could be assessed. In addition, the low exacerbation rate limited the ability to evaluate the impact of the addition of UMEC on the rate of asthma exacerbations. Finally, as with all clinical trials, the defined eligibility criteria limited the generalizability of the study results to some extent. Nonetheless, this analysis has provided the potential to assess the effects of FF/UMEC/VI vs FF/VI in a Japanese population with uncontrolled asthma.
Conclusion
In conclusion, consistent with the overall population, this analysis of the CAPTAIN study shows that once-daily single-inhaler FF/UMEC/VI reduces airflow obstruction and improves symptoms and asthma control in Japanese patients. Furthermore, no additional safety concerns have been found when UMEC is added to FF/VI in this patient population. As such, FF/UMEC/VI has a positive benefit-risk profile and provides a valuable treatment option for Japanese patients with inadequately controlled asthma.
Transparency
Declaration of funding
This study was funded by GlaxoSmithKline (GSK 205715, NCT02924688). GSK was involved at all stages of the study, including study design, data collection, analysis and interpretation, and preparation of the report.
Declaration of financial/other relationships
YN has received honoraria from GSK and Sanofi. SH has received honoraria from Astellas Pharma, GSK, Novartis Pharmaceuticals, AstraZeneca, Kyorin Pharmaceutical, and Sanofi. HS has no conflicts of interest to disclose. HO has received honoraria from Kyorin Pharmaceutical and Mylan EPD G.K. LAL was an employee of GSK at the time of the study and holds stocks or shares in GSK. JC, JT, TN and AF are employees of GSK, and JC and AF hold stocks or shares in GSK. A reviewer on this manuscript has disclosed being a former employee of GSK. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors take complete responsibility for the integrity of the data and accuracy of the data analysis. YN, SH, HS and HO were involved in the acquisition of data. LAL was involved in the conception or design of the study and in data analysis or interpretation. JC, JT, TN and AF were involved in data analysis or interpretation.
Supplemental Material
Download MS Word (1.2 MB)Acknowledgements
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Anne Errichelli, DPhil, of Fishawack Indicia Ltd., UK, part of Fishawack Health, and was funded by GSK. ELLIPTA and DISKUS are owned by or licensed to the GSK group of companies.
Data availability statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com
References
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2020 [cited 2020 Sep 15]. https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf.
- Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706.
- Nakamura Y, Tamaoki J, Nagase H, et al. Japanese guidelines for adult asthma 2020. Allergol Int. 2020;69(4):519–548.
- Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52(10):1073–1083.
- Davis J, Trudo F, Siddall J, et al. Burden of asthma among patients adherent to ICS/LABA: a real-world study. J Asthma. 2019;56(3):332–340.
- Lee LK, Obi E, Paknis B, et al. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55(2):208–219.
- Sulaiman I, Greene G, MacHale E, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51(1):1701126.
- Adachi M, Hozawa S, Nishikawa M, et al. Asthma control and quality of life in a real-life setting: a cross-sectional study of adult asthma patients in Japan (ACQUIRE-2). J Asthma. 2019;56(9):1016–1025.
- Nagase H, Adachi M, Matsunaga K, et al. Prevalence, disease burden, and treatment reality of patients with severe, uncontrolled asthma in Japan. Allergol Int. 2020;69(1):53–60.
- Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198–1207.
- Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst Rev. 2016;1:CD011721.
- Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69–84.
- Allen A, Siederer S, Yang S. Population pharmacokinetics of inhaled fluticasone furoate and vilanterol in adult and adolescent patients with asthma. Int J Clin Pharmacol Ther. 2016;54(4):269–281.
- Reddel HK, Taylor DR, Bateman ED, American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
- Virchow JC, Backer V, de Blay F, et al. Defining moderate asthma exacerbations in clinical trials based on ATS/ERS joint statement. Respir Med. 2015;109(5):547–556.
- Jones PW. St George's Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79.
- Juniper EF, Svensson K, Mörk AC, et al. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558.
- Tabberer M, von Maltzahn R, Bacci E, et al. Evaluation of the psychometric properties, scoring algorithm, and score interpretation of the E-RS: asthma in two clinical trials of moderate to severe asthma. Am J Respir Crit Care Med. 2020;201:A5624.
- FDA. TRELEGY ELLIPTA prescribing information; 2020 [cited 2020 Oct 6]. www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Trelegy/pdf/TRELEGY-ELLIPTA-PI-PIL-IFU.PDF.
- Kerstjens HAM, Maspero J, Chapman KR, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8(10):1000–1012.
- Virchow JC, Kuna P, Paggiaro P, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019;394(10210):1737–1749.
- Suzuki T, Fairburn-Beech J, Sato K, et al. Clinical characteristics, treatment patterns, disease burden, and persistence/adherence in patients with asthma initiating inhaled triple therapy: real-world evidence from Japan. Curr Med Res Opin. 2020;36(6):1049–1057.
- Bateman ED, Esser D, Chirila C, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol. 2015;136(4):914–922.
- Hozawa S, Ohbayashi H, Fowler A, et al. Long-term safety of once-daily single inhaler triple therapy with fluticasone furoate/umeclidinium/vilanterol in Japanese patients with asthma: a phase III open-label study. JSA/WOA Joint Congress; 2020 Sep 17–Oct 20.
