Abstract
Objective
Global treatment guidelines recommend treatment with oral anticoagulants (OACs) for patients with non-valvular atrial fibrillation (NVAF) and an elevated stroke risk. However, not all patients with NVAF and an elevated stroke risk receive guideline-recommended therapy. A literature review and synthesis of observational studies were undertaken to identify the body of evidence on untreated and undertreated NVAF and the association with clinical and economic outcomes.
Methods
An extensive search (1/2010–4/2020) of MEDLINE, the Cochrane Library, conference proceedings, and health technology assessments (HTAs) was conducted. Studies must have evaluated rates of nontreatment or undertreatment in NVAF. Nontreatment was defined as absence of OACs (but with possible antiplatelet treatment), while undertreatment was defined as treatment with only antiplatelet agents.
Results
Sixteen studies met our inclusion criteria. Rates of nontreatment for patients with elevated stroke risk ranged from 2.0–51.1%, while rates of undertreatment ranged from 10.0–45.1%. The clinical benefits of anticoagulation were reported in the evaluated studies with reductions in stroke and mortality outcomes observed among patients treated with anticoagulants compared to untreated or undertreated patients. Adverse events associated with all bleeding types (i.e. hemorrhagic stroke, major bleeding or gastrointestinal hemorrhaging) were found to be higher for warfarin patients compared to untreated patients in real-world practice. Healthcare resource utilization was found to be lower among patients highly-adherent to warfarin compared to untreated patients.
Conclusions
Rates of nontreatment and undertreatment among NVAF patients remain high and are associated with preventable cardiovascular events and death. Strategies to increase rates of treatment may improve clinical outcomes.
Introduction
Atrial fibrillation (AF), the most common form of arrhythmia with 5.2 million prevalent cases in the United States (US) in 2010, is predicted to affect more than 12 million people in the US by the year 2030Citation1. The prevalence of AF among the general population is approximately 1%, but it increases with age to approximately 9% for persons 80 years or olderCitation2. AF leads to more than 450,000 hospitalizations annually in the USCitation3, and contributes to more than 150,000 deaths each yearCitation4. Non-valvular AF (NVAF), defined as AF in the absence of moderate-to-severe mitral stenosis or a mechanical heart valveCitation5, accounts for 95% of all AF patientsCitation2.
AF has been associated with a four- to five-fold increased risk of ischemic stroke due to the high risk of clotting leading to thromboembolic eventsCitation6. An individual’s risk of stroke is measured by CHA2DS2-VASc score, which is a composite measure of the presence of age, history of cardiovascular comorbidities and acute events, non-cardiovascular comorbidities, and sex, with weights for each criterion. The score is commonly used in clinical practice to estimate the risk of stroke and, by extension, to determine the need for anticoagulationCitation5.
Anticoagulant guidelines issued by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society (AHA/ACC/HRS) in 2014 and 2019 recommended OACs, including vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs), for patients with an elevated risk of stroke (CHA2DS2-VASc score of ≥2 in men or ≥3 in women)Citation5,Citation7–11. Moreover, the 2014 AHA/ACC/HRS guidelines considered aspirin an option for patients with NVAF and a moderate risk of stroke (CHA2DS2-VASc score of 1) but not an option for patients with an elevated risk of stroke; patients with an elevated risk of stroke who were treated with aspirin only could therefore be considered an undertreated patient population.
Warfarin, a VKA, has historically been the first-line stroke prevention option in patients with NVAFCitation12; trials have shown that warfarin can reduce the relative risk of stroke by up to two-thirdsCitation6,Citation13. DOACs, a novel class of OACs including factor Xa and direct thrombin inhibitors, were first approved in 2010Citation14,Citation15–17 based on their noninferiority of efficacy and safety compared with warfarin in randomized controlled trialsCitation18–21. The 2019 AHA/ACC/HRS guidelines recommend DOACs as the first-line OAC for stroke prevention ahead of VKAs, including warfarinCitation5.
Although guidelines recommend treatment for NVAF with anticoagulants, a large proportion of patients in the US remain untreated. A study conducted by the National Cardiovascular Data Registry (NCDR) Practice Innovation and Clinical Excellence (PINNACLE) program found that only 55% of patients deemed eligible for warfarin were actually treated with that agentCitation22. Additionally, almost 35% of NVAF patients eligible for warfarin were not treated with either warfarin or antithrombotic or antiplatelet agents, which was discordant with treatment guidelines during the time of the study’s analysisCitation22. There are several reasons why patients with NVAF remain untreated, including physician underestimation of patient stroke risk, physician overestimation of bleeding risk, and patient reluctance to maintain adherence to OACs due to pharmacokinetics and interactions with food and other medicationsCitation23. Furthermore, educating physicians and patients regarding the benefits of anticoagulant treatment are crucial to increasing rates and compliance associated with anticoagulationCitation23.
When left untreated, NVAF poses a substantial burden to society, as stroke events are estimated to cost the US healthcare system about $34 billion each year in both direct medical costs and indirect productivity losses (e.g. absenteeism, presenteeism, disability)Citation24. From both clinical and economic perspectives, there remains a high unmet need despite current viable treatment options. Understanding the rates of anticoagulant treatment, as well as the burden posed by nonadherence to treatment guidelines, may further inform clinicians and patients about the benefits of anticoagulant treatment. The goals of this research project were three-fold: (1) to conduct a systematic literature review to identify rates of undertreatment or nontreatment with OACs in NVAF, and assess them over time, (2) evaluate the clinical and economic burden of undertreated or untreated NVAF, and (3) identify gaps in the literature corresponding to the clinical and economic consequences of undertreatment or nontreatment.
Methods
Inclusion and exclusion criteria
A global systematic literature search was conducted in accordance with guidelines developed by PRISMACitation25, and the study protocol was registered with PROSPERO (ID: CRD42020187381). An extensive search of the literature was conducted over a 10-year period, from 1 January 2010 to 15 April 2020, for observational studies evaluating the burden of untreated or undertreated NVAF. Sources included MEDLINE (PubMed, including Medline in-process), GoogleScholar, the Cochrane Library, and relevant cardiovascular or pharmacoeconomic/pharmacoepidemiologic conference proceedings. The following search terms were explored in the systematic search process: (“atrial fibrillation”) AND (“epidem*” OR “prevalence” OR “incidence” Or “burden” OR “cost” OR “economic”). The key search terms included “atrial fibrillation” in an effort to capture the full body of evidence relating to the study objectives. Studies were limited to those written in the English language. Only full-text publications of research studies including database analyses, systematic literature reviews, meta-analyses, and registry analyses were included in our review. Studies were excluded if they focused exclusively on valvular AF, did not have full-length publications or did not provide an abstract.
The literature search and study selection were conducted in accordance with relevant best practicesCitation25. Three reviewers (NA, CT, MS) applied criteria to search results to identify relevant studies. See the supplemental materials for more details.
Study populations must have included patients with an elevated stroke risk based on the risk score described in the publication, either CHADS2 (score ≥2 based on 2011 ACCF/AHA/HRS guidelinesCitation26) or CHA2DS2-VASc (score ≥2 based on 2014 AHA/ACC/HRS guidelinesCitation2). Patients with elevated stroke risk receive the strongest recommendations from global treatment guidelines with regard to initiating and continuing anticoagulation treatment, and thus were the focus of this review.
Treatment definitions
AHA/ACC/HRS treatment guidelines have evolved over time as DOACs have become more commonly used. The 2014 AHA/ACC/HRS treatment guidelines considered aspirin an option for patients with moderate risk of stroke and suggested that patients with high risk would be considered undertreated if treated only with aspirin. In contrast, the 2019 AHA/ACC/HRS treatment guidelines indicate that patients with NVAF, regardless of stroke risk, should not receive monotherapy with antiplatelets unless contraindicated to an OAC.
For the purpose of this analysis, the 2014 AHA/ACC/HRS treatment guidelines were used to define rates of treatment for two reasons. First, despite the change in the guidelines from 2014 to 2019, aspirin monotherapy for stroke prevention is still observed among those patients without an OAC contraindicationCitation5,Citation11. Second, the time horizon of this literature review dated back to 1 January 2010 and therefore included multiple iterations of guidelines. Using the current 2019 AHA/ACC/HRS guidelines for this analysis would preclude populations treated with antiplatelets. Applying this standard to older studies would exclude an important body of evidence and would not accurately reflect real-world prescribing behavior in prior years. Thus, inclusion of rates and outcomes related to aspirin use only is warranted.
Undertreated patients were defined as those high-risk patients treated with antiplatelet agents only. Untreated patients were defined as those who did not receive anticoagulant therapy; depending on the specificity of the publication under review, untreated patients may or may not have been treated with antiplatelets.
Study measures
For each study meeting our selection criteria, extracted study measures included rates of treatment by class (i.e. DOACs, warfarin, antiplatelets/undertreatment, nontreatment) and associated clinical and economic outcomes. Clinical outcomes included rates of ischemic stroke events, death, and bleeding events (i.e. hemorrhagic stroke events, major bleeding events, gastrointestinal hemorrhaging). Economic outcomes included healthcare resource utilization and direct medical costs corresponding to the clinical outcomes as well as indirect costs (i.e. lost productivity costs, caregiver costs). Treatment rates were stratified by geographic setting (i.e. US vs. ex-US) and separately by time period. The time period under evaluation was segmented into two periods, 1 January 2010–December 2012 (termed the “pre-DOAC era”) and January 2013–15 April 2020 (termed the “DOAC era”). The end of 2012 was used as the threshold time point since the three most prescribed DOACs were approved and on the marketCitation27.
Risk of bias assessment
The modified Newcastle-Ottawa Scale for cohort studies was used to assess the quality of studies reporting an association between treatment rates for NVAF and clinical and/or economic outcomesCitation28. Studies were graded on a scale of 0 (lowest quality) to 9 (highest quality) by a single reviewer and validated by a second reviewer.
Results
Search results
A total of 11,231 publications were reviewed for inclusion in this analysis, including peer-reviewed publications, non-peer-reviewed HTA documents, and conference abstracts and posters. After applying inclusion and exclusion criteria, our initial full-text review identified 43 manuscriptsCitation22,Citation29–69, published between 2011 and 2020 that evaluated the burden of NVAF in undertreated, untreated, and treated populations. provides a complete flowchart of the study selection methodology. Further review clarified that a subset of sixteen manuscripts stratified findings by stroke risk score which are the focus of this analysisCitation29–44. provides a detailed summary of the sixteen manuscripts.
Figure 1. Attrition chart using PRISMA criteria. Abbreviations. AF, atrial fibrillation; CADTH, Canadian Agency for Drugs and Technologies in Health; HTA, health technology assessment; ICER, Institute for Clinical and Economic Review; NICE, National Institute for Health and Care Excellence; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses.
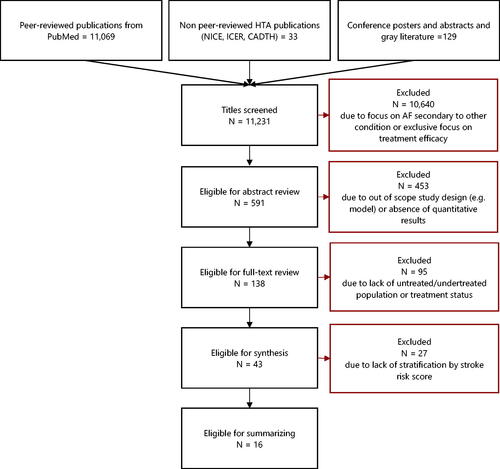
Table 1. Summary table of studies of the burden of nontreatment and undertreatment in NVAF.
Study characteristics
The majority of publications (15) were retrospective studies of claims databases, electronic health records, and preexisting registriesCitation30–44; the remaining study was a prospective evaluationCitation29.Seven studies focused on US populationsCitation30–36, two studies focused on UK populationsCitation37,Citation38, one study evaluated a multi-country populationCitation39, and the remaining six studiesCitation29,Citation40–44, covered other individual countries (e.g. Ireland, Japan, South Korea). All studies evaluated rates of treatment stratified by treatment class, including untreated patients. Several studies evaluated clinical outcomes pertaining to treatment, undertreatment, and nontreatment of AF, while one study also evaluated economic outcomes including resource utilization and direct costs of treatmentCitation30. Of these studies, 13Citation29,Citation31,Citation33–43 stratified study measures by CHA2DS2-VASc score while the remaining 3 studies stratified measures by CHADS2 scoreCitation30,Citation32,Citation44. All publications described high risk score as ≥2 on their respective scales.
Rates of undertreatment and nontreatment
Fourteen papersCitation29–39,Citation41–43 reported treatment rates for the NVAF subpopulation with elevated stroke risk. In patients with elevated stroke risk, the median rate of undertreatment was 35.3% (range: 12.1%Citation39 to 45.8%Citation36), while the median rate of nontreatment was 23.3% (range: 7.9%Citation39 to 51.1%Citation32). These studies used data from Ireland, New Zealand, South Korea, the United Kingdom, and the US, as well as combined global and pan-European data. A comparison between US and ex-US rates suggests higher rates of nontreatment in the US setting; the median rate of nontreatment in the US was 40% (range: 21% to 51%) while the median rate in ex-US settings was 20% (range: 11–43%).
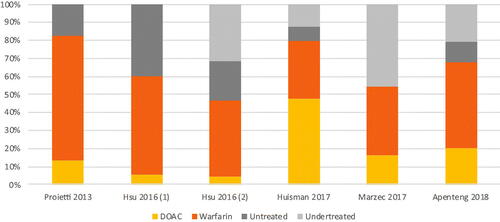
When broken down by era, the median rate of undertreatment decreased from 39.7% in the pre-DOAC era (range: 34.6%Citation29 to 45.1%Citation41) to 25.9% in the DOAC era (range: 12.1%Citation39 to 45.8%Citation36) (), while the median rate of nontreatment decreased from 33.6% in the pre-DOAC era (range: 13.4%Citation38 to 51.1%Citation32) to 17.4% in the DOAC era (range: 7.9%Citation39 to 40.2%Citation34) (). These median rates of undertreatment and nontreatment suggest declining trends in undertreated or nontreated NVAF patients with elevated stroke risk once DOACs became available.
Figure 2. Treatment rates by class among high-risk NVAF patients, pre-DOAC era. Abbreviations. DOAC, direct oral anticoagulants; NVAF, non-valvular atrial fibrillation.
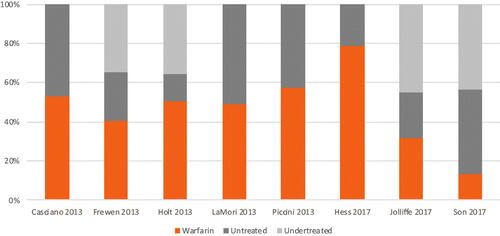
Figure 3. Treatment rates by class among high-risk NVAF patients, DOAC era. Abbreviations. DOAC, direct oral anticoagulants; NVAF, non-valvular atrial fibrillation.
Only Jolliffe 2016 reported reasons for nontreatment with OACs among patients with elevated stroke risk. The most frequently documented reason included decisions deferred to the primary care physician (15.6%), followed by the risk of falls or frailty (7.2%) and high risk of bleeding (6.6%); “no reason” was reported in 56.9% of these patientsCitation41.
Clinical consequences of undertreatment and nontreatment
Compared to treated patients with NVAF receiving anticoagulation, untreated patients had a significantly higher risk of adverse clinical outcomes such as strokes, mortality, and various composite outcomes, although patients on anticoagulants did have higher rates of bleeding compared to untreated patients.
Strokes and other ischemia were the most commonly reported clinical consequences of undertreatment or nontreatment of AF. Casciano 2013 found that high-risk patients without warfarin exposure had significantly higher rates of ischemic stroke than patients with low adherence (4.41 vs. 1.87 per 100 person-years, p < .001) or high adherence (4.41 vs. 0.72 per 100 person-years, p < .001) to warfarinCitation30. Similarly, patients without warfarin exposure had higher rates of transient ischemic attack than patients with low adherence (1.77 vs. 0.72 per 100 person-years, p < .001) or high adherence (1.77 vs. 0.46 per 100 person-years, p < .001) to warfarinCitation30. Additionally, in a mostly high-risk population comprised of 81% of patients with elevated risk, de Andrés-Nogales 2015 reported the incidence of ischemic stroke per 100 person-years to be highest for untreated patients (incidence: 3.1; 95% CI: 2.67–3.55) followed by patients treated with antiplatelets (incidence: 0.6; 95% CI: 0.49–0.77) and patients treated with DOACs (0.0; 95% CI: 0.0–10.4))Citation40.
Two studies reported mortality associated with nontreatment of AF. Hess 2017 reported that untreated high-risk patients were associated with higher adjusted annual rates of death (OR: 1.22; 95% CI: 1.05–1.41, p = .006) compared to patients on warfarinCitation31. In a mixed-risk population comprised of 50% of patients with elevated risk, Inoue 2014 also found that warfarin-treated patients had reduced all-cause mortality compared to untreated patients (OR: 0.50; 95% CI: 0.33–0.75; p < .001); antiplatelet use, however, did not significantly reduce risks of mortality (OR: 0.94; 95% CI: 0.66–1.34; p = .739)Citation44.
Three studies reported differences in bleeding risk for untreated patients compared to treated patients, with results indicating higher risks of bleeding among patients treated with antiplatelets or VKAs. de Andrés-Nogales 2015 reported incidence of bleeding events (i.e. hemorrhagic stroke, major bleeding, gastrointestinal hemorrhaging) per 100 person-years to be highest for patients treated with antiplatelets (incidence: 2.4; 95% CI: 2.0–2.8) or VKAs (incidence: 2.4; 95% CI: 2.1–2.7), followed by untreated patients (incidence: 1.7; 95% CI: 1.3–2.3) and patients treated with DOACs (incidence: 0.6; 95% CI: 0.0–3.6)Citation40. Inoue 2014 found that warfarin-treated patients had significantly higher rates of major hemorrhaging compared to untreated patients (OR: 2.35; 95% CI 1.12–4.93; p = .024)Citation44. Hess 2017 reported that untreated high-risk patients had significantly lower bleeding rates (OR: 0.35; 95% CI: 0.15–0.81, p = .0147) compared to patients on warfarinCitation31.
Economic consequences of undertreatment and nontreatment
Only one study compared healthcare resource utilization between high-risk patients treated with warfarin and untreated patients. Compared to untreated patients, Casciano 2013 found that patients highly-adherent to warfarin experienced significantly lower risks of inpatient hospitalizations (OR: 0.73; 95% CI: 0.61–0.86) and outpatient visits (OR: 0.84; 95% CI: 0.73–0.97) and shorter lengths of hospital stay (OR: 0.60; 95% CI: 0.55–0.66); adherence was measured according to the proportion of days coveredCitation30. No studies evaluated the resource utilization consequences of nontreatment compared to DOAC use. To the best of our knowledge, currently-published studies have not evaluated the burden of indirect costs (e.g. caregiver burden, loss of productivity) due to undertreatment or nontreatment of NVAFCitation45.
Risk of bias assessment
The risk of bias rating for each evaluated study is reported in the supplemental material (Supplemental Appendix, Table 1). Scores ranged from 6 to 8 (out of a total of 9 points), with most studies representing “good quality.”
Discussion
Overview
The results from this analysis indicate that median rates of nontreatment were higher in the US setting compared to ex-US settings (40% vs. 20%). Additionally, global rates of undertreatment and nontreatment in the high-risk population were lower in the DOAC era compared to the pre-DOAC era. More specifically, median rates of undertreatment and nontreatment were 16.4% and 11.1% in the DOAC era compared to 39.7% and 33.6% in the pre-DOAC era, respectively.
This review of observational studies showed that the clinical benefits of anticoagulation led to reductions in stroke and mortality outcomes among treated patients. Additionally, the risk of bleeding events (i.e. hemorrhagic stroke, major bleeding or gastrointestinal hemorrhaging) was found to be higher for patients treated with warfarin compared to untreated patients in real-world practice. Consistent with the reductions observed with stroke events, healthcare resource utilization was found to be lower among patients highly-adherent to warfarin compared to untreated patients.
Gaps and future opportunities
While the literature search identified important findings related to rates of treatment and corresponding clinical and economic consequences of undertreatment or nontreatment, the body of evidence was generally limited. Only three publications evaluated rates of treatment in the DOAC era, and few studies reported the clinical (four) and economic (one) outcomes associated with undertreatment or nontreatment. Therefore, there are notable gaps in the evidence base that warrant future study.
For instance, among the studies evaluating treatment rates in the DOAC era, either ex-US data or older US data was used for analysis. Proietti 2017 evaluated European AF patients in current cardiology practice in Belgium, Denmark, Netherlands, Norway, Poland, Romania, Greece, Italy, and PortugalCitation42; Huisman 2017 globally evaluated patients in Europe, Asia, North America (without further segmentation by country), Latin America, and Africa/Middle EastCitation39; and Apenteng 2018 studied UK patientsCitation37. Meanwhile, the three US studies used data from the PINNACLE database. Two of these studies used data through 2012, and one used data through 2014, which accounts only for the beginning of the DOAC period. Given the predicted size of the US population with AF by 2030Citation1, it would be important to evaluate treatment rates using newer US-specific data sources especially with regards to potential increases in DOAC market uptake in more recent years.
Of the few studies evaluating the impact of undertreatment and nontreatment on the clinical burden of NVAF, most analyses focus on warfarin as the sole OAC comparator. Only one study, de Andres Nogales 2015, included DOACs in its evaluation; however, only 1.3% of studied patients were prescribed DOACs which was likely a result of the enrollment period (May 2010-April 2012) coinciding with the very start of the DOAC eraCitation40. As a result, opportunities exist to compare outcomes in populations treated with DOACs with undertreated or untreated populations. This would provide an opportunity to evaluate real-world aspects of DOAC use that have been demonstrated within the context of clinical trials, such as superior control of bleeding as compared to warfarin, and in the realm of intracranial hemorrhage and hemorrhagic stroke specifically.
Only one study of the economic burden of NVAF undertreatment and nontreatment was identified in this literature search. Casciano 2013 was based on an evaluation of US administrative claims data from 2003 to 2007 and, as the authors note, the data set may have underrepresented the US Medicare populationCitation30. Additionally, the analysis reported economic outcomes based on levels of adherence to warfarin and did not consider the effects of DOAC treatment. Many of the same opportunities for evaluating the clinical effects of DOAC treatment would apply to future opportunities for economic studies of OAC. In addition, published studies stratifying the indirect cost burden of NVAF, such as lost productivity and the impact on caregivers, by treatment status (i.e. undertreatment, nontreatment, treatment) were not identified. An evaluation of the indirect cost burden could reveal additional significant burden of illness beyond direct medical costs.
Moreover, the study population evaluated in this review included a general population of NVAF patients. However, subpopulations such as those patients with NVAF who undergo percutaneous coronary intervention (PCI) are treated with OACs plus one or two antiplatelet agents which may impact bleeding risks and outcomesCitation70,Citation71. Therefore, the burden of undertreatment and nontreatment in these subpopulations may be different than in the general NVAF population described in this analysis; future research evaluating rates of treatment and corresponding outcomes in this subpopulation, as well as other subpopulations of patients with NVAF, is warranted.
Limitations
The scope of this study was restricted to the review of analyses of real-world observational data, which presented a few limitations. First, retrospective cohort studies evaluating real-world data may be subject to certain limitations including selection biases, differential losses to follow-up, as well as the inability to control for residual or unmeasured confounders (i.e. sociodemographic and/or clinical variables unavailable in the data), all of which may restrict the interpretation of study results. Second, rates of treatment varied across studies partially due to differences in regional real-world practice and treatment guidelines captured in each regional data set. For example, the six DOAC-era studies of treatment rates were based on data from three US studiesCitation34–36, two multinational studiesCitation39,Citation42, and one UK studyCitation37. Third, studies based on claims data were also limited by the fact that aspirin treatment is not reimbursed by payers (e.g. private US payers); as a result, researchers are unable to distinguish between patients on aspirin and untreated patients when analyzing this data type.
Additionally, despite general trends in results across the four studies reporting clinical outcomes, the individual studies were difficult to compare based on differences in the analytic populations as well as the presentation of results. In the assessments of stroke events, one study presented incidence rates per 100 person-years based on adherence to warfarinCitation30 and a second study presented incidence rates per 100 person-years based on anticoagulant treatment status (i.e. undertreated, untreated, treated with DOACs); even among the treated anticoagulant cohort, adherence levels were not reportedCitation40. In assessments of mortality, both studies presented adjusted odds of all-cause mortality; however, one study did so among patients with a high risk of stroke while the other paper did so among a mixed-risk population comprised of 50% of patients with a high risk of stroke. Those publications that evaluated mixed populations of patients based on stroke risk were included in our analysis due to the limited literature in this area. A meta-analysis of treatment rates and/or clinical outcomes would have provided an opportunity to statistically and succinctly combine results from the literature. However, it was deemed infeasible to do so, as described above, based on the heterogeneity of the geographic regions and data sources evaluated, population definitions (e.g. high vs. mixed risk of stroke), cohort definitions (e.g. based on warfarin adherence; based undertreatment/nontreatment), and measure definitions (e.g. presentation of incidence rates vs. odds ratios).
Finally, this literature review was guided by the 2014 AHA/ACC/HRS guidelines on treating AF, and readers should be aware of more recent updates to those guidelines when considering the findings. For instance, aspirin is no longer a recommended therapy for any stroke risk level per the 2019 AHA/ACC/HRS guidelines, and patients on aspirin who are described in this study as undertreated would now be considered untreated.
Conclusion
The limited evidence base suggests that the rate of treatment for NVAF has improved over time with the advent of DOACs. This is an important finding to consider given the increasing prevalence of AF as well as the association of undertreatment and nontreatment with adverse clinical and economic outcomes in this population. Ongoing efforts by clinicians, health insurers, and policy makers to develop programs to increase appropriate use of anticoagulation among NVAF patients with elevated stroke risk could lead to reductions in potentially preventable cardiovascular events and death, as well as the associated direct medical cost burden to healthcare systems. Contemporary research is warranted, however, to evaluate current clinical and economic consequences of undertreatment and nontreatment among NVAF patients with elevated stroke risk given the relatively limited evidence base, the evaluation of older data, and the absence of comparisons to patients treated with DOACs.
Transparency
Declaration of funding
This study was sponsored by the Bristol Myers Squibb-Pfizer Alliance.
Declaration of financial/other relationships
M Di Fusco, J Gillespie, I Shirkhorshidian, and M Cato are employees of Pfizer Inc. M Ferri and J Guo are employees of Bristol Myers Squibb. M Sussman, C Tao, and N Adair are employees of Panalgo who were paid consultants to the Bristol Myers Squibb-Pfizer Alliance in connection with the development of this manuscript. GD Barnes received consulting honoraria with regards to the development of the project design and interpretation. GD Barnes received no honoraria for his authorship activities of this manuscript.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MS, GDB, JDG, CYT, JAG, NA, and MDF were materially involved in the conception, design, analysis, and interpretation of the data. MS, JDG, CYT, NA, and MDF were involved in the drafting of the paper and revising it critically for intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
20010_NVAF_SLR_Manuscript_Supplemental_Materials_2021-08-20.docx
Download MS Word (21.6 KB)References
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285(18):2370.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a Report From the American Heart Association. Circulation. 2019;139(10):e56–e528.
- Centers for Disease Control and Prevention. Underlying cause of death, 1999–2018. 2020. [cited 2020 Mar 12]. https://wonder.cdc.gov/controller/datarequest/D76.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of THORACIC surgeons. Circulation. 2019;140(2):e125–e151.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983–988.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498.
- Joung B, Lee JM, Lee KH, et al. 2018 Korean guideline of atrial fibrillation management. Korean Circ J. 2018;48(12):1033–1080.
- Brieger D, Amerena J, Attia JR, et al. National heart foundation of Australia and cardiac society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Med J Aust. 2018;209(8):356–362.
- Group JJW. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013)–digest version. Circ J. 2014;78(8):1997–2021.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–e267. 2014
- Amin A. Oral anticoagulation to reduce risk of stroke in patients with atrial fibrillation: current and future therapies. Clin Interv Aging. 2013;8:75–84.
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–1457.
- Food & Drug Administration. Pradaxa Highlights of Prescribing Information. 2010. [cited 2020 Jul 16]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022512s000lbl.pdf.
- Food & Drug Administration. Xarelto highlights of prescribing information. 2012. [cited 2020 Jul 16]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022406s001s002s003lbl.pdf.
- Food & Drug Administration. Eliquis highlights of prescribing information. 2012. [cited 2020 Jul 16]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf.
- Food & Drug Administration. Savaysa highlights of prescribing information. 2015. [cited 2020 Jul 16]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817.
- Patel MR, Mahaffey KW, Garg J, et al. Investigators: Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Chan PS, Maddox TM, Tang F, et al. Practice-Level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol. 2011;108(8):1136–1140.
- Verdino RJ. Untreated atrial fibrillation in the United States of America: understanding the barriers and treatment options. J Saudi Heart Assoc. 2015;27(1):44–49.
- Centers for Disease Control and Prevention. Stroke facts. 2017.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097
- Fuster V, Rydén LE, Cannom DS, et al. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the heart rhythm society. J Am Coll Cardiol. 2011;57(11):e101–e198.
- U.S. Food and Drug Administration. Drugs@FDA: FDA-approved drugs. [cited 2020 Oct 21]. https://www.accessdata.fda.gov/scripts/cder/daf/.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: University of Ottawa; 2000.
- Frewen J, Finucane C, Cronin H, et al. Factors that influence awareness and treatment of atrial fibrillation in older adults. QJM Int J Med. 2013;106(5):415–424.
- Casciano JP, Dotiwala ZJ, Martin BC, et al. The costs of warfarin underuse and nonadherence in patients with atrial fibrillation: a commercial insurer perspective. J Manag Care Pharm. 2013;19(4):302–316.
- Hess PL, Kim S, Fonarow GC, et al. Absence of oral anticoagulation and subsequent outcomes among outpatients with atrial fibrillation. Am J Med. 2017;130(4):449–456.
- LaMori JC, Mody SH, Gross HJ, et al. Burden of comorbidities among patients with atrial fibrillation. Ther Adv Cardiovasc Dis. 2013;7(2):53–62.
- Piccini JP, Mi X, DeWald TA, et al. Pharmacotherapy in Medicare beneficiaries with atrial fibrillation. Heart Rhythm. 2012;9(9):1403–1408.
- Hsu JC, Maddox TM, Kennedy K, et al. Aspirin instead of oral anticoagulant prescription in atrial fibrillation patients at risk for stroke. J Am Coll Cardiol. 2016;67(25):2913–2923.
- Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: Insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1(1):55–62.
- Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69(20):2475–2484.
- Apenteng PN, Gao H, Hobbs FR, et al. Temporal trends in antithrombotic treatment of real-world UK patients with newly diagnosed atrial fibrillation: findings from the GARFIELD-AF registry. BMJ Open. 2018;8(1):e018905.
- Holt TA, Hunter TD, Gunnarsson C, et al. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross-sectional survey. Br J Gen Pract. 2012;62(603):e710–e717.
- Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF Registry Phase 2. J Am Coll Cardiol. 2017;69(7):777–785.
- de Andrés-Nogales F, Oyagüez I, Betegón-Nicolás L, et al. Status of oral anticoagulant treatment in patients with nonvalvular atrial fibrillation in Spain. REACT-AF Study. Rev Clin Esp. 2015;215(2):73–82.
- Jolliffe E, Fu V, Lanford J, et al. Burden of atrial fibrillation: a retrospective review of patients presenting to acute medical services. Intern Med J. 2016;46(10):1166–1171.
- Proietti M, Laroche C, Opolski G, et al. 'Real-world' atrial fibrillation management in Europe: observations from the 2-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace. 2017;19(5):722–733. euw112.
- Son MK, Lim N-K, Kim HW, et al. Risk of ischemic stroke after atrial fibrillation diagnosis: a national sample cohort. Bishopric NH, ed. PLoS One. 2017;12(6):e0179687.
- Inoue H, Atarashi H, Okumura K, et al. Impact of gender on the prognosis of patients with nonvalvular atrial fibrillation. Am J Cardiol. 2014;113(6):957–962.
- Martin AL, Reeves AG, Berger SE, et al. Systematic review of societal costs associated with stroke, bleeding and monitoring in atrial fibrillation. J Comp Eff Res. 2019;8(14):1147–1166.
- Amin A, Keshishian A, Xie L, et al. Real-world comparison of major bleeding risk among untreated non-valvular atrial fibrillation patients and those initiating apixaban, dabigatran, rivaroxaban, or warfarin. J Am Coll Cardiol. 2016;67(13):668.
- Atzema CL, Dorian P, Fang J, et al. A clinical decision instrument to predict 30-day death and cardiovascular hospitalizations after an emergency department visit for atrial fibrillation: the atrial fibrillation in the emergency room, part 2 (AFTER2) study. Am Heart J. 2018;203:85–92.
- Boggon R, Lip GYH, Gallagher AM, et al. Resource utilization and outcomes in patients with atrial fibrillation: a case control study. Appl Health Econ Health Policy. 2012;10(4):249–259.
- Boriani G, Diemberger I. Globalization of the epidemiologic, clinical, and financial burden of atrial fibrillation. Chest. 2012;142(6):1368–1370.
- Hayden DT, Hannon N, Callaly E, et al. Rates and determinants of 5-year outcomes after atrial fibrillation–related stroke. Stroke. 2015;46(12):3488–3493.
- Johansson C, Hägg L, Johansson L, et al. Characterization of patients with atrial fibrillation not treated with oral anticoagulants. Scand J Prim Health Care. 2014;32(4):226–231.
- Kabra R, Girotra S, Vaughan Sarrazin M. Refining stroke prediction in atrial fibrillation patients by addition of African-American ethnicity to CHA 2 DS 2-VASc score. J Am Coll Cardiol. 2016;68(5):461–470.
- Kim D, Yang P-S, Jang E, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018;104(24):2010–2017.
- Kim YG, Choi J-I, Boo KY, et al. Impact of age on thromboembolic events in patients with non-valvular atrial fibrillation. Clin Cardiol. 2020;43(1):78–85.
- Kloosterman M, Oldgren J, Conen D, et al. Characteristics and outcomes of atrial fibrillation in patients without traditional risk factors: an RE-LY AF registry analysis. Europace. 2020;22(6):870–877.
- Kotowycz MA, Filion KB, Joza J, et al. In-Hospital management of atrial fibrillation: the CHADS2 score predicts increased cost. Can J Cardiol. 2011;27(4):506–513.
- Komatsu T, Tachibana H, Satoh Y, et al. Relationship between CHA2DS2-VASc scores and ischemic stroke/cardiovascular events in Japanese patients with paroxysmal atrial fibrillation without receiving anticoagulant therapy. J Cardiol. 2012;59(3):321–328.
- Lin L-Y, Lee C-H, Yu C-C, et al. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation—a nation wide database analysis. Atherosclerosis. 2011;217(1):292–295.
- Yu‐Sheng L, Tien‐Hsing C, Ching‐Chi C, et al. Different implications of heart failure, ischemic stroke, and mortality between nonvalvular atrial fibrillation and atrial flutter—a view from a national cohort study. J Am Heart Assoc. 2017;6(7):e006406.
- Maggioni AP, Dondi L, Andreotti F, et al. Four-year trends in oral anticoagulant use and declining rates of ischemic stroke among 194,030 atrial fibrillation patients drawn from a sample of 12 million people. Am Heart J. 2020;220:12–19.
- Mazurek M, Huisman MV, Rothman KJ, et al. Gender differences in antithrombotic treatment for newly diagnosed atrial fibrillation: the GLORIA-AF registry program. Am J Med. 2018;131(8):945–955.e3.
- Ogilvie IM, Welner SA, Cowell W, et al. Characterization of the proportion of untreated and antiplatelet therapy treated patients with atrial fibrillation. Am J Cardiol. 2011;108(1):151–161.
- Raji MA, Lowery M, Lin Y-L, et al. National utilization patterns of warfarin use in older patients with atrial fibrillation: a population-based study of Medicare part D beneficiaries. Ann Pharmacother. 2013;47(1):35–42.
- Redfors B, Gray WA, Lee RJ, et al. Patients with atrial fibrillation who are not on anticoagulant treatment due to increased bleeding risk are common and have a high risk of stroke. JACC Clin Electrophysiol. 2017;3(12):1369–1376.
- Sakamoto Y, Okubo S, Nito C, et al. Insufficient warfarin therapy is associated with higher severity of stroke than no anticoagulation in patients with atrial fibrillation and acute Anterior-Circulation stroke. Circ J. 2018;82(5):1437–1442.
- Song X, Sander SD, Johnson BH, et al. Impact of atrial fibrillation and oral anticoagulation on hospital costs and length of stay. Am J Health Syst Pharm. 2012;69(4):329–338.
- Steinberg BA, Kim S, Fonarow GC, et al. Drivers of hospitalization for patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J. 2014;167(5):735–742.e2.
- Suzuki S, Yamashita T, Okumura K, et al. Incidence of ischemic stroke in Japanese patients with atrial fibrillation not receiving anticoagulation therapy-pooled analysis of the Shinken Database, J-RHYTHM Registry, and Fushimi AF Registry. Circ J. 2015;79(2):432–438.
- Witassek F, Springer A, Adam L, et al. Health-related quality of life in patients with atrial fibrillation: the role of symptoms, comorbidities, and the type of atrial fibrillation. PLoS One. 2019;14(12):e0226730.
- Corpataux N, Spirito A, Gragnano F, et al. Validation of high bleeding risk criteria and definition as proposed by the academic research consortium for high bleeding risk. Eur Heart J. 2020;41(38):3743–3749.
- Gragnano F, Calabrò P, Valgimigli M. Is triple antithrombotic therapy, or rather its duration and composition, the true culprit for the excess of bleeding events observed in patients with atrial fibrillation undergoing coronary intervention? Eur Heart J. 2019;40:216–217.
