Abstract
Objective
The aims of the survey were to assess first experiences of Swiss COPD patients switching from the disposable to the new reusable Respimat inhaler, and to evaluate physicians´ and patients´ views of the new training material.
Methods
Patients with a confirmed diagnosis of COPD using a disposable Respimat inhaler for at least three months were included. Patients´ demographics, COPD stage, current treatment, and comorbidities relevant for the handling of the device were assessed. Further, patients were trained on the reusable Respimat by placebo inhaler, patient brochure, video cards/demo films and SMS reminder service. After at least one cartridge change, patients gave comprehensive feedback on their satisfaction with the reusable Respimat and physicians evaluated the need for re-training.
Results
235 patients participated in the survey. Of these, 37% suffered from comorbidities restricting the handling of the Respimat. 216 (92%) patients had a better overall satisfaction with the reusable than with the disposable Respimat. Dose counter (86%), monthly preparation (81%) and daily handling (77%) were also assessed as better by most of the patients. In 80% of cases, the user ability was stated as better than for the disposable Respimat. Less than 15% of the patients required further training. Placebo inhaler was the mostly preferred training material by both, physicians (in 86% of the patients) and patients (75%). In patients with comorbidities affecting inhaler handling, overall satisfaction was also better in 86% of the patients.
Conclusion
The majority of patients were satisfied with the new reusable Respimat device and proper handling could be attained using the provided training material, even in patients with restricting comorbidities.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common progressive respiratory disease mainly characterized by persistent airflow limitation leading to significant symptom burden in affected patientsCitation1. The main goal in COPD treatment is achievement of disease control and consequently, an improvement of health-related quality of life. Numerous inhaler devices for maintenance treatment in COPD patients are available on the market, but each class of device has a different approach to inhalation and therefore, requires distinct handling techniques. However, the correct usage of the respective inhaler is a prerequisite for achievement of maximal clinical benefit.
The Respimat Soft Mist Inhaler (SMI) (Boehringer Ingelheim International GmbH, Ingelheim, Germany) is well accepted by patients which is an important factor for treatment complianceCitation2–5. Based on physicians´ and patients’ feedback, the first, disposable Respimat was updated to a reusable version with an improved usability, a simplified assembly and an optimized dose counter while still maintaining sufficient drug deposition to the lungsCitation6. The main difference is that (1) the device lock is now reusable. (2) The dose indicator showing the remaining doses was applied to the cartridge. (3) When the cartridge is used up, the device automatically locks and (4) the safety catch releases to push off the clear base of the inhaler as a clear indication for the patient that a cartridge change is necessary. (5) To improve the handling of the Respimat inhaler, the clear base was modified giving a longer grip. This eases the twisting of the device. Further, the enlarged dose counter can be received by the base. (6) A cartridge counter was also included to indicate a used-up deviceCitation6. Since numerous studies have shown that up to 80% of patients use their inhaler devices incorrectlyCitation7,Citation8, it is suggested that patients need proper training with adequate training material such as placebo inhaler, patient brochures, and video films to be able to handle the inhaler device accurately. Recent studies underline this assumption that an optimized patient teaching might have positive effects on treatment adherence and exacerbation frequencyCitation9,Citation10. Consequently, well-performed and repeated patient training can be considered as an important cornerstone for successful COPD maintenance treatmentCitation11–13. However, even medical staff often lack knowledge regarding the correct handling of the various kinds of inhaler devices. Consequently, they are not able to teach patients sufficiently on inhaler utilizationCitation14–17. Therefore, assessment of patients’ acceptance of and satisfaction with provided training material is an important point to optimize teaching conditions. In addition to the training aspect, several conditions such as cognitive disorders or physical disabilities represent further important factors influencing the accurate handling of the inhaler device and should be considered while introducing an inhaler in such a patientCitation15,Citation18–20.
In Switzerland, the phasing out of the disposable device in favor of the reusable Respimat was started in November 2019. To ensure correct usage of the new Respimat inhaler device, specific training material for handling and inhalation was released along with the launch of the new Respimat. The aim of this survey was to evaluate physicians’ and patients’ views on the provided training material and to assess the first experience of patients switching from the disposable to the reusable Respimat with special consideration of co-existing conditions relevant for the correct handling of the inhaler device.
Methods
Participants and ethical considerations
COPD patients from all over Switzerland, inhaling medication (either Spiriva or Spiolto) from the disposable Respimat inhaler were eligible to participate in the survey. Inclusion criteria were (1) a confirmed COPD diagnosis and (2) a treatment utilizing the current disposable Respimat for at least three months prior to the survey. Prior to inclusion, eligible patients had to provide their consent. The local ethics committee (Ethikkommission Nordwest- und Zentralschweiz [EKNZ], Basel, Switzerland, No. EKNZ BASEC 2019-00921) was informed about the survey.
Study design
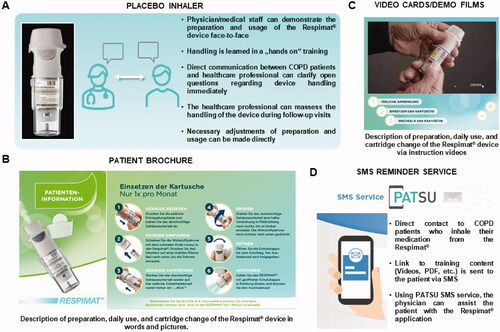
The survey comprised two visits. It was created as simple, qualitative questionnaire to evaluate the real-life perceptions in Swiss patients after they had to switch from the disposable to the reusable Respimat. Questions based on the Ease of Handling Questionnaire which was developed by BoehringerCitation21 and were adjusted after supervision by clinicians to be short and easy to fill in, and to be also suitable for patients with cognitive disorders. The survey was not validated. The questions were scored without a threshold on a 5 point-Likert scale. At Visit 1, patients’ demographics, COPD stage, and current treatment scheme were documented by the physician (Supplementary material 1). Additionally, presence of the following comorbidities was assessed: arthritis/arthrosis, a rheumatological disorder, dementia/Alzheimer’s disease, mental disorders, cognitive disorders, Parkinson’s (or other Parkinson’s like) disease, and other not further specified diseases relevant for handling the reusable Respimat inhaler. Different training materials were introduced to the patient by the treating physician who had previously been trained on the educational material, including placebo inhaler, patient brochure, video cards/demo films and reminder service via SMS (PATient SUpport service = PATSU) (details shown in ). Afterwards, patients’ and physicians’ preferred training material concerning the handling of the reusable Respimat inhaler was assessed by survey questions. Visit 2 was performed during clinical routine after the patient had changed the cartridge at least once but not more than three times. At visit 2, the patient’s experience with the reusable Respimat was assessed by use of a simple survey comprising questions with pre-defined answers (much better, slightly better, equal, slightly worse, and much worse) about dose counter, daily handling, monthly preparation, environmental friendliness, and overall satisfaction. Finally, it was recorded whether the treating physician suggested an extra training for the patient to ensure proper use of the Respimat.
Figure 1. Training material used for patients´ education. Overview about the training material provided by Boehringer for the launch of the reusable Respimat which was used in the current survey for patients´ education. (A) Placebo inhaler. (B) Patient brochure (partly shown). (C) Video cards/demo films (partly shown). (D) SMS reminder service (PATSU). Abbreviation. PATSU, PATient SUpport service.
For subgroup analyses, patients were stratified according to their concomitant diseases relevant for handling the reusable Respimat inhaler. “Group mobility” (Groupmob) comprised patients with at least one concomitant disease limiting mobility. In “group cognitive disorders” (Groupcog), patients with at least one concomitant disease that was classified as mental and/or cognitive disorder were included. “Group both mobility and cognitive disorders” (Groupboth) contained patients with at least one concomitant disease limiting mobility and at least one concomitant disease classified as mental and/or cognitive disorder. Diseases limiting mobility were arthritis/arthrosis, a rheumatological disorder, and Parkinson’s disease. Mental and/or cognitive disorders limiting the ability to use the inhaler adequately were dementia/Alzheimer’s disease, mental disorders, and cognitive disorders without further specification.
Statistics
Data were analyzed using SAS version 9.4 (NC, United States). Analyses were performed in a descriptive and explorative manner and were carried out in all patients who fulfilled the inclusion criteria and had given their consent. All data were expressed as N and %. Due to the descriptive character of this survey, no additional statistical analyses were performed.
Results
Demographics
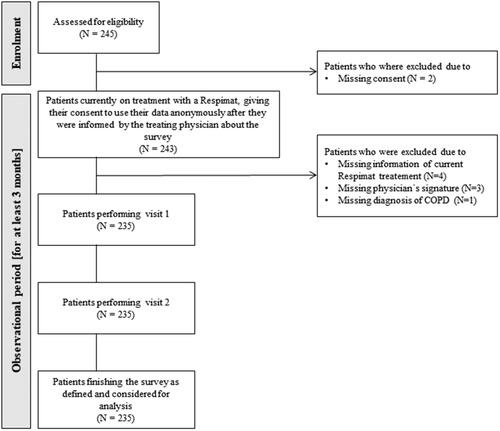
In this Non-Trial-Activity (NTA), 245 patients were screened for eligibility () by 35 different physicians. Thereof, 10 patients were excluded from the survey due to missing consent (N = 2), missing information of current Respimat treatment (N = 4), missing physician’s signature (N = 3), and missing diagnosis of COPD (N = 1, ). In total, 235 patients participated in the survey (). All those patients performed both visits and were considered for analysis (beside one patient who was excluded for evaluation of visit 2 due to missing signature of the respective physician at visit 2. ).
An overview of patients’ demographics, COPD characteristics, and current inhaled COPD treatment is given in . 61.7% of the patients were male (). Regarding the therapy relevant classification of COPD, most of the patients belonged to the GOLD group B (37.0%) or GOLD group A (28.1%, ). 71.9% of the patients used Spiolto Respimat for inhaled therapy during the last three months before visit 1 and 28.5% of the patients inhaled with Spiriva Respimat at survey inclusion (). Comorbidities in the total patient population were hypertension (53.2%), cardiovascular disease (35.7%), or diabetes (24.7%, ).
Table 1. Patient demographics, COPD characteristics and current inhaled therapy for COPD treatment (all patients, N = 235).
Table 2. Concomitant diseases with effect on handling and ability to inhale with the Respimat inhaler and further comorbidities (all patients, N = 235).
Concomitant diseases relevant for the handling and ability to use the Respimat inhaler
36.6% of the study population (N = 86) suffered from concomitant diseases probably affecting the handling and use of the Respimat inhaler. An overview is given in .
Patients’ evaluation of the new reusable Respimat inhaler in the total patient population
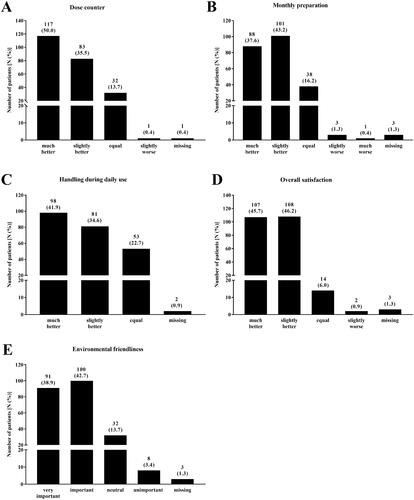
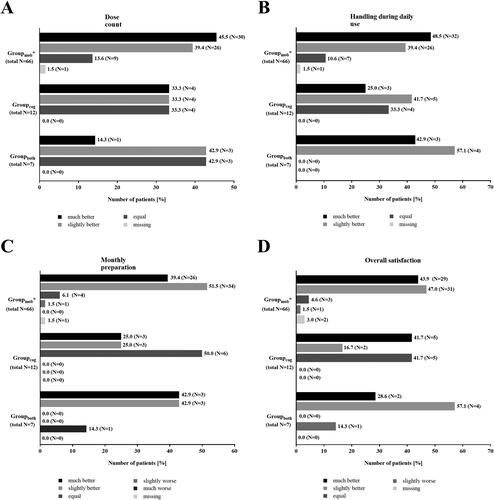
85.5% of the patients rated the dose counter of the new reusable Respimat better than that of the disposable Respimat (). The monthly preparation was also stated as better by most of the patients (80.8% of the patients, ). Similar results were observed regarding the handling during daily use (76.5% slightly or much better, ). None of the patients rated the daily handling of the reusable Respimat as inferior to the handling of the disposable Respimat (). In total, 91.9% of the patients rated the overall satisfaction with the new reusable Respimat as better as with the disposable Respimat (). Last item for evaluation was the environmental friendliness. For most of the patients (81.6%), environmental friendliness of the reusable Respimat was important or very important ().
Figure 3. Patients´ evaluation of the new reusable Respimat inhaler (all patients, N = 234*). Patients were asked to evaluate the new reusable Respimat inhaler with focus on dose counter (A), monthly preparation (B), handling during daily use (C) after changing at least once the cartridges at home, and overall satisfaction (D). Further they were asked how important environmental friendliness is to them (E). Shown is the number of patients who chose the respective answer. Percentages are given in brackets. *1 Patient was excluded due to missing signature of the respective physician at visit 2.
Physicians’ evaluation of the new reusable Respimat
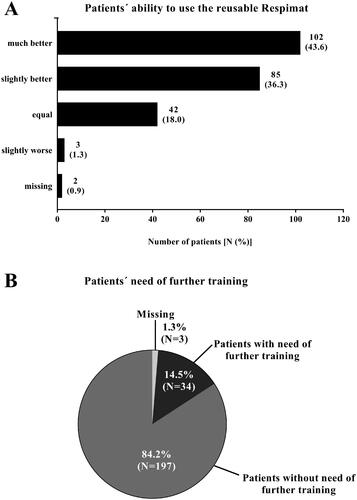
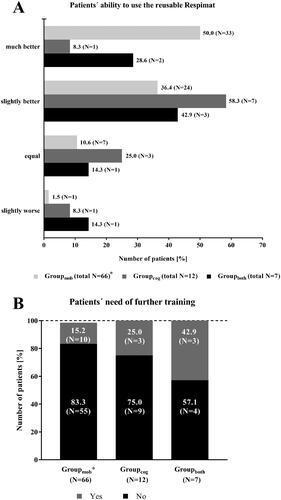
Physicians judged the ability of the patients to use the new reusable Respimat inhaler in 79.9% of the patients to be better than for the previous versions (). In 1.3% of the patients, the patient’s user ability was assessed as slightly worse compared to previous versions of the Respimat. For 84.2% of the patients, physicians indicated no need of further training on using the new reusable Respimat. However, 14.5% of the patients, required further training ().
Figure 4. Medical evaluation of the new reusable Respimat compared to the previous version (all patients, N = 235). Physicians were asked to report the ability of the patient to use the new reusable Respimat inhaler (A) and whether the patients need further training (B) after changing the cartridges at least once at home. (A) Given is the number of patients (percentages are given in brackets). (B) Given is the number of patients in %. Abbreviation. N, number of patients.
Evaluation of the offered training materials
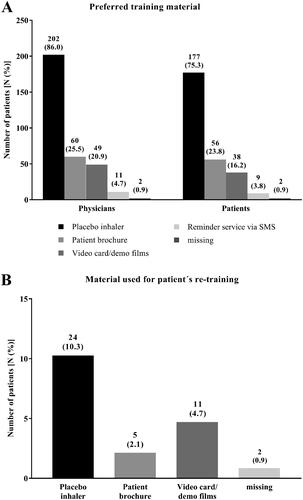
All training materials (placebo inhaler, patient brochure, video card/demo films, and the reminder service by SMS) were used by the physicians to teach the correct operation with the new reusable Respima inhaler. Physicians mostly preferred the placebo inhaler (in 86.0% of the cases) as training material, followed by the patient brochure (25.5%) and the video cards/demo films (20.9%, ). Similar results were observed in the patient population with 75.3% favoring the placebo inhaler, 23.8% the patient brochure, and 16.2% the video card/demo films (). For re-training of the patients, the placebo inhaler was most often documented as the preferred training material ().
Figure 5. Physicians´ and patients´ preferred training material (all patients, N = 235). Physicians and patients were asked for their preferred training material to demonstrate the correct usage of the Respimat inhaler and to learn how to use it (A). Given is the number of physicians as well as the number of patients who preferred the respective training material. (B) Physicians´ report which training material was used for patient´s re-training. Percentages are given in brackets. Abbreviation. N, number of patients.
Subgroup analysis stratified by concomitant diseases affecting the handling of the Respimat inhaler
In total, 86 patients (36.6%) of the study population had concomitant diseases affecting the handling of the Respimat inhaler. Demographic and disease details of those patients are provided in . Most of the patients belonged to Groupmob (77.9%).
Table 3. Subgroup analysis: patients with concomitant diseases affecting the handling of the Respimat inhaler and the ability to inhale (grouped, N = 86).
In Groupmob and Groupcog, majority of patients stated the dose counter as better (Groupmob: 84.9%, Groupcog: 66.6%). In Groupboth, 85.8% of the patients indicated it as slightly better or equal in comparison to previous versions (). Daily handling was reported as better in all patients from Groupboth and in most of the patients of Groupmob and Groupcog (Groupmob: 87.9%, Groupcog: 66.7%, ). The share of patients reporting the daily use as equivalent to previous Respimat versions was higher in Groupcog (33.3%%) as in Groupmob (10.6%, ). The monthly preparation of the Respimat inhaler was assessed as better in 90.9% of the patients from Groupmob and in 85.8% of the patients from Groupboth (). In Groupcog, each 50.0% of the patients stated the monthly preparation as better or equally compared to the disposable version (). More than 85.0% of the patients in Groupmob and Groupboth reported a better overall satisfaction with the new reusable Respimat inhaler (). In Groupcog, the share of patients was lower (58.4%, ).
Figure 6. Subgroup analysis – patients with concomitant diseases effecting the handling of the Respimat inhaler and the ability to inhale: Patients´ evaluation of the new reusable Respimat inhaler (N = 85*). Graphs show the patients´ evaluation of dose counter (A), handling during daily use (B), monthly preparation (C), and overall satisfaction (D) with the Respimat inhaler after they had changed 1–3 cartridges at home using a scale with pre-defined answers (much better, slightly better, equal, slightly worse, and much worse). Given is the percentage of patients who chose the respective answer. *1 Patient in Groupmob was excluded due to missing signature of the respective physician at visit 2. Abbreviations. N, number of patients; mob, Group mobility; cog, group cognitive disorders; both, group mobility and cognitive disorders.
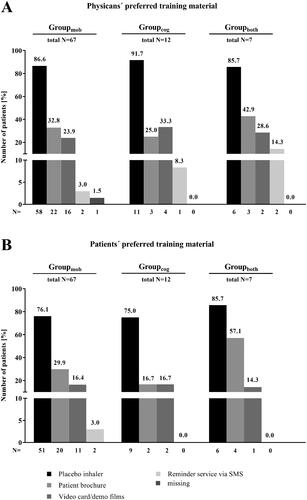
Subgroup analysis revealed that in all three subgroup categories, both, physicians (), and patients (), preferred mostly the placebo inhaler for training of Respimat inhaler handling (physicians: 85.7 to 91.7%, patients: 75.0 to 85.7%). In Groupboth, physicians favored the reminder service by SMS in a greater share of patients (14.3%) than in the other two subgroup categories (Groupmob: 3.0%, Groupcog: 8.3%, ). However, in all three subgroup categories the proportion of patients indicating the reminder service by SMS as preferred training material was ≤ 3.0% (). Patient brochure was favored by 57.1% of the patients from Groupboth (Groupmob: 29.9%, Groupcog: 16.7%, ). In the physicians’ assessment, the patient brochure was also most often considered for patients of Groupboth (42.9 vs. 32.8% [Groupmob], vs. 25.0% [Groupcog], ).
Figure 7. Subgroup analysis – Patients with concomitant diseases effecting the handling of the Respimat inhaler and the ability to inhale (N = 86): Preferred training material – physicians vs. patients. Physicians and patients were asked for their preferred training material to demonstrate the correct usage of the Respimat inhaler and to learn how to use it. Given is the percentage of patients for whom the physician has favored the respective training option (A) as well as the number of patients who preferred the respective training material (B) in regard with their concomitant disease. Abbreviations. N, number of patients; mob, group mobility; cog, group cognitive disorders; both, group mobility and cognitive disorders.
Physicians assessed the user ability as better in most of the patients of all three subgroup categories (). In Groupboth, for 14.3% of the patients, the user ability was documented as slightly worse, in the other two subgroup categories, the share of patients with such a result was smaller (Groupmob: 1.5%, Groupcog: 8.3%, ). Physicians recommended an additional training in 42.9% of the patients from Groupboth. In Groupmob and Groupcog, the share of patients was smaller (Groupmob: 15.2%, Groupcog: 25.0%, ). For the renewed teaching, the use of the placebo inhaler was most often documented for the patients in all three subgroup categories (data not shown).
Figure 8. Subgroup analysis – Patients with concomitant diseases effecting the handling of the Respimat inhaler and the ability to inhale: Medical evaluation of the new reusable Respimat compared to the previous version (N = 85). Physicians were asked to report the ability of the patient to use the new reusable Respimat inhaler (A) and whether the patients need further training (B) after changing the cartridges at least once and maximum 3 times at home. *1 Patient in Groupmob was excluded due to missing signature of the respective physician at visit 2. Abbreviations. N, number of patients; mob, group mobility; cog, group cognitive disorders; both, group mobility and cognitive disorders.
Discussion
The present survey assessed first experience of patients switching from the disposable to the reusable Respimat in Swiss clinical practice. Most of the patients were satisfied or very satisfied with the new reusable Respimat in comparison to the disposable device regarding inhaler preparation and handling resulting in a great overall satisfaction with the updated device version. In clinical studies, it was consistently shown that the disposable Respimat soft mist inhaler is well accepted by COPD patientsCitation2,Citation6,Citation22,Citation23. This is mainly due to its user-friendly handling and comfortable inhalation, but despite all that, patients’ and physicians’ feedback indicated a preference for a reusable inhaler that is even easier to useCitation6. Therefore, all approved features of the disposable Respimat have been retained in the updated version and have been supplemented with new features such as an improved dose counter or the ability for cartridge change to fulfil patients’ and physicians’ desires/wishes. Some studies recently demonstrated that patient satisfaction and acceptance of the new reusable Respimat is very goodCitation6,Citation21. The present survey supports those findings since a great overall satisfaction concerning the updated Respimat device in Swiss COPD patients was observed.
In the current survey, patients were included who were designated to switch from the disposable to the reusable Respimat device. In a study by Dreher et al. Citation21, switching patients also showed a high level of satisfaction with the updated Respimat but regarding convenience, patients rated it as worse than naïve patients and patients who had been on a reusable device at study inclusion. DreherCitation21 suggested that those results were caused by the fact that these patients were required to switch from their familiar disposable inhaler device. In the present survey, only switching patients were included, thus, no comparisons were made between switching patients and those who were Respimat naïve or patients who had been on a reusable device before survey inclusion. However, the present survey confirmed that patients who switched were mostly satisfied with the new reusable device.
Since patients and physicians indicated that they would prefer a reusable inhaler device, the new Respimat device was designed to be used with up to six cartridgesCitation6. This feature lowers the already low product carbon footprint of the disposable Respimat by up to 71.0%Citation24. The environmental friendliness was also rated as important by more than 80.0% of the patients in the current survey. A previous study demonstrated that the frequency of cleaning the reusable device did not affect drug distribution and delivered volume, even if cleaning was performed less often than recommendedCitation6. This inhaler attribute in combination with an easy cleaning procedure is also an important factor to ensure patients’ handling satisfactionCitation25. Today it is well accepted, that therapeutic success of inhalation therapy depends more than expected on a patient’s perception, preference, and satisfaction with the inhalerCitation26,Citation27 because higher satisfaction with the device seems to contribute markedly to an increased treatment adherence in COPD patientsCitation3,Citation28–30.
Beside suboptimal treatment adherence, numerous studies have shown that incorrect usage of inhaler devices is one of the most common problems in COPD treatmentCitation8,Citation31. Unfortunately, handling failure often results in insufficient disease control which in turn leads to reduced willingness for treatment adherence as patients do not see any treatment success with the prescribed medicationCitation3,Citation32,Citation33. Thus, the correct usage of the respective inhaler is mandatory to achieve maximal clinical benefit and patient’s satisfaction. Various publications regarding poor treatment adherence in COPD patients indicated that the willingness to adhere to designated treatment can be improved when patients received training on the correct usage of the prescribed inhaler deviceCitation10,Citation12,Citation29,Citation34. In turn, an adequate treatment outcome will contribute to higher treatment satisfaction. Beside symptom reduction, also exacerbation rates seem to be lower in well-trained patientsCitation10, possibly due to the consistent proper use of the prescribed inhaler. Training material to demonstrate the correct handling of the inhaler device includes placebo inhaler, patient brochures, and video films. With the launch of the new reusable Respimat, such training material was provided so that physicians were able to teach the patients adequately in handling the new version of the Respimat device. In the present survey, placebo inhaler, patient brochures, video films/demo cards and a reminder service via SMS were offered for patients’ training. At follow up, most of the patients had a better ability to use the Respimat and only a small proportion of patients were indicated for further training. Those results underline the findings of previous studies that adequate training improves user capabilitiesCitation9,Citation11,Citation12,Citation29,Citation34,Citation35. In the present survey, placebo inhaler was the preferred training material for both physicians and patients. It enables “hands-on” training on the real-world device and thus, possible handling errors can directly be addressed during training. Previous studies also showed a good acceptance of placebo inhaler use for training of device handlingCitation35. Video films/demo cards were also recommended for patient education on inhaler usage. A recent study by Luley et al.Citation35 showed that device handling was markedly improved in elderly patients after an intensive video-assisted training of 8 days. Generally, several studies showed that a multimedia training approach accompanied by verbal comments was more effective than an exclusive written or verbal explanationCitation35,Citation36. However, in the current survey, it was not assessed how often the patients downloaded such video films, thus, we could only speculate if the quality of the provided video films might have been not sufficient for successful support of the patients or if the download of the films was too complicated for the patients, both possible reasons for preferring the placebo inhaler much more than video films in the current survey. Demo cards are frozen images, and they are more unlikely to adequately depict every single handling step, which might be the reason for lower preference in patients and physicians in comparison to the placebo inhaler in the current survey. Patient brochures were preferred in about 25.0% of both patients and physicians in the present survey. They represent helpful tools as they improve inhaler performance, but it has been suggested that only providing written patient brochures is likely to be inadequate for learning the correct handling of the inhaler deviceCitation37,Citation38. This suggestion is amplified by the fact that patient brochures are unable to correct handling errors which was demonstrated previouslyCitation38. Thus, it is recommended to use patient brochures as additional training materials, especially in elderly patients who might favor the opportunity to look up specific handling steps when they prepare the inhaler device at home. Corresponding to this, in the present survey, placebo inhaler use was most often documented for re-training of the patients since it enables the patient to carry out the handling under a physician’s supervisionCitation36. Interestingly, only a small proportion of patients preferred the SMS service in the current survey but as there was no assessment of the frequency of usage of the SMS service or a detailed evaluation of the user friendliness of this service, the reason why patients preferred the SMS service less remains unclear. Nevertheless, effective training is only possible if the trainee has sufficient knowledge regarding handling of the respective inhalerCitation14. Due to the large scale of different inhaler devices offered for COPD treatment, it is even difficult for physicians to operate all of them correctly. However, better knowledge of common handling errors can help physicians to identify their occurrence among patients and to step in promptly during follow-up visitsCitation13,Citation39. Even among medical staff, a lack of knowledge regarding inhaler device handling exists. Thus, handling instructions should also be provided to healthcare professionals before they start training patients with a specific inhaler device to ensure a high training quality.
Previous studies described that specific comorbidities such as cognitive disorders or physical disabilities reduce patient capabilities to handle the Respimat properlyCitation15,Citation18–20,Citation35. Thus, the current survey assessed in affected patients to what extent they are satisfied with the updated Respimat device in comparison to the previous version. In total, most of the patients included in the subgroup analysis were satisfied with the handling and preparation of the new reusable Respimat and reported a great overall satisfaction. COPD patients are mainly geriatric patients with impaired vision, cognitive decline and/or motor/sensory disturbances of their fingers. Therefore, they are often faced with difficulties in handling inhaler devices and operating errors are predictableCitation35. Consequently, achievement of treatment success will be problematic and patient satisfaction as well as patient’s willingness to adhere to designated treatment will be massively reduced. Further, insufficient disease control and an impaired QoL in those patients can be observed. The updated Respimat was optimized in accordance with patient’s feedback and therefore, an improved usability of the Respimat even for patients with impaired mobility was designated during developmentCitation6. Results of the current survey showed that most of the patients with conditions probably affecting the handling of the Respimat device were able to use the updated Respimat correctly. Additionally, the suitability of the provided training material was demonstrated as most of the patients were able to operate the inhaler device without need for further training at Visit 2. Thus, the updated version of the Respimat can be considered as an adequate inhaler device even in patients with conditions limiting mobility and cognitive functions. Further, the importance of appropriate educational training with suitable training material was shown.
This study has some limitations. Patients included in the current survey had been on a Respimat device before, thus most of the patients were familiar with the general usage of the inhaler device which could have influenced the results regarding the need of further training. Due to the phasing out of the disposable version of the Respimat device, all patients had to switch to the new, reusable device version. Thus, a parallel arm for patients still being on the disposable Respimat was not possible although this would have been an interesting objective to assess the suitability of adequate training material independently from the used version of the Respimat inhaler. Further, patients participated on a voluntary basis and were not chosen in a randomized way, therefore, patients might have been included in the survey who had a greater willingness for treatment adherence and who were still satisfied with the disposable Respimat device. In addition to that, no control group with patients who were naïve to a Respimat device was included in the survey. In this survey, only one question was asked to determine patient’s preferred training material. This is a limitation as it does not ask for the patient's view on each individual training material in detail which might have been interesting to assess. For subgroup analysis, comorbidities affecting handling of the inhaler device were assessed using simple questions. They had not been validated by disease specific questionnaires or medical examination, so that physicians had to rely on the patient's statement to have at least one of the given conditions. The number of patients with cognitive disorder or both, cognitive and mobility disorders was indeed low in this study. Those low numbers of patients showed that it is difficult to include patients with cognitive disorders in studies/surveys who are still able to answer questionnaires in a credible way. However, it is important to assess whether these patients use their inhaler devices correctly because this is mandatory for sufficient clinical benefit. Our results showed that patients with cognitive disorders (alone or in combination with mobility disorders) absolutely do not want to use SMS service, which suggests that they are overwhelmed with this kind of support due to their cognitive limitations. Those results suggested differences between the preference of training material in the different subgroups, therefore it is important to evaluate each subgroup category individually. Further, the share of patients preferring the patient brochure was higher in those with both, cognitive and mobility disorders, showing that those patients had other needs regarding adequate training material than patients of the other 2 subgroup categories. Finally, patient satisfaction was assessed after at least one and a maximum of three cartridge changes, thus only satisfaction directly after switching was reported. Long-term satisfaction and statements concerning consistent treatment adherence were not possible due to the short observational period. However, due to the descriptive, multicenter design of this survey including different physicians from various regions in Switzerland, we believe that our patient sample adequately represents the real-world clinical situation in Switzerland.
Conclusions
Overall, most of the patients were satisfied with the new reusable Respimat device. The provided training material ensured proper device handling by most of the patients. The placebo inhaler was the preferred training tool for both, physicians, and patients. The present survey showed an improvement in user convenience of the updated Respimat device due to better handling features of the inhaler device. Adequate training is mandatory in COPD patients taking inhaler-based maintenance treatment to ensure proper handling and sufficient drug deposition in the lungs. This is an important factor since patient satisfaction is based on disease control and therapeutic benefit. In turn, treatment adherence improves therapeutic success and ensures long-term COPD control resulting in improved quality of life.
Transparency
Declaration of funding
This work was supported by the Boehringer Ingelheim (Schweiz) GmbH, Basel, Switzerland. Medical writing assistance was provided by Dr. Katharina Bakhaus, Alcedis GmbH, Gießen, Germany, funded by Boehringer Ingelheim (Schweiz) GmbH, Basel, Switzerland.
Declaration of financial/other relationships
AT has received lecture and advisory board fees as well as payment for travel/accommodation and meeting expenses from Boehringer Ingelheim, Sanofi, Astra Zeneca, Phillips Respironics and OM Pharma. She is in a business relationship with SOS Oxygene and Löwenstein Medical. CC received payment for travel/accommodation/meeting expenses as well as lecture fee from AstraZeneca and Mundipharma. SS is employee of Boehringer Ingelheim (Schweiz) GmbH, Basel, Switzerland. JPD is employee of Boehringer Ingelheim (Schweiz) GmbH, Basel, Switzerland. PNC has received lecture and advisory board fees from Cipla, Lupin, and Zydus Cadilla. JDL is supported by grants from the Swiss National Science Foundation (SNF160072 and 185592) as well as by Swiss Personalised Health Network (SPHN 2018DR108). JDL has also received unrestricted grants from AstraZeneca AG Switzerland, Boehringer Ingelheim GmbH Switzerland, GSK AG Switzerland, and Novartis AG, Switzerland. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
JPD and JDL were involved in the conception and design of the survey. All authors were involved in the analysis and interpretation of the data, drafting of the manuscript, and revising it critically for intellectual content. All authors approved of the final version of this manuscript and agreed to be accountable for all aspects of the work.
Supplementary_Material_1_NTA_Switch.pdf
Download PDF (381.5 KB)Acknowledgements
The authors thank Dr. Katharina Bakhaus, Alcedis GmbH, Gießen, Germany for medical writing assistance.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- goldcopd.org [Internet]. Fontana (WI): Global Initiative for Chronic Obstructive Lung Disease; 2021 [cited 21 April 2021]. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
- Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with respimat soft mist inhaler. Int J Chron Obstruct Pulmon Dis. 2009;4:381–390.
- Chrystyn H, Small M, Milligan G, et al. Impact of patients' satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108(2):358–365.
- Hanada S, Wada S, Ohno T, et al. Questionnaire on switching from the tiotropium HandiHaler to the respimat inhaler in patients with chronic obstructive pulmonary disease: changes in handling and preferences immediately and several years after the switch. Int J Chron Obstruct Pulmon Dis. 2015;10:69–77.
- Miravitlles M, Montero-Caballero J, Richard F, et al. A cross-sectional study to assess inhalation device handling and patient satisfaction in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:407–415.
- Dhand R, Eicher J, Hänsel M, et al. Improving usability and maintaining performance: human-factor and aerosol-performance studies evaluating the new reusable Respimat inhaler. Int J Chron Obstruct Pulmon Dis. 2019;14:509–523.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):1601794.
- Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and Meta-analysis. Chron Respir Dis. 2020;17:1479973119901234.
- Gregoriano C, Dieterle T, Breitenstein AL, et al. Use and inhalation technique of inhaled medication in patients with asthma and COPD: data from a randomized controlled trial. Respir Res. 2018;19(1):237.
- Gregoriano C, Dieterle T, Breitenstein AL, et al. Does a tailored intervention to promote adherence in patients with chronic lung disease affect exacerbations? A randomized controlled trial. Respir Res. 2019;20(1):273.
- Aksu F, Şahin AD, Şengezer T, et al. Effect of training by a physician on dynamics of the use of inhaler devices to improve technique in patients with obstructive lung diseases. Allergy Asthma Proc. 2016;37(5):98–102.
- Price D, Keininger DL, Viswanad B, et al. Factors associated with appropriate inhaler use in patients with COPD – lessons from the REAL survey. Int J Chron Obstruct Pulmon Dis. 2018;13:695–702.
- Yawn BP, Mintz ML, Doherty DE. GOLD in practice: Chronic obstructive pulmonary disease treatment and management in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2021;16:289–299.
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360–1374.
- Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51(2):158–172.
- Sanchis J, Corrigan C, Levy ML, ADMIT Group, et al. Inhaler devices - from theory to practice. Respir Med. 2013;107(4):495–502.
- Lavorini F. Inhaled drug delivery in the hands of the patient. J Aerosol Med Pulm Drug Deliv. 2014;27(6):414–418.
- Frohnhofen H, Hagen O. Handgrip strength measurement as a predictor for successful dry powder inhaler treatment: application in older individuals with COPD. Z Gerontol Geriatr. 2011;44(4):245–249.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495–502.
- Ciciliani AM, Langguth P, Wachtel H. Handling forces for the use of different inhaler devices. Int J Pharm. 2019;560:315–321.
- Dreher M, Price D, Gardev A, et al. Patient perceptions of the re-usable Respimatt soft mist™ inhaler in current users and those switching to the device: a real-world, non-interventional COPD study. Chron Respir Dis. 2021;18:1479973120986228.
- Schürmann W, Schmidtmann S, Moroni P, et al. Respimat soft mist inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfaction. Treat Respir Med. 2005;4(1):53–61.
- Dal Negro RW, Povero M. Acceptability and preference of three inhalation devices assessed by the handling questionnaire in asthma and COPD patients. Multidiscip Respir Med. 2016;11(1):7.
- Hänsel M, Bambach T, Wachtel H. Reduced environmental impact of the reusable Respimat soft mist™ inhaler compared with pressurised metered-dose inhalers. Adv Ther. 2019;36(9):2487–2492.
- Davis KH, Su J, González JM, et al. Quantifying the importance of inhaler attributes corresponding to items in the patient satisfaction and preference questionnaire in patients using combivent respimat. Health Qual Life Outcomes. 2017;15(1):201.
- Ding B, Small M, Scheffel G, et al. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma-COPD overlap syndrome consulting for routine care. COPD. 2018;13:927–936.
- Schreiber J, Sonnenburg T, Luecke E. Inhaler devices in asthma and COPD patients - a prospective cross-sectional study on inhaler preferences and error rates. BMC Pulm Med. 2020;20(1):222.
- Small M, Anderson P, Vickers A, et al. Importance of inhaler-device satisfaction in asthma treatment: real-world observations of physician-observed compliance and clinical/patient-reported outcomes. Adv Therapy. 2011;28(3):202–212.
- López-Campos JL, Quintana Gallego E, Carrasco Hernández L. Status of and strategies for improving adherence to COPD treatment. Int J Chron Obstruct Pulmon Dis. 2019;14:1503–1515.
- Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–472.
- Zhang W, Xu L, Gao S, et al. Technical evaluation of soft mist inhaler use in patients with chronic obstructive pulmonary disease: a cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2020;15:1471–1479.
- Darbà J, Ramírez G, Sicras A, et al. The importance of inhaler devices: the choice of inhaler device may lead to suboptimal adherence in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:2335–2345.
- Kocks JWH, Chrystyn H, van der Palen J, et al. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim Care Respir Med. 2018;28(1):43.
- Takemura M, Mitsui K, Itotani R, et al. Relationships between repeated instruction on inhalation therapy, medication adherence, and health status in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:97–104.
- Luley MC, Loleit T, Knopf E, et al. Training improves the handling of inhaler devices and reduces the severity of symptoms in geriatric patients suffering from chronic-obstructive pulmonary disease. BMC Geriatr. 2020;20(1):398.
- Press VG, Arora VM, Shah LM, et al. Teaching the use of respiratory inhalers to hospitalized patients with asthma or COPD: a randomized trial. J Gen Intern Med. 2012;27(10):1317–1325.
- Wilson EA, Park DC, Curtis LM, et al. Media and memory: the efficacy of video and print materials for promoting patient education about asthma. Patient Educ Couns. 2010;80(3):393–398.
- Alsomali HJ, Vines DL, Stein BD, et al. Evaluating the effectiveness of written dry powder inhaler instructions and health literacy in subjects diagnosed with COPD. Respir Care. 2017;62(2):172–178.
- Braido F, Chrystyn H, Baiardini I, Respiratory Effectiveness Group, et al. “Trying, But Failing” – the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract. 2016;4(5):823–832.