Abstract
After fecal microbiota transplantation (FMT) to treat Clostridioides difficile infection (CDI), cognitive improvement is noticeable, suggesting an essential association between the gut microbiome and neural function. Although it is known that the gut microbiome is linked with cognitive function, whether FMT may lead to cognitive improvement in patients with neurodegenerative disorders remains to be elucidated. We present the case of a 90-year-old woman with Alzheimer's dementia and severe CDI who underwent FMT. Cognitive function testing (Mini-Mental State Examination, Montreal Cognitive Assessment, and Clinical Dementia Rating assessment) was performed one month before FMT and one week and one month after FMT. We collected the patients’ fecal samples before FMT and 3 weeks after FMT to compare the microbiota composition. The 16S rRNA gene amplicons were analyzed using the QIIME2 platform (version 2020.2) and the Phyloseq R package. The linear discriminant analysis effect size was performed to determine the taxonomic difference between pre- and post-FMT. Functional biomarker analysis using the Kruskal–Wallis H test was performed between the pre- and post-FMT. The cognitive function tests after FMT showed an improvement compared to the tests before the procedure. FMT changed the microbiota composition in recipient feces. We found that the genera were reported to be associated with cognitive function. In addition, short-chain fatty acids were found to be significantly different between before and after FMT. This finding suggests the presence of an association between the gut microbiome and cognitive function. Further, it emphasizes the need for clinical awareness regarding the effect of FMT on the brain-gut-microbiome axis and its potential as a therapy for patients with dementia.
Introduction
The brain-gut-microbiome axis has recently gained importance because of the associations reported with neurological diseasesCitation1. In several neurological disorders, such as Parkinson’s disease, Alzheimer’s disease (AD), autism spectrum disorder, multiple sclerosis, and amyotrophic lateral sclerosis, the composition of gut microbiota differs in patients compared to healthy controls, indicating a pathophysiological role of the gut microbiome in the central nervous system functionsCitation2,Citation3. Decreased diversity and richness in the gut microbiota composition are accompanied by disease aggravation, including worsening cognitive functionsCitation2. The progression of neurological disorders may be reduced by gut microbiota interventions, such as fecal microbiota transplantation (FMT). Animal studies have confirmed the positive impact of FMT in cognitive function disordersCitation4. However, no association between FMT and cognitive improvement has been established clinically. Herein, we describe a case of cognitive improvement following FMT.
Case report
A 90-year-old woman with a history of diabetes mellitus (DM), hypertension (HTN), and chronic kidney disease was admitted for diarrhea and fever (37.8 °C). She took linagliptin (5 mg/qd) for DM treatment (admission HbA1c 6.3) and a calcium channel blocker (10 mg/qd) for HTN treatment (admission vital sign; 139/77 [mmHg]-88 [beat/min]-20 [breath/min]). The patient had experienced a gradual decline in memory and cognition over the previous 5 years and had been prescribed donepezil (10 mg/qd) for AD () after a diagnosis made by a neurologist based on brain imaging (imaging assessment for dementia) findings, clinical symptoms, and cognitive function test results. The patient was not taking any other medications, except donepezil, that could affect alertness or awareness. She no longer appeared to enjoy socializing (Geriatric Depression Scale [GDS] 23, Supplemental Table 1) and sometimes required assistance with basic tasks. However, she had no prior psychiatric history or history of alcohol or tobacco abuse.
Table 1. Clinical characteristics of fecal microbiota transplantation.
One month before admission, she underwent cognitive function evaluations (Mini-Mental State Examination [MMSE], Montreal Cognitive Assessment [MoCA], and Clinical Dementia Rating [CDR] assessment) for ∼30 min by the neurologist (Supplemental Table 1). The cognitive tests revealed significant impairments in the areas of short-term memory, semantic skills, attention, non-verbal learning, and response inhibition (MMSE 15, MoCA 11, CDR 1; , ).
Figure 1. Timeline of the fecal microbiota transplantation and cognitive function tests. The cognitive function test scores improved after fecal microbiota transplantation (Mini-Mental State Examination [MMSE], red line; Montreal Cognitive Assessment [MoCA], green line; Clinical Dementia Rating [CDR], blue line).
![Figure 1. Timeline of the fecal microbiota transplantation and cognitive function tests. The cognitive function test scores improved after fecal microbiota transplantation (Mini-Mental State Examination [MMSE], red line; Montreal Cognitive Assessment [MoCA], green line; Clinical Dementia Rating [CDR], blue line).](/cms/asset/fc1e75be-ba07-4c82-9072-96a19410cc89/icmo_a_1957807_f0001_c.jpg)
Table 2. Cognitive function test scores before and after fecal microbiota transplantation.
On the third day of hospitalization, she was diagnosed with Clostridioides difficile infection (CDI) based on a positive stool test (). She was treated unsuccessfully with vancomycin (oral, 125 mg/qid) and metronidazole (oral, 500 mg/tid) for 10 days (). She was diagnosed with severe CDI, and after failed treatment with several antibiotics and aggravation of symptoms determined by a positive CDI stool test, she underwent FMT following FMT indicationCitation5. She did not present with any delirium due to CDI or medication as determined by a delirium assessment scale (4 A’s test [4AT] score 1)Citation6.
The FMT donor was a 27-year-old man with no gastrointestinal or other health problems, not using drugs and antibiotics. He underwent blood, stool, and psychological tests and responded to a specific questionnaire for donor selection according to FMT guidelinesCitation7,Citation8. Donated stool (50–70 g) was filtered to make a stool suspension diluted with sterile saline (0.9%) for FMT and stored at −80 °C. The stool suspension was strained and poured into a sterile container. Stool suspension (60 g) was thawed at 37 °C for 6 h before FMT. The recipient underwent bowel preparation, and the stool suspension was applied during a colonoscopy performed by an experienced gastroenterologistCitation5. After FMT, she was checked to monitor any side effects of FMT in the microbiome center.
Following the first FMT, her severe gastrointestinal symptoms improved, and a stool test for CDI was negative. One month after the first FMT, her cognitive functions slightly improved (MMSE 18, MoCA 12, CDR 1; , ).
Three months after the first FMT, the patient experienced watery diarrhea, fever (38.0 °C), continuous abdominal pain, and progressively worsening conditions. However, she did well blood sugar test control for DM treatment (HbA1c 6.1) and her vital signs were stable (140/82 [mmHg]–88 [beat/min]–20 [breath/min]). She was diagnosed with a recurrent severe CDI for a positive CDI stool test that was unsuccessfully treated with antibiotics. She underwent a second FMT with the same modalities as the first transplantationCitation5.
Her MMSE, MoCA, and CDR scores before the second FMT procedure were 20, 16, and 0.5, respectively, an improvement over her results immediately after the first FMT. In addition, similar to the previous admission, during the second admission also, she did not present with delirium (4AT score 1). Her cognitive functions were deemed not affected by her general physical condition (). One week after the second FMT, her severe gastrointestinal discomfort improved with negative CDI stool results. Her scores were stable (MMSE 20, MoCA 17, and CDR 0.5), and she reported a marked improvement in mood (GDS 17) and daily living activities and showed more expressive affection ().
Table 3. Laboratory findings of fecal microbiota transplantation.
Discussion
This is an interesting case report describing cognitive improvement in a patient with AD after FMT for recurrent severe CDI. Our results provide evidence that gut microbiome composition alterations may affect humans’ cognitive function and provide additional clinical evidence supporting the association between the gut microbiome and cognitive function.
Most previous studies in animal models have confirmed the correlation between the gut microbiome and cognitive functionCitation4. The gut microbiome may contribute to the pathogenesis of cognitive function through various mechanisms. In a mouse model of AD, amyloid-beta (Aβ) pathology and neuroinflammation were increased in mice with decreased cognitive function as seen in humans. As a result of injecting healthy mouse feces into these mice, cognitive function was improved. Recipient mice with cognitive decline demonstrated decreased cerebral Aβ. These microbial-derived changes have essential modulate host homeostasis, including blood-brain barrier and intestinal integrity about 4 weeks after FMT, in addition to results showing improved cognitive function. FMT treatment improved cognitive deficits and reduced Aβ deposition in the miceCitation4. These protective effects may be related to reversing changes in the gut microbiota and short-chain fatty acids (SCFA)Citation9, as enteric neuron receptors for SCFA facilitate metabolite regulation related to gastrointestinal motility, rapidly transmit information about metabolites crossing the epithelial barrier to the brain, and provide information about metabolites’ concentrations in the gut lamina propria.
Neurotransmitters and amyloid plaques in the brain are metabolized by gut microbiota. Previous studies presented that reduced glutamate levels are associated with impaired cognitive functionCitation10. According to those results, decreased hippocampal glutamate levels in mild cognitive impairment (MCI) and AD were associated with episodic memory performance. A pilot study demonstrated that benzoate could improve cognition in patients with early-phase AD or MCI. Therefore, further studies are warranted to evaluate the relationship between cognitive impairment and d-glutamate levels in the brain. Moreover, increased gamma-aminobutyric acid (GABA) levels have been found in ADCitation11. In particular, GABA is synthesized and metabolized in the gut microbiome. In addition, Aβ plaques are significant indicators of ADCitation12. Molecular mimicry occurs between humans and microbiota in cross-activation immune cells, such as T or B cells.
We analyzed the microbiota of frozen fecal samples to compare the microbiota of our patients to those of patients enrolled in other studies (KCT0004423). We collected the patients’ fecal samples before FMT and 3 weeks after FMT to compare the microbiota composition. The microbiome analysis of this patient was performed after the first FMT. Metagenomic DNA was then extracted from our patient’s fecal samples, and amplification of the V3–V4 region of the bacterial 16S rRNA gene was conducted using barcoded universal primers. Sequence data for the 16S rRNA gene amplicons were analyzed using the QIIME2 platform (version 2020.2) and Phyloseq R packageCitation13. Mean sequence counts were 101,695 in both forward reads and reverse reads. The α-diversity difference was significant (p = .04, ). This indicates that FMT changed the species composition in the recipient feces. The β-diversity did not show a significant difference, per the results of the permutational multivariate analysis of variance (PERMANOVA) test (p = .142, ).
Figure 2. Microbiome analysis of the pre- and post-fecal microbiota transplantation (FMT). (A) The α-diversity representing Shannon diversity index. (B) The β-diversity representing principal coordinates analysis. (C) Linear discriminant analysis (LDA) effect size analysis (LDA score > 2.0). *p-Value < .05, LDS: linear discriminant analysis.
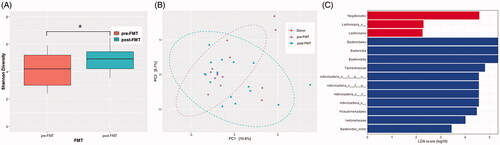
The linear discriminant analysis effect size was performed to determine the taxonomic difference between pre- and post-FMT. Bacteroidales, Bacteroidia, Tannerellaceae, and Actinobacteria were the more enriched taxonomy after FMT. These genera were reported to be associated with cognitive function ()Citation14–16.
Functional biomarker analysis using the Kruskal-Wallis H test was performed between the pre- and post-FMT groups by using EzBio-Cloud Apps (ChunLab Inc., Seoul, Korea). Pentose phosphate cycle, the functional pathway associated with the production of SCFA, was found to be significantly different between before and after FMT (p = .026)Citation17.
Therefore, we suggest that FMT corrected the microbiome dysbiosis of the recipient and that FMT altered the abundance of microbiota affecting the brain-gut-microbiome axis. These beneficial effects may be related to changes in the gut microbiota and an increase in short-chain free acids, known as profitable brain metabolitesCitation18.
This report shows a case of a patient who experienced a step-wise improvement in the cognitive domains of orientation, attention, and short-term memory, in addition to daily living ability, following repeated FMT for recurrent severe CDI. Whether FMT may have a continuous and cumulative effect on cognitive function when repeated is challenging to confirm. Additional evaluation is underway to examine the effects and mechanisms of FMT to improve cognitive function and daily living ability, such as the AD Assessment Scale–Cognitive Subscale (ADAS-cog) or Severe Impairment Battery (SIB)Citation19,Citation20. We are also planning to recruit more patients to study the relationship between FMT and cognitive function.
According to studies to date, FMT is known as a relatively safe procedure. Although nausea was noted for 3 h after the first FMT during 1 h in our patient (), she improved nausea after using anti-emetics. Then, she did not present any side effects after FMT. This supports previous reports that reported that acute side effects, such as fever, abdominal pain, and abdominal distension, may occur after FMT. Therefore, patient monitoring after FMT is necessaryCitation21.
In conclusion, our case provides evidence of the benefit of FMT in patients with cognitive decline and the efficacy of FMT. Furthermore, it suggests an association between the gut microbiome and cognitive function and its potential as a therapy for AD.
Transparency
Declaration of funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No.2021R1F1A1049320) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (No.HI20C1488).
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contributions
SH Park and SH Choi: conception and organization of the research project. SH Park, JH Lee, JB Shin, BR Cha, SJ Lee, KS Kwon, and SH Choi: execution of the research project. SH Park, JS Kim, and YW Shin: analysis and interpretation. SH Park and JH Lee: writing of the first draft of the manuscript. SH Park, JH Lee, JB Shin, BR Cha, SJ Lee, KS Kwon, and SH Choi: review and critique of the manuscript. All authors: contributed to the article and approved the submitted version.
Reviewer disclosure
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_Information_highligted_version.docx
Download MS Word (21.3 KB)Acknowledgements
The authors would like to thank the patient and her family. This case study was conducted according to the tenets of the Declaration of Helsinki and approved by the Institutional Review Board of Inha University Hospital (2020-11-004). Our patient’s family provided informed written consent.
References
- Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480.
- Hazan S. Rapid improvement in Alzheimer's disease symptoms following fecal microbiota transplantation: a case report. J Int Med Res. 20;48(6):300060520925930.
- Vendrik KEW, Ooijevaar RE, de Jong PRC, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10(98):98.
- Sun J, Xu J, Ling Y, et al. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019;9(1):189.
- Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580.
- Shenkin SD, Fox C, Godfrey M, et al. Delirium detection in older acute medical inpatients: a multicentre prospective comparative diagnostic test accuracy study of the 4AT and the confusion assessment method. BMC Med. 2019;17(1):138.
- Cammarota G, Ianiro G, Kelly CR, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68(12):2111–2121.
- Brandt LJ. American Journal of Gastroenterology lecture: intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am J Gastroenterol. 2013;108(2):177–185.
- Martin CR, Osadchiy V, Kalani A, et al. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133–148.
- Chang CH, Lin CH, Lane HY. D-glutamate and gut microbiota in Alzheimer’s disease. IJMS. 2020;21(8):2676.
- Wu Z, Guo Z, Gearing M, et al. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer's [corrected] disease model. Nat Commun. 2014;5:4159.
- Rapsinski GJ, Newman TN, Oppong GO, et al. CD14 protein acts as an adaptor molecule for the immune recognition of Salmonella curli fibers. J Biol Chem. 2013;288(20):14178–14188.
- Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857.
- Tang W, Meng Z, Li N, et al. Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and hippocampus-dependent behaviors. Front Cell Infect Microbiol. 2020;10:611014.
- Gureev AP, Syromyatnikov MY, Ignatyeva DA, et al. Effect of long-term methylene blue treatment on the composition of mouse gut microbiome and its relationship with the cognitive abilities of mice. PLoS One. 2020;15(11):e0241784.
- Novotny M, Klimova B, Valis M. Microbiome and cognitive impairment: can any diets influence learning processes in a positive way? Front Aging Neurosci. 2019;11:170.
- den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340.
- Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020; 11:25.
- Lin CH, Chen PK, Wang SH, et al. Effect of sodium benzoate on cognitive function among patients with behavioral and psychological symptoms of dementia: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2021;4(4):e216156.
- Schmitt FA, Saxton J, Ferris SH, et al. Evaluation of an 8-item Severe Impairment Battery (SIB-8) versus the full SIB in moderate to severe Alzheimer's disease patients participating in a donepezil study. Int J Clin Pract. 2013;67(10):1050–1056.
- van Beurden YH, de Groot PF, van Nood E, et al. Complications, effectiveness, and long term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile infection. United European Gastroenterol J. 2017;5(6):868–879.
