Abstract
Objective
In the absence of a head-to-head study, we assessed the comparative effectiveness of pegcetacoplan, a targeted C3 complement inhibitor, vs. ravulizumab, a C5 complement inhibitor, among patients with paroxysmal nocturnal hemoglobinuria (PNH) previously treated with eculizumab using matching-adjusted indirect comparison methodology.
Methods
Individual patient data from the PEGASUS study (NCT03500549) comparing pegcetacoplan and eculizumab enabled adjustment for baseline differences compared with published results from the ALXN1210-PNH-302 study (NCT03056040), comparing ravulizumab and eculizumab. Adjusted differences and 95% confidence intervals (CIs) were computed via weighted Wald tests for comparisons of pegcetacoplan vs. ravulizumab, anchored to the common comparator eculizumab.
Results
Sixty-eight patients from PEGASUS (36 pegcetacoplan; 32 eculizumab) and 195 from ALXN1210-PNH-302 (97 ravulizumab; 98 eculizumab) were included. Compared with ravulizumab, treatment with pegcetacoplan was associated with more transfusion avoidance (adjusted difference [95% CI] = +71.4% [53.5%, 89.3%]), hemoglobin level stabilization (+75.5% [56.4%, 94.6%]), lactate dehydrogenase (LDH) level normalization (+64.0% [41.8%, 86.1%]), and fewer blood transfusions (−5.7 units [−7.2, −4.2]). Additionally, patients who received pegcetacoplan experienced clinically meaningful improvements in fatigue (+8.2 points [3.8, 12.6]), global health status (+9.6 points [0.1, 19.0]), physical functioning (+11.5 points [3.6, 19.5]), and fatigue symptoms (−13.3 points [−23.7, −3.0]), compared with ravulizumab. Mean change from baseline in LDH level was not significantly different for pegcetacoplan vs. ravulizumab.
Conclusions
Results suggest that among patients previously treated with eculizumab, clinical, hematological, and quality of life endpoints were better for patients who received the C3 complement inhibitor pegcetacoplan vs. patients who received ravulizumab, a C5 complement inhibitor.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, chronic, and life-threatening hemolytic disease caused by a somatic mutation in bone marrow stem cells. The blood cells produced by these stem cells lack protective proteins at their surface, and as a result, are overly susceptible to activation or destruction by the complement systemCitation1. In the United States (U.S.), the prevalence of PNH is estimated to be approximately 12–13 cases per 1,000,000 personsCitation2. If left untreated, the 10-year mortality rate is approximately 24–29%. Eculizumab (Soliris), a C5 complement inhibitor approved for first-line treatment in patients with PNH, is associated with sustained improvements in intravascular hemolysis leading to lactate dehydrogenase (LDH) level normalization improvement, anemia reduction, reduced thromboembolic events, transfusion avoidance, survival, and quality of life (QoL) measures such as fatigue and global health statusCitation3–8. In addition to eculizumab, the C5 complement inhibitor ravulizumab (Ultomiris) was recently approved by the Food and Drug Administration (FDA) for treatment of PNHCitation9. Ravulizumab is a new, modified C5 complement inhibitor, with a longer terminal half-life than that of eculizumab. It has been shown to be non-inferior to eculizumab for outcomes including change in LDH, LDH level normalization, transfusion avoidance, fatigue, breakthrough hemolysis, and hemoglobin level stabilizationCitation10,Citation11. Based on the results of the multicenter, randomized, open-label ALXN1210-PNH-302 study (NCT03056040), ravulizumab represents a switching agent for complement inhibitor-experienced patients with PNH who were on stable treatment with eculizumabCitation10.
While treatment with eculizumab and ravulizumab has improved clinical outcomes and QoL measures, a substantial proportion of patients with PNH who receive these C5 complement inhibitors may continue to develop clinical manifestations of diseaseCitation12,Citation13. For example, in a retrospective analysis of 141 patients with PNH who had received treatment with eculizumab for ≥13 months, 72% of patients remained anemic and 36% required ≥1 transfusion per yearCitation14. Additionally, evidence from clinical trials and observational studies suggests that up to 27% of patients receiving eculizumab will experience breakthrough hemolysisCitation7,Citation15,Citation16, and 25–35% of patients continue to require transfusions despite treatment with eculizumabCitation13.
The C3 targeting complement inhibitor pegcetacoplan (developed by Apellis Pharmaceuticals, Inc. ‘Apellis’; Waltham, MA, USA) has the potential to address the remaining clinical burden and unmet medical need in PNH patients treated with C5 complement inhibitors by mitigating intra- and extra-vascular hemolysis. C3 represents the central node for all complement pathways, and insufficient regulation in patients with PNH can prompt numerous detrimental inflammatory and immunostimulatory processes. Pegcetacoplan targets C3 and thereby acts upstream in the complement cascade in contrast to eculizumab and ravulizumab targeting C5, downstream to C3. In consequence to this, C3 inhibition by pegcetacoplan has a broader effect on subsequent complement cascade inhibition as compared to C5 inhibition by eculizumab and ravulizumab. Broader complement inhibition by pegcetacoplan may mitigate both intravascular hemolysis (which is mediated by the terminal complement protein C5 of the complement cascade and membrane attack complex formation) and extravascular hemolysis (which is mediated by opsonization with C3 fragments and subsequent phagocytosis), and result in improved hematologic benefit in patients with PNH relative to current approved treatmentsCitation17.
Pegcetacoplan has been investigated in PNH patients previously treated with eculizumab. The PHAROAH study (NCT02264639) is an open‐label, phase Ib, prospective, non‐randomized trial investigating pegcetacoplan as add-on therapy to eculizumabCitation18. Four patients were enrolled in an extension study and received treatment with pegcetacoplan for 2 years. Among these patients, pegcetacoplan was well‐tolerated and resulted in improved hematological and symptom responses. Transfusion avoidance was achieved in all four patients who completed the study. Additionally, three patients had LDH levels ≤1.5× upper limit of normal (ULN). Also, change from baseline in Functional Assessment of Chronic Illness Therapy (FACIT)‐Fatigue total score was >3 points in three of the four patients. Apellis conducted the PEGASUS study, a phase III randomized, open-label trial assessing the clinical efficacy and safety of pegcetacoplan compared with eculizumab among patients who had previously received eculizumab (NCT03500549). Results at week 16 demonstrated that pegcetacoplan was superior to eculizumab for change in hemoglobin level, and non-inferior for transfusion avoidance and change in absolute reticulocyte countCitation17.
While pegcetacoplan has been compared directly to eculizumab in a clinical trial setting in patients previously treated with eculizumabCitation17, there are evidence gaps for treatment outcomes with pegcetacoplan compared to other treatment options of patients with PNH. The purpose of this study was to assess the evidence gap that there is no head-to-head comparison of pegcetacoplan and ravulizumab among complement inhibitor-experienced patients who were previously treated with eculizumab (the PEGASUS study was initiated in June 2018; however, ravulizumab was not approved until December 2018). While eculizumab and ravulizumab share the same mechanism of action, there is no randomized controlled trial specifically evaluating pegcetacoplan vs. ravulizumab, and in the absence of such a direct comparison, clinicians may not be able to make conclusions for patients just based on the shared mechanism of action for C5 complement inhibitors. Therefore, to address this gap and provide relevant evidence, the comparative effectiveness of pegcetacoplan and ravulizumab among patients who were previously treated with eculizumab was evaluated through matching-adjusted indirect comparison (MAIC). This method uses individual patient data (IPD) from the PEGASUS study and aggregate results that have been published for the ALXN1210-PNH-302 study. The ALXN1210-PNH-302 study was ongoing at the time this MAIC was conducted, and given the interest in generating timely evidence comparing pegcetacoplan vs. ravulizumab, it was not feasible to wait for the completion of the study to begin relevant data requisition processes for IPD (or determine if it was possible) from the ALXN1210-PNH-302 study. Health technology assessment agencies have acknowledged MAIC as a valid and robust method of indirect comparison that can adjust for cross-trial differences, reduce bias, and provide reliable point estimates of treatment effectsCitation19. By combining IPD from the PEGASUS study and aggregate data from the ALXN1210-PNH-302, the MAICs described here provide timely comparative evidence for treatment outcomes of pegcetacoplan vs. ravulizumab among complement-inhibitor experienced patients with PNH that may inform clinicians and payer organizations.
Methods
Data sources and study populations
Supplemental Table 1 provides a summary of key study design elements for the PEGASUS and ALXN1210-PNH-302 study. The PEGASUS study is a prospective, multi-center, randomized, phase III, open-label study comparing pegcetacoplan and eculizumab among patients with PNH who had previously been treated with eculizumab. Patients enrolled in the PEGASUS study received stable-dose eculizumab for ≥3 months prior to screening and continued to have hemoglobin level <10.5 g/dL. A total of 80 patients were randomized to receive either monotherapy pegcetacoplan (n = 41) or monotherapy eculizumab (n = 39) for 16 weeks during the randomized controlled period, after a 4 week run-in period during which patients were treated with both pegcetacoplan and eculizumab. The ALXN1210-PNH-302 study is a prospective, multi-center, randomized, phase III, open-label study comparing ravulizumab and eculizumab among patients with PNH who had previously been treated with eculizumab. One hundred ninety-five patients were randomized to receive either monotherapy ravulizumab (n = 97) or monotherapy eculizumab (n = 98) for 26 weeks.
The patients included in this MAIC were ≥18 years of age, previously treated with eculizumab (PEGASUS study: ≥3 months; ALXN1210-PNH-302 study: ≥6 months), received meningococcal vaccination, had absolute neutrophil count >500/mm3 at screening, had adequate platelet count at screening (PEGASUS study: >50,000/mm3; ALXN1210-PNH-302 study >30,000/mm3), and did not have a previous history of bone marrow transplantation. In addition, IPD from the PEGASUS study were re-analyzed and patients with LDH level ≤1.5× ULN at screening and without major adverse vascular events (MAVE) in the 6 months prior to treatment were selected to align more closely with the patients enrolled in the ALXN1210-PNH-302 study.
Study endpoints
Definitions for clinical, hematological, fatigue, and QoL endpoints were similar across both the PEGASUS and ALXN1210-PNH-302 studies (refer to for a complete description of endpoint definitions). Clinical and hematological endpoints include transfusion avoidance, number of packed red blood cells (PRBCs) transfused, hemoglobin level stabilization, change from baseline in LDH level, and LDH level normalization (in the PEGASUS study, LDH level normalization was assessed in the absence of transfusions; in the ALXN1210-PNH-302 study, LDH level normalization was assessed regardless of transfusion status). Endpoints related to fatigue and QoL include fatigue (FACIT-Fatigue Scale), global health status, physical functioning, and fatigue symptoms (all measured via the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 [EORTC QLQ-C30]).
Table 1. Comparison of endpoint definitions.
Statistical methods
Given the presence of a common comparator arm (i.e. eculizumab) for both the PEGASUS and ALXN1210-PNH-302 studies, anchored comparisons incorporating information for all patients including pegcetacoplan and eculizumab treatment arms in the PEGASUS study, and ravulizumab and eculizumab treatment arms in the ALXN1210-PNH-302 study, were conducted in accordance with guidance from the National Institute for Care and Health Excellence (NICE)Citation19. Baseline demographic and clinical characteristics were summarized using frequencies and percentages for categorical variables, and means and standard deviations (SDs) for continuous variables before and after matching procedures. Following NICE guidance, baseline characteristics were classified as an effect modifier, a purely prognostic variable, or neither an effect modifier nor a purely prognostic variable, based on clinical input regarding biological plausibility (refer to Supplemental Table 2 for variable classifications used in these analyses)Citation19. Because between-study differences in purely prognostic variables do not affect inference of anchored MAICs, baseline matching procedures adjusted for effect modifiers only in order to avoid loss of precision due to over-matchingCitation19.
A propensity score model using logistic regression was used to estimate the likelihood of enrollment in the ALXN1210-PNH-302 study vs. the PEGASUS study. Weights were assigned such that the weighted means and proportions of baseline characteristics in the PEGASUS study exactly matched the means and proportions of the baseline characteristics reported in the ALXN1210-PNH-302 study. The weight of each patient enrolled in the PEGASUS study was equal to the inverse odds of their enrollment in the ALXN1210-PNH-302 study vs. the PEGASUS study. Due to the small sample size, it was not possible to adjust for all effect modifiers. Model adequacy was assessed through visual inspection of histograms of patient weights and effective sample size (ESS). In the absence of technical guidance regarding minimum ESS, models that retained at least 50% of the initial PEGASUS population while adjusting for important effect modifiers were selected. Additionally, the distribution of baseline demographic and clinical characteristics was compared across patient groups following matching. Because effect modifier classification varied by type of endpoint (refer to Supplemental Table 2), comparisons of clinical and hematological endpoints vs. fatigue and QoL endpoints were adjusted for separate baseline characteristics. Comparisons of clinical and hematological endpoints were adjusted for patient age at first study infusion, female sex, White race, Asian race, history of aplastic anemia, and LDH level at baseline (i.e. the means and proportions of these effect modifiers matched exactly for pegcetacoplan vs. ravulizumab patients). Patient race was determined through self-identification and categories defined by trial investigators included White, Asian, African American, and other/multiple races. Among these categories, Asian and White race represented the two largest self-reported categories and were prioritized for inclusion during matching procedures. In addition, evidence demonstrates that differences in clinical characteristics (e.g. history of MAVEs, thromboembolisms, red blood cell transfusions, and corticosteroid use) exist between patients who identify as Asian versus non-AsianCitation20,Citation21. Comparisons for fatigue and QoL endpoints were adjusted for patient age at first study infusion, weight, history of aplastic anemia, and LDH level at baseline. Clinical effect modifiers including hemoglobin level and history of transfusions were not matched on due to reduced ESS and the presence of extreme patient weights.
Before matching, Wald tests and 95% confidence intervals (CIs) were used to compare categorical and continuous outcomes (i.e. chi square and z tests, respectively). After matching, outcomes were compared between balanced treatment groups using statistical tests that incorporated weights generated during matching. Weighted Wald tests with 95% CIs were used for comparisons of categorical and continuous outcomes (i.e. weighted chi square and weighted z tests, respectively). Unanchored comparisons, which do not incorporate data for patients randomized to receive eculizumab, were conducted as sensitivity analyses. Per NICE guidance, effect modifiers and purely prognostic variables were included as matching variables in order to reliably predict endpoints through unanchored analysesCitation19.
Results
Study characteristics
Because IPD were available for the PEGASUS study, additional inclusion criteria were applied to patients enrolled in the PEGASUS study to ensure alignment with patients enrolled in the ALXN1210-PNH-302 study. After implementing criteria related to LDH and MAVEs, 36 patients from the pegcetacoplan arm and 32 patients from the eculizumab arm were included in this analysis (Supplemental Table 3). Because LDH was >1.5× ULN at screening, 12 patients (5 pegcetacoplan; 7 eculizumab) were excluded from the analysis. No patients in the PEGASUS study had MAVEs in the 6-month period prior to treatment. One hundred ninety-five patients were included from the ALXN1210-PNH-302 study: 97 ravulizumab patients, and 98 eculizumab patients.
Baseline characteristics
Prior to matching, the distribution of effect modifiers including patient age, race, weight, history of aplastic anemia, and LDH level was similar for patients randomized to receive pegcetacoplan in the PEGASUS study vs. ravulizumab in the ALXN1210-PNH-302 study (). Compared with patients who received ravulizumab, a greater proportion of pegcetacoplan patients were female (69.4% vs. 48.5%) and had a history of transfusions during the year before the study (72.2% vs. 13.4%). Mean hemoglobin was also lower for patients who received pegcetacoplan vs. ravulizumab (8.7 g/dL vs. 11.1 g/dL, respectively).
Table 2. Overview of study baseline characteristics before and after matching (clinical and hematological endpoints).
Table 3. Overview of study baseline characteristics before and after matching (fatigue and quality of life endpoints).
Endpoints
Clinical and hematological endpoints
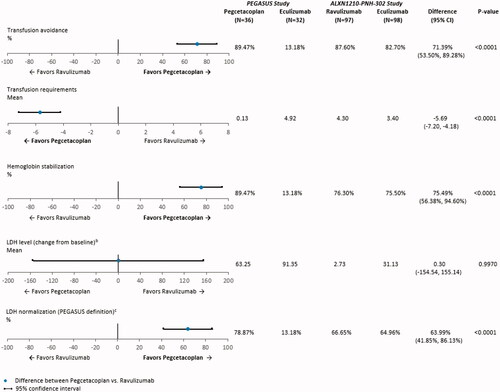
After anchoring on eculizumab, treatment with pegcetacoplan was associated with statistically significant improvements on numerous clinical and hematological endpoints when compared with ravulizumab (). The adjusted difference in the proportion of patients with transfusion avoidance was 71.4% (95% CI: 53.5%, 89.3%; p < .0001) for pegcetacoplan vs. ravulizumab, indicating that pegcetacoplan was associated with 71.4% more transfusion avoidance than ravulizumab. Compared with ravulizumab, pegcetacoplan was associated with a significant decrease in the mean number of units of PRBCs transfused during follow-up; on average, patients who received pegcetacoplan had 5.7 fewer units of PRBCs (95% CI: −7.2, −4.2; p < .0001) transfused during treatment than patients who received ravulizumab. The adjusted difference in the proportion of patients who achieved hemoglobin level stabilization was 75.5% (95% CI: 56.4%, 94.6%; p < .0001), suggesting pegcetacoplan was associated with 75.5% more hemoglobin level stabilization than ravulizumab. In addition, compared with ravulizumab, pegcetacoplan was associated with 64.0% more LDH level normalization in the absence of transfusions (95% CI: 41.9%, 86.1%; p < .0001). The adjusted mean difference in change from baseline in LDH level was not significant for pegcetacoplan vs. ravulizumab (0.3 U/L; 95% CI: −154.5 U/L, 155.1 U/L; p = .9970).
Figure 1. Anchored comparisons of clinical and hematological endpoints through Week 16 (PEGASUS study) and Week 26 (ALXN1210-PNH-302 study) after matchinga. Abbreviations. CI: confidence interval; LDH, lactate dehydrogenase; SD, standard deviation. Notes. aThe following baseline characteristics were matched on: age at first infusion of study drug, female, White, Asian, history of aplastic anemia, and LDH. bChange from baseline in LDH level was examined for Week 16 (Day 113) for the ALXN1210-PNH-302 study. Baseline mean and SD for LDH level were reported in of the Kulasekararaj et al.Citation10 publication. Week 16 (Day 113) mean and 95% CI for LDH level were extracted from Supplemental Figure S3 of the Kulasekararaj et al.Citation10 publication. The SD for LDH level at Week 16 (Day 113) was calculated using the following equation: √(N)*(upper limit of CI – lower limit of CI)/3.92. During follow-up, LDH level was available at Week 16 (Day 113) for 94 patients who received ravulizumab and 96 patients who received eculizumab in the ALXN1210-PNH-302 study. cIn the PEGASUS study, LDH level normalization is defined as the proportion of patients who achieved LDH level ≤1× ULN (226 U/L) in the absence of transfusions from baseline through the end of follow-up. In the ALXN1210-PNH-302 study, LDH level normalization is defined as the proportion of patients who achieved LDH level ≤1× ULN (246 U/L), with or without transfusions. Week 16 (Day 113) mean and 95% CI for the proportion of patients with LDH level normalization in the ALXN1210-PNH-302 study were extracted from of the Kulasekararaj et al. Citation10 publication.
Fatigue and quality of life endpoints
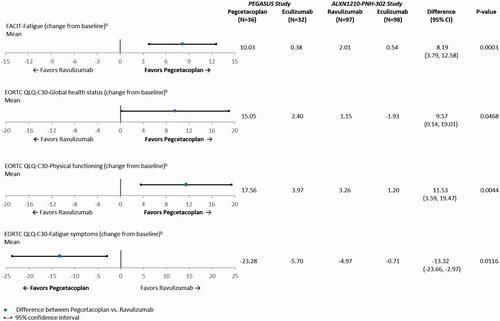
Outcomes related to fatigue and QoL (i.e. global health status, physical functioning, and fatigue symptoms) all showed statistically significant adjusted mean differences favoring pegcetacoplan when compared with ravulizumab (). The adjusted difference in mean change from baseline in FACIT-Fatigue was 8.2 points (95% CI: 3.8, 12.6; p = .0003). Thus, compared with ravulizumab, pegcetacoplan was associated with an improvement in fatigue that is ∼3 times greater than the clinically meaningful threshold of 3 points. The adjusted difference in mean change from baseline in global health status (EORTC QLQ-C30) was 9.6 points (95% CI: 0.1, 19.0; p = .0468), which suggests that pegcetacoplan was associated with an improvement of 9.6 points in global health status when compared with ravulizumab. In addition, compared with ravulizumab, patients who received pegcetacoplan reported a significant improvement from baseline in physical functioning (EORTC QLQ-C30) (11.5 points; 95% CI: 3.6, 19.5; p = .0044). Finally, the adjusted difference in mean change from baseline in fatigue symptoms (EORTC QLQ-C30) favored pegcetacoplan, and was associated with an alleviation of fatigue symptoms by 13.3 points (95% CI: −23.7, −3.0; p = .0116) when compared with ravulizumab.
Figure 2. Anchored comparisons of fatigue and quality of life endpoints through Week 16 (PEGASUS study) and Week 26 (ALXN1210-PNH-302 study) after matchinga. Abbreviations. CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; FACIT, Functional Assessment of Chronic Illness Therapy; LDH, lactate dehydrogenase. Notes. aThe following baseline characteristics were matched on: age at first infusion of study drug, weight, history of aplastic anemia, and LDH. bData were available for 67 of 68 patients in the PEGASUS study.
Anchored comparisons based on the unweighted sample before matching produced similar results for all clinical, hematological, fatigue, and QoL endpoints (data not shown). Unanchored comparisons that excluded patients randomized to receive eculizumab in both studies were consistent in magnitude and direction of effect as the anchored comparisons (data not shown).
Discussion
In the absence of a head-to-head randomized controlled trial, indirect treatment comparisons may be conducted in order to generate comparative evidence for patient outcomes across different treatment options. However, indirect comparisons that examine aggregate data only are limited in their interpretation due to the bias from cross-trial differences in patient populations or study designs. By combining IPD from one trial and aggregate data from a second trial, MAICs are capable of addressing such limitations to reduce bias and provide reliable point estimatesCitation22. The present MAIC is the first study to compare the effectiveness of pegcetacoplan vs. ravulizumab in complement inhibitor-experienced patients who had previously been treated with eculizumab. After anchoring on the common comparator eculizumab and adjusting for relevant baseline demographic and clinical characteristics that were considered effect modifiers, results show that relative to ravulizumab, pegcetacoplan is associated with statistically significant and clinically meaningful improvements across numerous clinical, hematological, and QoL endpoints.
Compared with ravulizumab, the results of this MAIC demonstrated that pegcetacoplan was associated with increased hemoglobin level stabilization, more transfusion avoidance, and fewer units of PRBCs transfused during treatment, after anchoring to eculizumab. Hemoglobin levels are an important indicator of disease severity and are impacted by underlying intra- and extra-vascular hemolysis and bone marrow function in patients with PNHCitation23,Citation24. In addition, transfusion requirements represent an important measure of disease severity and hemolytic activity both before and during treatment with complement inhibitorsCitation11. During the PEGASUS study, patients being anemic despite pre-study eculizumab treatment achieved hemoglobin level stabilization in conjunction with the reduction in or complete avoidance of transfusions following the initiation of pegcetacoplanCitation17. Broader complement cascade inhibition of C3 by pegcetacoplan may mitigate both intravascular and extravascular hemolysis, and as such result in improved hematologic benefit in patients with PNH relative to eculizumab.
LDH, a marker of intravascular hemolysis and disease activity, is used to evaluate response to treatment, and its level has been shown to decrease along with the reduction of the hemolytic rateCitation23. While the increase in LDH level from baseline was greater for both pegcetacoplan and eculizumab patients in the PEGASUS study relative to the increases reported in the ALXN1210-PNH-302 study, these measures were accompanied by wide confidence intervals, and therefore a high level of uncertainty. Moreover, when comparing pegcetacoplan with ravulizumab, the results of this MAIC did not indicate a clinically meaningful difference in LDH level between the two treatments. While LDH has been a leading primary endpoint in clinical trials investigating terminal C5 agents such as ravulizumab and eculizumab, the clinical meaningfulness of this endpoint by itself is limited (i.e. LDH levels may remain elevated even during effective treatment with C5 complement inhibitorsCitation24, and are elevated in several conditions other than hemolysisCitation23). In addition to intravascular hemolysis, all patients receiving C5 agents experience C3-mediated extravascular hemolysis. Serum bilirubin represents one hematological biomarker of extravascular hemolysis and is typically elevated among patients with PNH treated with C5 complement inhibitors. This endpoint was not reported in the ALXN1210-PNH-302 study; however, C5 agents are not expected to mitigate extravascular hemolysis. In order to more completely evaluate hematological benefit in PNH patients, future studies, especially those comparing terminal C5 agents and proximal C3 agents such as pegcetacoplan, should examine serum bilirubin and other relevant markers of intravascular and extravascular hemolysis.
Compared with ravulizumab, pegcetacoplan was associated with a significant increase in the proportion of patients who achieved LDH level normalization (64.0%). This result may be conservative, as the ALXN1210-PNH-302 study did not exclude patients who had received a transfusion during follow-up from the efficacy analyses. In a sensitivity analysis, the definition of LDH level normalization was revised to match the ALXN1210-PNH-302 study definition, which was agnostic to transfusions of PRBCs (i.e. patients who received a transfusion during follow-up were not excluded in the measurement of LDH level normalization). Results confirm the primary analysis and show that regardless of transfusion status during follow-up, pegcetacoplan was associated with significantly more LDH level normalization (adjusted difference = 57.4%; 95% CI: 35.3%, 79.5%; p < .0001; data not shown). Because it is tied to reference values in the general population (i.e. normal range), LDH level normalization is a meaningful outcome from a clinical perspective because it better reflects actual patient improvement as opposed to endpoints that incorporate stabilization alone. Reports from the International PNH Registry and others have identified increases in LDH levels as a prognostic indicator in PNHCitation25–28. In addition, patients with PNH having elevated LDH levels are at increased risk of experiencing complications of PNH associated with increased risk of thromboembolism, mortality, and poor QoLCitation25. Based on the results of this MAIC, treatment with pegcetacoplan, targeting C3, evidenced a more robust control of intravascular hemolysis while also controlling for extravascular hemolysis among patients previously treated with eculizumab compared to patients on ravulizumab and prior eculizumab treatment, both targeting C5.
While previous clinical trials have primarily focused on the impact of treatment on the clinical and hematological endpoints described above, patients with PNH may still experience other symptoms. Patients with PNH have a greater, clinically meaningful, level of fatigue, as assessed via FACIT-Fatigue, compared with the U.S. general population and non-anemic cancer patientsCitation29,Citation30. In addition, the level of QoL, as measured by the EORTC QLQ-C30, is clinically meaningfully lower than the U.S. general population, and similar to that reported by patients with cancerCitation31,Citation32. Fatigue is a common effect of hemolytic anemia reported among ∼80% of patients with PNH, and may have a profound effect on patients’ QoL and restrict their ability to perform normal daily activitiesCitation25. For example, in a cross-sectional analysis of data collected through the International PNH Registry, the proportion of patients who had been hospitalized during the 6 months prior to completing the baseline survey was significantly greater for patients who reported experiencing fatigue compared with those who had not (∼25% vs. ∼15%; p < 0.01)Citation25. The results of this MAIC suggest that compared with ravulizumab, pegcetacoplan was associated with an improvement in fatigue that is ∼3 times greater than what is considered a clinically meaningful change (i.e. ≥3 points). The improvements in global health status, physical functioning, and fatigue symptoms were also clinically meaningful (i.e. ≥10 points) when comparing pegcetacoplan vs. ravulizumab. Thus, in addition to the mitigation of ongoing hemolysis, the results presented here provide further support that compared with ravulizumab, patients who were treated with pegcetacoplan experience greater control of disease symptoms and improved therapeutic benefit related to fatigue and QoL. In addition to the clinical, hematological, and QoL endpoints examined in this study, future research should investigate how pegcetacoplan impacts morbidity and mortality among patients with PNH.
As with other analyses that utilize MAIC methodology, the results of this study are subject to limitations. While numerous inclusion and exclusion criteria are consistent across the PEGASUS and ALXN1210-PNH-302 studies, there are some differences in the study design and enrollment criteria. Hemoglobin level <10.5 g/dL at screening was required for enrollment in the PEGASUS study, but there was no requirement related to hemoglobin for the ALXN1210-PNH-302 study. As a result, mean baseline hemoglobin level was 8.7 g/dL among PEGASUS patients, and approximately 11.0 g/dL among ALXN1210-PNH-302 patients. However, to improve the similarity between the patient samples of the PEGASUS and ALXN1210-PNH-302 studies, IPD from the PEGASUS study were re-analyzed to incorporate additional clinical characteristics associated with disease severity specific to the ALXN1210-PNH-302 study as inclusion/exclusion criteria (i.e. LDH level ≤1.5× ULN at screening and history of MAVE in the 6 months prior to treatment). While differences in study design could not be fully accounted for (i.e. demonstrating non-inferiority of ravulizumab to eculizumab among stable patients vs. demonstrating superiority of pegcetacoplan vs. eculizumab among patients with decreased hemoglobin), appropriate MAIC adjustment was conducted to the extent feasible with the data, and it is noted that despite lower baseline hemoglobin among patients in PEGASUS, improved efficacy outcomes were demonstrated both within the trial and across the comparison to ALXN1210-PNH-302. Additionally, differences in route of administration, treatment administration schedule, dosing regimen, and treatment modifications for the PEGASUS and ALXN1210-PNH-302 studies cannot be adjusted for in statistical analyses. Matching procedures were limited to the baseline demographic and clinical characteristics published in the ALXN1210-PNH-302 study, and residual confounding may exist due to the presence of unreported or unobserved cross-trial differences. In addition, not all baseline clinical characteristics classified as effect modifiers could be matched on (i.e. hemoglobin level and history of transfusions prior to study) due to imbalance across studies and impact on effective sample size. It was not possible to examine the primary efficacy endpoint for the PEGASUS study (i.e. change from baseline in hemoglobin level) because such data were not reported by the ALXN1210-PNH-302 study. The different length of follow-up for the PEGASUS study (16 weeks) and the ALXN1210-PNH-302 study (26 weeks) may result in underestimation of endpoints. Future analyses of PEGASUS study data collected through 48 weeks of follow-up may be conducted to confirm the results presented here. Finally, as is common for rare diseases, sample sizes for the studies included in this MAIC were small. However, even for small sample sizes, MAIC methodology reduces bias and provides reliable estimates of statistical uncertaintyCitation33.
Conclusions
This was the first study, to our knowledge, to examine the comparative effectiveness of pegcetacoplan vs. ravulizumab in patients with PNH who had previously received eculizumab. While direct comparisons from head-to-head, randomized trials are considered the ‘gold standard’ for generating comparative evidence for two pharmacological treatments, the MAIC described in this study enabled a timely, statistically robust comparison of numerous outcomes for two treatments, pegcetacoplan and ravulizumab, not studied in the same trial. After anchoring on eculizumab, results show an improvement in transfusion avoidance, hemoglobin level stabilization, intravascular hemolysis, fatigue, and QoL measures, and a reduction in PRBCs transfused, for patients who received the C3 inhibitor pegcetacoplan vs. patients who received the C5 inhibitor ravulizumab. For clinicians assessing treatment options, results show improved benefits of switching to pegcetacoplan vs. ravulizumab, among patients previously treated with eculizuma.
Transparency
Declaration of funding
This study was funded by Apellis Pharmaceuticals, Inc.
Declaration of financial/other relationships
Sujata P. Sarda, Nikita Mody-Patel, Sangeeta Krishnan, and Scott Baver are employees of Apellis Pharmaceuticals, Inc., and own stock/stock options. Rachel H. Bhak, Colin Kunzweiler, Christopher W. Yee, Sanjana Sundaresan, Natalia Swartz, and Mei Sheng Duh are employees of Analysis Group Inc., which received research funding from Apellis Pharmaceuticals, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Authors contributed to the conception and design of the study; drafting and critical revision of the article for important intellectual content; and approved the final version. All authors agree to be accountable for all aspects of this study. RHB: all of the above; NM-P: all of the above; SB: all of the above; CK: all of the above; CWY: all of the above; SS: all of the above; NS: all of the above; MSD: all of the above; SK: all of the above; SPS: all of the above.
REVISED_SUPP_MAT__clean_-Bhak-Manuscript-CMRO-MAIC_of_pegcetacoplan_in_second-line_PNH-07.19.2021.docx
Download MS Word (57.4 KB)Acknowledgements
Apellis Pharmaceuticals, Inc. and Swedish Orphan Biovitrum AB (‘Sobi’) reviewed and provided feedback on the manuscript.
Data availability statement
For data requests, please contact Rachel H. Bhak ([email protected]).
References
- Brodsky RA. Stem cell transplantation for paroxysmal nocturnal hemoglobinuria. Haematologica. 2010;95(6):855–856.
- Jalbert JJ, Chaudhari U, Zhang H, et al. Epidemiology of PNH and real-world treatment patterns following an incident PNH diagnosis in the US. Blood. 2019;134(Supplement_1):3407–3407.
- Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–1847.
- Hill A, Hillmen P, Richards SJ, et al. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(7):2559–2565.
- Hillmen P, Elebute M, Kelly R, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85(8):553–559.
- Hillmen P, Muus P, Duhrsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–4128.
- Hillmen P, Muus P, Roth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(1):62–73.
- Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243.
- Alexion Pharmaceuticals, Inc. Ultomiris [prescribing information]. Boston (MA): Alexion Pharmaceuticals, Inc.; 2018.
- Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019;133(6):540–549.
- Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530–539.
- DeZern AE, Dorr D, Brodsky RA. Predictors of hemoglobin response to eculizumab therapy in paroxysmal nocturnal hemoglobinuria. Eur J Haematol. 2013;90(1):16–24.
- Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811.
- McKinley CE, Richards SJ, Munir T, et al. Extravascular hemolysis due to C3-Loading in patients with PNH treated with eculizumab: defining the clinical syndrome. Blood. 2017;130(Supplement 1):3471–3471.
- Nakayama H, Usuki K, Echizen H, et al. Eculizumab dosing intervals longer than 17 days may be associated with greater risk of breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria. Biol Pharm Bull. 2016;39(2):285–288.
- Peffault de Latour R, Fremeaux-Bacchi V, Porcher R, et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125(5):775–783.
- Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037.
- de Castro C, Grossi F, Weitz IC, et al. C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am J Hematol. 2020;95(11):1334–1243.
- Phillippo D, Ades A, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. Decision Support Unit, ScHARR, University of Sheffield: NICE Decision Support Unit; 2016.
- Jang JH, Okamoto S, Sakurai M, et al. Comparison of baseline clinical characteristics between Asian Vs. Non-Asian patients with paroxysmal nocturnal hemoglobinuria (PNH) from international PNH registry. Blood. 2017;130(Supplement 1):3472–3472.
- Sakurai M, Jang JH, Chou WC, et al. Comparative study on baseline clinical characteristics of Asian versus non-Asian patients with paroxysmal nocturnal hemoglobinuria. Int J Hematol. 2019;110(4):411–418.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
- Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Markers. 2015;2015:635670.
- Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157.
- Schrezenmeier H, Muus P, Socie G, et al. Baseline characteristics and disease burden in patients in the international paroxysmal nocturnal hemoglobinuria registry. Haematologica. 2014;99(5):922–929.
- Lee JW, Jang JH, Kim JS, et al. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean registry. Int J Hematol. 2013;97(6):749–757.
- Jang JH, Kim JS, Yoon SS, et al. Predictive factors of mortality in population of patients with paroxysmal nocturnal hemoglobinuria (PNH): results from a Korean PNH registry. J Korean Med Sci. 2016;31(2):214–221.
- Yenerel MN, Muus P, Wilson A, et al. Clinical course and disease burden in patients with paroxysmal nocturnal hemoglobinuria by hemolytic status. Blood Cells Mol Dis. 2017;65:29–34.
- Cella D, Eton DT, Lai J-S, Peterman AH, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561.
- Cella D, Lai J, Chang CH, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538.
- Snyder CF, Blackford AL, Sussman J, et al. Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients’ supportive care needs. Qual Life Res. 2015;24(5):1207–1216.
- Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 reference values. Brussels (Belgium): EORTC Quality of Life Group; 2008.
- Cheng D, Ayyagari R, Signorovitch J. The statistical performance of matching-adjusted indirect comparisons. 2019: arXiv:1910.06449. [cited 2019 Oct 1]. https://ui.adsabs.harvard.edu/abs/2019arXiv191006449C.
