Abstract
Objectives
Long-term real-world management of inflammatory rheumatic diseases remains unclear, especially with the advent of new treatment options. This study characterizes the number of advanced treatments used by patients with selected rheumatic diseases (rheumatoid arthritis [RA], psoriatic arthritis [PsA], ankylosing spondylitis, juvenile idiopathic arthritis) and provides a contemporary portrait of treatment patterns and therapeutic sequencing among patients with RA and PsA.
Method
Patients were selected from a large US claims database and classified into disease subsamples based on the latest rheumatic diagnosis recorded before/on the day of initiation of the first advanced treatment (index date). The total number of advanced treatments was assessed within the first 5 years following the index date. Treatment patterns and therapeutic sequencing were assessed over the first 2 years.
Results
Approximately 20% of patients received ≥2 distinct advanced treatments during the first year following index date – the proportion increased to almost 50% among patients with 5 years of observation. Most patients (RA: 76.8%; PsA: 88.7%) initiated a tumor necrosis factor as the first advanced treatment. Over the first 2 years after the index date, 1/3 of RA and PsA patients switched to another advanced treatment. More than 50% initiated a second treatment with the same mechanism of action (MOA). A small proportion of patients received a biosimilar.
Conclusion
Despite advent of treatments with different MOA, cycling between treatments with the same MOA was common. Further studies with longer data follow-up would be needed to assess the impact of higher adoption of biosimilars on treatment patterns/sequencing.
Introduction
It is estimated that approximately 1.71 billion people are living with rheumatic diseases worldwideCitation1. With the aging of the population, the number of patients living with rheumatic diseases is expected to continue to increase over the next decades. In the United States (US), it is projected that by 2040, 78 million (26%) of adults aged 18 years or older would have received a diagnosis for some form of rheumatic diseases – compared to 54.4 million in 2013–2015Citation2–4. Rheumatic diseases can significantly impair mobility and daily functioning, and thus considerably impact the overall patient’s quality of life. Rheumatic diseases are reported as a leading cause of morbidity and disability worldwideCitation1,Citation5–7.
Rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS) and juvenile idiopathic arthritis (JIA) are the most frequent forms of inflammatory rheumatic diseasesCitation8. There is currently no curative treatment option for patients with inflammatory rheumatic diseases. Treatments aim to manage pain/symptoms and reduce inflammation to limit joint/structural damage and improve daily functioning and well-beingCitation8–12. The American College of Rheumatology (ACR) and the European League against Rheumatism (EULAR) typically advocate for a treat-to-target approach to manage patients with inflammatory rheumatic diseasesCitation9–12. Pharmacological treatments for the management of these patients typically comprise different classes of medications including non-steroidal anti-inflammatory drugs (NSAIDs), conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologic DMARDs (bDMARDs) and janus kinase inhibitors (JAKi). The choice of treatment varies depending on the disease activity state/severity and prognostic factors, with bDMARDs and JAKi being typically reserved for patients with higher activity state. For example, for RA, which is one of the most common inflammatory rheumatic diseases, guidelines typically recommend initiation of csDMARDs (e.g. methotrexate) along with short-term glucocorticoids following clinical diagnosis. For patients without satisfactory response within 3–6 months, depending on the patient’s prognostic factors (disease activity, joint damage) guidelines recommend change or addition of a second csDMARD, a bDMARD or JAKi. For patients who failed to achieve satisfactory response within 3 to 6 months of initiation of the second treatment, a switch to a new bDMARD or JAKi (from a different or same class) is then recommendedCitation11.
The treatment landscape for patients with inflammatory rheumatic diseases has drastically evolved over the last decades. The advent of biosimilars (providing access to biologics at lower costs) and the increasing numbers of advanced treatment options (i.e. tumor necrosis factor inhibitor (TNFi), non-TNFi and JAKi) with different mechanisms of actions (MOA) and route of administrations (oral vs. injectable) have improved access to optimal care, especially for patients with a more severe disease form. Optimal treatment choices and therapeutic sequencing strategies are tailored to patients. Notwithstanding guideline recommendations, in addition to disease activity state, prognostic factors and safety, other considerations such as patient comorbidity profile, patient preferences, treatment history, and costs can also impact treatment decisionsCitation9–11,Citation13. It remains unclear how physicians and patients approach the long-term management of inflammatory rheumatic diseases in a real-world setting, especially with the advent of new advanced treatment options.
Accordingly, the objective of this study was twofold. First, to characterize the overall number of different advanced treatments (JAKi, TNFi, non-TNFi) that patients with selected rheumatic diseases (RA, PsA, AS, and JIA) use in the first 5 years from initiation of the first advanced treatment (Phase I). Second, among subsamples of patients with RA and PsA, to characterize treatment patterns and therapeutic sequencing following initiation of the first advanced treatment (Phase II).
Methods
Data source and study design
This study used data from the IBM MarketScanFootnotei Commercial Database between 1 January 2014, and 31 July 2020. The MarketScan Commercial and Medicare Supplemental Databases represent health services of approximately 130 million employees, dependents, and retirees in the US with primary or Medicare supplemental coverage through privately insured fee-for-service, point-of-service, or capitated health plans. The databases include de-identified data and are compliant with the US Health Insurance Portability and Accountability Act (HIPAA). Analyses were conducted using the Instant Health Data (IHD) platform developed by Panalgo.
The study was descriptive in nature. Analyses were carried out separately for each of the selected inflammatory rheumatic diseases (i.e. RA, PsA, AS and JIA). For patients in each of these subsamples, the index date was defined as the date of initiation of the first advanced treatment (i.e. TNFi, non-TNFi or JAKi) () and the treatment initiated on that date as the index treatment. The baseline period was defined as the 6-month prior to the index date. The number of advanced treatments used by patients was assessed within 1, 2, 3, 4 and 5 years following the index date, among subgroups of patients with continuous health care plan enrollment over the periods of interest. Treatment patterns and therapeutic sequencing were assessed among the subsamples of patients with RA and PsA, over the first 2 years following the index date.
Table 1. List of treatments.
Patient selection criteria
Phase I analyses
Patients with ≥1 pharmacy or medical claim for one of the selected advanced treatments and with ≥2 medical claims (at least 30 days apart) associated with a diagnosis codeFootnoteii for RA, PsA, AS or JIA were selected.Footnoteiii Except for patients in the JIA subsample, all patients were required to be ≥18 years of age as of the index date. Continuous health care plan enrollment was required for ≥6 months prior to and ≥12 months after the index date. Patients using advanced treatment combination therapy during the observation period or with pharmacy claims with invalid/incomplete days of supply information for the selected advanced treatments were excluded.
Selected patients were classified into subsamples (i.e. RA, PsA, AS and JIA) based on the latest rheumatic diagnosis recorded before or on the day of initiation of the first advanced treatment.
Phase II analyses
Phase II analyses were limited to the subsamples of patients with RA and PsA. To allow sufficient time to observe treatment changes and therapeutic sequencing, the RA and PsA subsamples were limited to patients with continuous health care plan enrollment for ≥2 years after the index date.
Study outcomes
Phase I analyses
Patient demographic and clinical characteristics were described for the full samples of RA, PsA, JIA and AS patients with ≥1 year of continuous health plan enrollment following the index date. The number of different advanced treatments used was assessed within 1, 2, 3, 4, and 5 years following the index date. For each drug, the originator and the biosimilars were considered as a one treatment.
Phase II analyses
Patient demographics and clinical characteristics, stratified by the treatment class initiated on the index date (i.e. TNFi, non-TNFi or JAKi), were described for the RA and PsA subsamples. Treatment patterns and therapeutic sequencing during the 2 years following the index date were also assessed.
More specifically, switching rates and days to first switch were assessed and reported overall and stratified by the treatment class initiated on the index date. A treatment switch was defined as the initiation of a new advanced treatment. Days to first switch was defined as the number of days between the initiation of the index treatment to the date of initiation of a new advanced treatment. Among patients who did not switch, the proportion of patients who remained treated with the index treatment (with or without temporary treatment interruption) versus discontinued the index treatment and remained untreated (without any advanced treatments) were reported.
Therapeutic sequencing and treatment received within each line was also characterized, both at the class level and at the drug level. A line of therapy was defined as the period between the initiation of the advanced treatment until its discontinuation or a switch to another advanced treatment. For each drug, the brand and the biosimilars were considered as a unique treatment. However, the proportion of patients treated with biosimilars was also assessed separately.
Results
Phase I analyses
Overall, a total of 23,212 RA patients, 8,856 PsA patients, 1,388 JIA patients, and 3,070 AS patients had at least one year of continuous health care plan enrollment following the index date and were included in the analyses (full sample, ). Patient demographics and clinical characteristics for the full sample are described in .
Table 2. Number of unique advanced treatments used.
Table 3. Baseline patient demographics and clinical characteristics – full sample of patients with at least 1-year follow-up period.
Within the first 5 years after the index date, the mean number of advanced treatments received by patients varied from approximately 1.2 during the first year to 1.8 during the five years after the index date. This trend was similar across all studied indications. While around 20% of patients received two or more advanced treatments during the first year of observation, this proportion increased with the duration of the observation period, reaching almost 50% during the five-year observation period (22.1 vs. 46.7% for RA patients; 18.8 vs. 45.4% for JIA patients; 22.4 vs. 48.1% for PsA patients and 20.5 vs. 45.2% for AS patients) ().
Phase II analyses
A total of 13,846 RA patients and 5,167 PsA patients met the selection criteria for inclusion for the Phase II analyses. TNFi were the most common class of advanced treatments initiated on the index date (RA: 10,631 [76.8%]; PsA: 4,585 [88.7%]) followed by non-TNFi (RA: 2,243 [16.2%]; PsA: 567 [11.0%]). Only a minority of patients initiated a JAKi on the index date (RA: 972 [7.0%]; PsA: 15 [0.3%]) ( and ). Among the RA subsample, the most common index treatments were adalimumab (35.6%) and etanercept (29.1%), followed by tofacitinib (7.0%) and rituximab (6.6%). Adalimumab (50.5%) and etanercept (27.9%), were also the most common index treatments received in the PsA subsample, followed by ustekinumab (5.4%) and infliximab (5.0%). All patients who initiated a JAKi on the index date among the RA and PsA subsamples were treated with tofacitinib.
Table 4. Treatment received by line of therapy – among RA patients over the 2-year follow-up period.
Table 5. Treatment received by line of therapy – among PsA patients over the 2-year follow-up period.
Median age as of the index date was 54 years old in the RA subsample and 50 years old in the PsA subsample ( and ). The median number of days between the first observed RA/PsA diagnosis and the initiation of the first advanced treatment was 256 days in the RA subsample and 140 days in the PsA subsample. For both subsamples, the shortest time was observed among patients who initiated a TNFi (RA: median = 245 days; PsA: median = 133 days) and the longest among those who initiated a JAKi (RA: median = 375.5 days; PsA: median = 252 days). Hypertension was the most frequent comorbid conditions observed during the baseline period (RA: 33.8%, PsA: 31.5%) followed by hyperlipidemia (RA: 21.9%. PsA: 20.3%) and diabetes (RA: 13.7%; PsA: 13.1%). More than one third of PsA patients had a diagnosis for psoriasis recorded during the baseline period (results not shown).
Table 6. Baseline patient demographics and clinical characteristics – RA patients.
Table 7. Baseline patient demographics and clinical characteristics – PsA patients.
Over the 2-year observation period, 32.3% of patients in the RA subsample and 33.9% of patients in the PsA switched to another advanced treatment (). Among patients who switched, most patients switched within the first year following the index date (median time: 270 days for both RA and PsA). More than half of patients who switched initiated another advanced treatment with the same MOA ( and ). Most common treatment switches were between etanercept and adalimumab – in more than 40% of cases in both the RA and PsA subsamples. Ultimately, 60.8 and 54.6% of RA and PsA patients who switched, initiated a treatment with a different MOA than the one initiated on the index date.
Figure 1. Switch rates patients with 2-year follow-up period – stratified based on index medication. (a) RA patients. (b) PsA Patients. Notes: JAKi: Janus kinase inhibitors; TNFi: Tumor necrosis factor inhibitors. Figure 1b: results for the JAKi cohort only were not reported due to the small sample size.
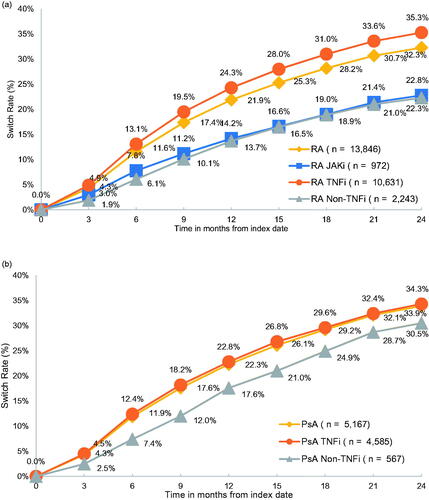
Table 8. 25 Most frequent treatment sequences at the class level – RA patients with 2-year follow-up period.
Table 9. 25 Most frequent treatment sequences at the class level - PsA patients with 2-year follow-up period.
Among patients who did not switch, the majority (RA: 62.0%, PsA: 62.6%) were still treated with the index treatment at the end of the observation period, with or without treatment interruption (mean duration of treatment interruption was 75.6 days for RA, 80.7 days for PsA). Among patients who discontinued the index treatment and did not receive any other advanced treatments (RA: 38.0%; PsA: 37.4%), treatment discontinuation occurred on average within the first year following the initiation of the index treatment (RA: mean = 298.1 days; PsA mean = 299.4 days).
The most common treatment sequences observed among the RA subsample involved the initiation of a TNFi or non-TNFi without further treatment (49.7 and 12.6%, respectively) or the initiation of a TNFi followed by a switch to another TNFi (9.9%) (). Among the PsA subsample, the majority of patients initiated a TNFi without further treatment (58.3%). Other common treatment sequences observed were the initiation of a TNFi followed by a switch to another TNFi (11.6%) or the initiation of a TNFi followed by a switch to non-TNFi (8.9%) (). In both the RA and the PsA subsamples, most patients who initiated a JAKi on the index date did not receive any further treatments ( and ).
Most common treatment sequences observed at the drug level over the 2-year observation period are presented in . Among the RA subsample, the five most common treatment sequences were all single-line treatments, i.e. adalimumab (22.8%), etanercept (18.7%), rituximab (6.1%), tofacitinib (5.4%), as well as abatacept (4.6%) without further treatments. Among the PsA subsample, two treatment sequences stood out, i.e. adalimumab (33.3%) and etanercept (18.2%) in the first-line therapy without further treatment ().
Figure 2. Top 20 treatment sequences at the drug level – patients with 2-year follow-up period. (a) RA patients. (b) PsA patients.
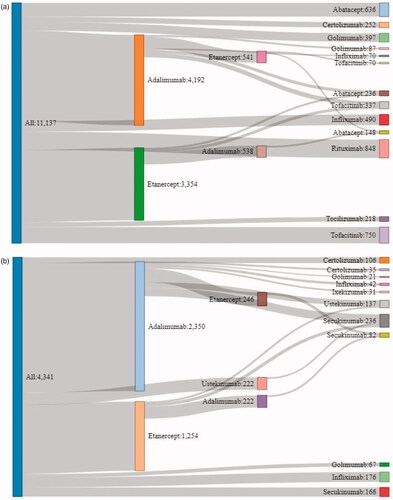
Irrespective of the treatment sequences, the proportion of patients treated with non-TNFi and JAKi increased in later lines. Non-TNFi and JAKi represented respectively 43.8 and 22.2% of treatments used in third line among RA patients and 52.3 and 5.1% among PsA patients ( and ).
The only biosimilar observed over the period covered by the study were infliximab biosimilars. Across all lines of therapy, a small proportion of patients treated with infliximab received a biosimilar (4.1% of RA patients and 4.6% of PsA patients). However, an increasing trend in the use of biosimilars was observed in the most recent years (results not shown).
Discussion
Using a large administrative claim database, this study provides a contemporary portrait of treatment patterns and therapeutic sequencing among patients with inflammatory rheumatic diseases treated with advanced treatments in the US.
Results showed that among patients with RA, PsA, AS and JIA, around 20% received two or more distinct advanced treatments during the first year following initiation of the first advanced treatment. This proportion increased with the duration of the observation period to reach almost 50% among patients with 5 years of observation.
Among the subsamples of RA and PsA patients, the vast majority (76.8% of RA patients and 88.7% of PsA patients) initiated a TNFi on the index date - mainly adalimumab and etanercept. A small proportion of patients initiated a JAKi (RA: 7.0%; PsA: 0.3%) as first line advanced treatment – however, the proportion increased in later lines, especially among RA patients, to reach over 20% in third and fourth lines after the index date. In both subsamples, within the first 2 years following the index date, most patients remained on the first advanced treatment (RA: 42.0%; PsA: 41.3%) or discontinued treatment and remained untreated (RA: 25.7%; PsA: 24.7%). Approximately 1/3 of RA and PsA patients switched to another advanced treatment.
To the best of our knowledge, no study has yet assessed real-world treatment patterns and sequencing among RA and PsA patients across the full spectrum of advanced treatments, i.e. including TNFi, non-TNFi and JAKi as well as biosimilars. However, the high proportion of RA and PsA patients initiating first-line advanced treatment on adalimumab and etanercept is consistent with the literature. Adalimumab and etanercept are well-established TNFi and generally reported as the most common treatments initiated among patients starting advanced treatmentsCitation14–17. Switching rates found in the current study are also generally consistent with those reported in the literatureCitation15,Citation18. In a recent study by Wu et al., assessing treatment patterns in PsA patients treated with bDMARDs (TNFi and non-TNFi) versus apremilast, authors reported a 33.3% switching rate at 2 years after treatment initiation in the bDMARDs cohortCitation15 – the switching rate among PsA patients was 32.3% at 2 years in the current study. Similarly, the one year switching rate found in the current study among the subsample of RA patients (21.9%) is also generally in line with the rates reported in the literature, ranging between 11 and 18%, although closer to the upper end of the spectrumCitation19–21.
Despite the advent of alternative treatment options with different MOA, results from the current study showed that cycling between treatments with the same MOA is commonCitation22,Citation23. More than half of patients who initiated a second treatment initiated an advanced treatment with the same MOA (e.g. TNFi -> TNFi). This finding is consistent with the literature demonstrating that cycling within the same MOA (especially among TNFi) is more common than switching to a different MOACitation16,Citation23,Citation24. Treating patients toward remission can involve multiple treatment switchesCitation11. Evidence suggests that switching to a treatment with a different MOA may lead to better outcomes, however among patients who failed to reach a satisfactory response to advanced treatments, a change to a treatment within the same class is still, in fact, recommended as an option (along with a change to a different class) in treatment guidelinesCitation9–11,Citation22,Citation23. In the current study, a little more than half of RA and PsA patients who switched, eventually initiated (in second or later lines) a treatment with a different MOA than the treatment initiated on the index date. Different reasons could explain why patients are switching to a different treatment, including lack of response, adverse events, cost considerations as well as physician/patients’ preference. Although, administrative claims databases provide rich information about real-life treatment patterns and therapeutic sequencing, there is no information available to confirm reasons for treatment decisions. Further study would be warranted to better understand reasons/factors associated with treatment changes and the choice of the subsequent advanced treatment including cycling between treatments with the same MOA.
Results also showed that although the use of biosimilars displayed an increasing trend over time, over the 2-year observation period, a small proportion of patients received a biosimilar (4.1% of RA patients and 4.6% of PsA patients treated with infliximab). Different reasons could explain the relatively low rate of biosimilars use in the US. Among them, the recent approval of biosimilars and the lack of preferred coverage among private health plans could delay the uptake of biosimilars. Although, substitution policies for biosimilars are being considered by many plans to reduce expenditures, the adoption of such policies is relatively low and varies a lot across the different plans in the USCitation25. Indeed, a recent report assessing coverage for biosimilars versus reference products among commercial plans in the US indicated that, in 2019, preferred coverage for biosimilars, i.e. biosimilar coverage ahead of the reference product, was adopted in only 14% of the decisionsCitation25. Although reasons for coverage decisions are not available, cycles of negotiation of reimbursement contracting agreements and private agreements including negotiated rebates might have contributed to delay the biosimilar uptake among patients with private insurance coverage. Further study would be warranted to assess patterns of use of biosimilars among patients who are not privately insured.
This study was subject to some limitations. First, claims data do not provide information on disease severity, which may affect treatment patterns and sequencing. Second, patients included in the study were identified based on the presence of a diagnosis code for rheumatic diseases. However, in administrative claim databases, diagnosis codes are recorded for billing purposes and may reflect suspected rather than confirmed diagnoses. Third, results showed that 1 in 4 patients discontinued their index treatment and remained untreated until the end of the 2 years observation period. Treatment patterns and sequencing evaluated in the current study focus on advanced treatments. It possible that among patients who stopped their first advanced treatment and remained untreated, a portion of them were later treated with other non-advanced treatments (e.g. conventional DMARDs). In addition, some of the discontinuations may be temporary (e.g. to manage adverse events or following clinical remission). It is possible that some of the patients eventually restarted an advanced treatment (e.g. after the reappearance of clinical manifestations), but by that we do not observe the patient long enough to capture the next treatment. Fourth, although administrative claims databases provide rich information about real-life treatment patterns and therapeutic sequencing, there is no information available to confirm reasons for treatment decisions and treatment changes. Fifth, the identification of the first line advanced treatment (index date) was based on the first pharmacy/medical claim for an advanced treatment observed over the period covered by the data – at least 6 months of continuous health care plan enrollment before the index date was required for all patients. It is, however, possible that some patients received advanced treatments prior to the start of the period covered by the data. Finally, the study sample consisted of privately insured employees and their dependents from the US. Prescribing patterns and access to care may differ for patients who are not privately insured. Accordingly, the generalizability of the findings is limited to a population of patients insured by private payers and may not be generalizable to the overall population of patients with rheumatic diseases. Further study would also be warranted to better understand how prescribing patterns vary across countries.
Conclusion
This is the first study to provide a comprehensive and contemporary portrait of treatment patterns and therapeutic sequencing among a large sample of patients with inflammatory rheumatic diseases treated with advanced treatments in the US. Results showed that among patients with RA, PsA, AS, and JIA initiating an advanced treatment, switching to different treatments is common − 20% patients received two or more distinct advanced treatments during the first year following initiation of their first advanced treatment. Despite the advent of treatments with different MOA, cycling between treatments with the same MOA was frequent. Further studies with longer data follow-up would be needed to assess the impact of higher adoption of biosimilars on treatment patterns/sequencing.
Transparency
Declaration of funding
Funding for this research was provided by Pfizer Inc.
Declaration of financial/other relationship
Dario Ponce de Leon, Juan Manuel Reyes, Esteban Chiarello and Rebecca Levin are employees of Pfizer Inc. and may own stocks/stock options. Geneviève Gauthier and Francis Vekeman are employees of StatLog Inc. which has received consultancy fees from Pfizer Inc. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors made substantial contribution to the study conception and design. Analyses were performed by Geneviève Gauthier and Francis Vekeman - all authors contributed to the interpretation of the results. All authors contributed to the development of the manuscript and maintained control over the final content.
Acknowledgements
Writing/editorial assistance was provided by Marie-Noëlle Robitaille from StatLog Inc.
Data availability statement
Data supporting the findings of this study are covered under a license agreement. Accordingly, data cannot be made public and/or shared.
Notes
i MarketScan is a registered trademark of IBM Corporation in the United States, other countries, or both.
ii RA: ICD-10 codes M05.x, M06.0x, M06.2x, M06.3x, M06.8x, and M06.9; ICD-9 codes 714.0, 714.1, 714.2, 714.81; AS: ICD-10 code M45.x; ICD-9 code 720.0; PSA: ICD-10 code L40.5x; ICD-9 code 696.0; JIA: ICD-10 codes M08.0x, M08.2x, M08.3, M08.4x, M08.8x, M08.9x; ICD-9 code 714.3x
iii At least one diagnosis was required to occur during the 6 months prior to or one the index date.
References
- World Health Organization. Fact sheet musculoskeletal conditions [Internet]; 2021 [cited 2021 Apr 6]. Available from: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.
- Hootman JM, Helmick CG, Barbour KE, et al. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582–1587.
- Center for Disease Control and Prevention. Arthritis-related statistics [Internet]; 2021 [cited 2021 Apr 6]. Available from: https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm
- Barbour KE, Helmick CG, Boring M, et al. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation — United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–253.
- Theis KA, Roblin DW, Helmick CG, et al. Prevalence and causes of work disability among working-age U.S. adults, 2011–2013, NHIS. Disabil Health J. 2018;11(1):108–115.
- American College of Rheumatology. 2015 Workforce study of rheumatology specialists in the United States [Internet]; 2016. Available from: https://www.rheumatology.org/Learning-Center/Statistics/Workforce-Study.
- Cieza A, Causey K, Kamenov K, et al. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2021;396(10267):2006–2017.
- American College of Rheumatology. Rheumatic diseases in America: the problem. The impact. The answers [Internet]; 2021 [cited 2021 Apr 6]. Available from: https://www.rheumatology.org/Learning-Center/Publications-Communications/White-Papers.
- Singh JA, Guyatt G, Ogdie A, et al. 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. J Psoriasis Psoriatic Arthritis. 2019;4(1):31–58.
- Singh JA, Saag KG, Bridges SLJ, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2015;68(1):1–25.
- Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–S699.
- Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the american college of rheumatology/spondylitis association of america/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599–1613.
- Hifinger M, Hiligsmann M, Ramiro S, et al. Patients' preferences and economic considerations play an important role in treatment decisions: a discrete choice experiment among rheumatologists. Rheumatology. 2017;56(1):68–76.
- Tymms K, Littlejohn G, Griffiths H, et al. Treatment patterns among patients with rheumatic disease (rheumatoid arthritis (RA), ankylosing spondylitis (as), psoriatic arthritis (PsA) and undifferentiated arthritis (UnA)) treated with subcutaneous TNF inhibitors. Clin Rheumatol. 2018;37(6):1617–1623.
- Wu JJ, Pelletier C, Ung B, et al. Treatment switch patterns and healthcare costs in Biologic-Naive patients with psoriatic arthritis. Adv Ther. 2020;37(5):2098–2115.
- Bonafede M, Fox KM, Watson C, et al. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther. 2012;29(8):664–674.
- Youssef P, Marcal B, Button P, et al. Reasons for biologic and targeted synthetic disease-modifying antirheumatic drug cessation and persistence of second-line treatment in a rheumatoid arthritis dataset. J Rheumatol. 2020;47(8):1174–1181.
- Zhang HF, Gauthier G, Hiscock R, et al. Treatment patterns in psoriatic arthritis patients newly initiated on oral nonbiologic or biologic disease-modifying antirheumatic drugs. Arthritis Res Ther. 2014;16(4):420–410.
- Gu T, Mutebi A, Stolshek BS, et al. Cost of biologic treatment persistence or switching in rheumatoid arthritis. Am J Manag Care. 2018;24(8 Spec No):SP338–SP435.
- Degli Esposti L, Favalli EG, Sangiorgi D, et al. Persistence, switch rates, drug consumption and costs of biological treatment of rheumatoid arthritis: an observational study in Italy. Clinicoecon Outcomes Res. 2017;9:9–17.
- Wilke T, Mueller S, Lee SC, et al. Drug survival of second biological DMARD therapy in patients with rheumatoid arthritis: a retrospective non-interventional cohort analysis. BMC Musculoskelet Disord. 2017;18(1):1–10.
- Gottenberg JE, Brocq O, Perdriger A, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. J Am Med Assoc. 2016;316(11):1172–1180.
- Chastek B, Becker LK, Chen CI, et al. Outcomes of tumor necrosis factor inhibitor cycling versus switching to a disease-modifying anti-rheumatic drug with a new mechanism of action among patients with rheumatoid arthritis. J Med Econ. 2017;20(5):464–473.
- Chastek B, Chen CI, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis changing biologics in the USA. Adv Ther. 2017;34(11):2422–2435.
- Chambers JD, Lai RC, Margaretos NM, et al. Coverage for biosimilars vs reference products among US commercial health plans. JAMA. 2020;323(19):1972–1973.
