Abstract
Background
It is unclear whether 90-day supply fills with rivaroxaban result in better adherence and persistence compared to 30-day supply fills. We assessed patients’ adherence and persistence to rivaroxaban at 12- and 24-months in nonvalvular atrial fibrillation (NVAF) patients whose rivaroxaban prescriptions were filled every 30- vs. 90-days.
Methods
Using the IBM MarketScan Commercial and Medicare Supplemental data sets, we identified adult NVAF patients with ≥12-months of continuous insurance coverage who filled a prescription in May 2018 and their immediate subsequent prescription for rivaroxaban for the same days’ supply. We propensity score-matched 30- and 90-day rivaroxaban interval fill patients and compared the percentage with a proportion of days covered (PDC) ≥80%, mean PDC, and percentage persistent to rivaroxaban therapy over 12- and 24-months of follow-up.
Results
Following propensity score matching, 2237 patients were included in the rivaroxaban 30- and 90-day supply fill cohorts. The proportion of patients with a PDC ≥80% was greater in the 90-day vs. 30-day cohort at both 12-months (odds ratio [OR] = 1.75, 95% confidence interval [CI] = 1.54–1.97) and 24-months (OR = 1.78, 95%CI = 1.58–2.00), as were mean PDC values (absolute difference in mean PDC = 9.4%, 95%CI = 8.2–10.7% at 12-months and 11.2%, 95%CI = 9.5–12.9% higher at 24-months, respectively). Persistence to rivaroxaban was not found to significantly differ between the 30- and 90-day supply cohorts at 12- or 24-months (assuming a 30-day permissible gap); however, greater persistence was observed with 90-day fills at both time points when a 14-day gap was utilized (HR = 1.22, 95%CI = 1.10–1.36 at 12-months and HR = 1.12, 95%CI = 1.02–1.22 at 24-months).
Conclusions
Dispensing 90-day supply fills with rivaroxaban appears to increase the proportion of patients achieving acceptable (PDC ≥80%) adherence as well as mean adherence compared to 30-day supply fills. Ninety-day rivaroxaban fills may also result in improved persistence vs. 30-day fills.
Introduction
In routine clinical practice, one quarter or more of nonvalvular atrial fibrillation (NVAF) patients have been found to have suboptimal adherence to direct-acting oral anticoagulation (DOAC) therapyCitation1,Citation2. This is of immense concern since suboptimal adherence to oral anticoagulation among NVAF patients has been associated with both a higher risk of stroke and mortalityCitation1,Citation2.
Previous research suggests that providing chronic medications to patients as larger (i.e. 90-day supply fills) compared to more traditional 30-day fills may result in better medication adherence and persistenceCitation3. However, there is a paucity of data evaluating the impact of larger days’ supply fills on adherence and persistence to DOACs in patients with NVAF. The primary objective of our present study was to compare medication adherence and persistence to rivaroxaban over 12- and 24-months among NVAF patients whose rivaroxaban prescriptions are filled every 30- vs. 90-days.
Methods
Study design and data sources
We performed a retrospective cohort analysis using IBM MarketScan databases from May 1, 2017 through June 30, 2020. The IBM MarketScan database utilized combined two separate data sets, the commercial claims and encounters (CCAE) and the Medicare supplemental and coordination of benefits (MDCR) data sets, to cover all age groups. These data sets contain claims from approximately 160 contributing employers (i.e. approximately 40 health plans of government and public organizations) and represent approximately 263 million livesCitation4. All patients in the IBM Marketscan CCAE data set receive their insurance through their employer. All patients in the MDCR data set have Medicare supplemental (Part C) plans in addition to their Medicare Part A and B plans. IBM MarketScan data captures enrollment records, demographics, International Classification of Diseases, Ninth- and Tenth-Revision (ICD-9 and ICD-10) diagnosis codes, procedure codes, admission and discharge dates, outpatient medical services data, and prescription dispensing records. All IBM MarketScan data are de-identified and thus comply with the Health Insurance Portability and Accountability Act of 1996 to preserve patient anonymity and confidentiality.
Participants
Eligible patients included adults with ≥12-months of continuous medical and prescription insurance coverage, who filled a prescription for rivaroxaban at an NVAF dose (15 mg or 20 mg once daily) during May 2018 (this index date chosen to assure a contemporary patient cohort with a sufficient proportion of 90-day cohort patients and early enough to allow for 24-month follow-up of all patients), and had ≥1 inpatient or outpatient ICD codes in any position for atrial fibrillation (AF). In addition to these eligibility criteria, patients had to have filled their first and immediate subsequent prescription for rivaroxaban for the same days’ supply. We subsequently stratified rivaroxaban users into two groups, a 30 ± 5-day and a 90 ± 5-day fill cohort, based on days’ supply for their May 2018 rivaroxaban prescription. The requirement for two consecutive prescriptions of the same days’ supply was used to reduce the likelihood of 30- and 90-day cohort crossover. We excluded patients with greater than one prescription claim for rivaroxaban during May 2018, those appearing in both the CCAE and MDCR data sets during the study period, patients with claims for a non-NVAF approved rivaroxaban dose, hyperthyroidism, venous thromboembolism, valve replacement or stenosis, rheumatic heart disease, recent orthopedic knee or hip replacement, recent AF ablation or cardioversion within the prior 2-months, having an alternative anticoagulant indication, or who were pregnant during the 12-month baseline period. The present study’s design allowed for the inclusion of both new and experienced users of rivaroxaban. Since increased dosing frequency (twice vs. once daily) of DOACsCitation2,Citation5 and the use of warfarin itself are associated with poorer adherence and persistenceCitation6, this study will be restricted to rivaroxaban users only.
Adjustment for confounders
To adjust for potential confounding on observed variables between the 30- and 90-day rivaroxaban fill cohorts, we calculated propensity scores based upon multivariable logistic regression including demographics, comorbidities, and concurrent outpatient co-medication useCitation7. Variables entered in the propensity score model are depicted in . Since dependence on billing codes was required to identify covariates, we utilized endorsed and/or validated coding algorithms whenever possibleCitation8,Citation9. We performed 1:1 nearest neighbor propensity-score matching (using a caliper of 0.25 standard deviations of the logit of the propensity score) with residual differences between cohorts assessed via absolute standardized differences (ASD, a value <0.10 considered well-balanced)Citation7. Propensity score matching was performed using the “MatchIT” package and R statistical software (version 3.6.1, The R Project for Statistical Computing).
Table 1. Baseline characteristics after propensity score matching.
Outcomes
The primary outcome for this study was rivaroxaban medication adherence. Adherence was measured in the 30- and 90-day supply fill cohorts using the PDC metric. Specifically, PDC was defined as the ratio of the number of days a patient is covered by the medication (rivaroxaban) to the number of follow-up days they are eligible to have the medicationCitation10,Citation11. We assessed the percentage of patients with a rivaroxaban PDC ≥80% and mean ± standard deviation (SD) PDC over 12- and 24-months of follow-up (index date was the first qualifying rivaroxaban prescription in May 2018). Additional secondary outcomes included the proportion of patients that were persistent to rivaroxaban therapy (assuming a permissible gap of 30-days within the measurement period) over 12- and 24-months follow-up.
Statistical analysis
Baseline characteristics were analyzed using descriptive statistics. Categorical data were reported as percentages and continuous data as means ± SDs or medians with their accompanying interquartile ranges (IQR) (when appropriate). In the propensity score-matched population, we calculated the odds ratio (OR) with 95% confidence intervals (CIs) for achieving a PDC ≥80% using logistic regression (since ASDs were <0.1 for all covariates, only the 30- vs. 90-day supply fill cohort indicator was entered into the model as an independent variable). Mean PDC at 12- and 24-months between the 30- and 90-day supply fill cohorts were compared using independent samples t-tests. For the adherence outcome, we performed subgroup analysis stratified by rivaroxaban use experience. Due to the relatively small number of new rivaroxaban starters identified, new starters during May 2018 and those with ≤6-months of prior use were classified as “less experienced” and those with >6 months prior use of rivaroxaban “more experienced.” We also performed subgroup analysis stratified by whether patients were identified in the CCAE or MDCR data set.
Persistence in the 90-day vs. 30-day supply fill (referent) cohort through 12- and 24-months was assessed using Cox proportion hazard regression (30- vs. 90-day supply fill cohort indicator was the only independent variable in the regression model) and was reported as hazard ratio (HR) with accompanying 95%CIs. We performed additional analyses to test the robustness of our persistence findings, by changing the permissible gap from 30-days to either 14- or 45-days.
Finally, a sensitivity analysis whereby we perform propensity score overlap weighting instead of 1:1 propensity score matching (to maximize sample size and prevent overweighting of propensity score outliers) was performedCitation12. Whereas propensity score matching operates by taking each treated study participant and finding the closest propensity score match among controls within a caliper (or bound), overlap weighting assigns weights to each patient that are proportional to the probability of that patient belonging to the opposite treatment group. Ninety-day supply fill patients were weighted as 1 − propensity score and 30-day supply fill patients as the propensity score. These weights are smaller for extreme propensity score values so that outliers who are nearly always treated (propensity score near 1) or never treated (propensity score near 0) do not dominate results and worsen precision, as may occur with inverse probability weightingCitation7,Citation12. Overlap weighting also leads to exact balance on the mean of every measured covariate when the propensity score is estimated by logistic regression. Overlap weighted analyses of PDC ≥80% and mean PDC were performed using logistic regression and an independent samples t-test, respectively. Persistence was assessed using Cox regression utilizing a robust estimatorCitation12.
Data management and statistical analysis were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R statistical software (version 3.4.3, The R Project for Statistical Computing). This report of our findings was written to comply with “the reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology” statementCitation13.
Results
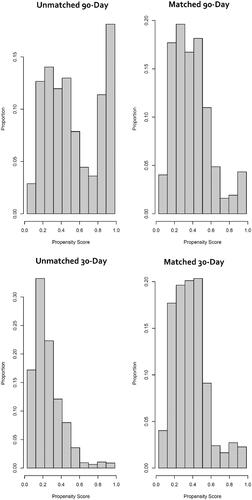
In total, 10,510 NVAF patients filled a 30- or 90-day supply prescription for rivaroxaban (15 or 20 mg) during May 2018 and had a subsequent rivaroxaban fill of the same days’ supply. After exclusion of patients <18 years of age, those with valvular heart disease, hyperthyroidism, recent ablation or cardioversion, an alternative indication for anticoagulation, >1 claim for rivaroxaban in May 2018, appearing in both the CCAE and MDCR or who were pregnant in the prior 12-months, 8823 patients were eligible for analysis (n = 5696 in the 30-day and n = 3127 in the 90-day supply cohort). Following 1:1 propensity score matching, 2237 patients were included in each of the two cohorts (30- and 90-day rivaroxaban day’s supply). The distributions of propensity scores in each cohort before and after 1:1 propensity score matching is depicted in . Not all patients in either cohort were included in the 1:1 matched analysis (). The characteristics of the 1:1 propensity score-matched 30- and 90-day rivaroxaban interval cohorts are depicted in . All covariates had an ASD <0.1; and consequently, the 30- and 90-day cohorts were deemed well matched. The Median (IQR) time of available post-qualifying rivaroxaban prescription follow-up was 730 days (345, 730).
Results of the PDC analyses in the 1:1 propensity score-matched analysis population are available in . The proportion of patients with a PDC of at least 80% was significantly greater in the 90-day vs. 30-day cohort at both 12-months (OR = 1.75) and 24-months (OR = 1.78). Mean PDC was also significantly higher in the 90-days supply fill cohort compared to the 30-day cohort at 12- and 24-months (9.4% and 11.2% higher, respectively). Subgroup analysis stratified by rivaroxaban experience (n = 607 new or ≤6-months of rivaroxaban use; n = 3867 users of rivaroxaban for >6-months) was consistent with the overall study findings, with no significant interaction between the 30- and 90-day interval fill cohorts in adherence over 12- or 24-months (p-value for interaction >.05 for all) (). Subgroup analysis stratified by CCAE and MDCR patients (n = 1800 and 2674, respectively) was consistent with the overall study findings; however, the odds of having a PDC ≥ 80% at 24-months with 90- vs. 30-day fills was greater in MDCR compared to CCAE patients (OR = 2.04 vs. OR = 1.45, p-value for interaction = .01) (). In both the CCAE and MDCR cohorts, adherence was significantly better in 90-day fill patients.
Table 2. Propensity score-matched results.
Table 3. Subgroup analysis stratified by rivaroxaban experience in propensity score-matched results.
Table 4. Subgroup analysis stratified by commercial claims and encounters or medicare supplemental and coordination of benefits data sets in propensity score-matched results.
At 12-months, 83.4% of rivaroxaban users, regardless of fill interval, persisted on therapy (mean rivaroxaban persistency days at 12-months was 334 ± 80 days). At 24-months, 76.4% were still refilling their rivaroxaban prescription (mean rivaroxaban persistency days at 24-months was 623 ± 212 days). Persistence to rivaroxaban was not found to significantly differ between the 30- and 90-day cohorts at 12- or 24-months assuming a 30-day or larger permissible gap; however, significant differences were observed at both 12-months (HR = 1.22) and 24-months (HR = 1.12) when a permissible gap of 14-days was allowed.
Sensitivity analysis using overlap weighting in lieu of 1:1 propensity score matching resulted in similar overall conclusions to the base-case analyses for adherence and persistence outcomes; albeit the improvement in persistence (assuming a 30-day gap) at 12-months associated with 90-day rivaroxaban fills reached statistical significance (HR= 1.15; 95%CI = 1.02–1.31) in the overlap weighted analysis ().
Table 5. Results of overlap weighting sensitivity analysis.
Discussion
The present study’s findings suggest that 90-day supply fills (vs. 30-day fills) are associated with new- and experienced rivaroxaban users with NVAF having 75–78% increased odds of achieving acceptable adherence (PDC ≥80%) and a 9–11% higher mean PDC at 12- and 24-months. It also demonstrated that more than three-quarters of rivaroxaban users with NVAF (regardless of days’ supply cohort) remained persistent to treatment through 24-months. While no significant difference in persistence was observed between the 90- and 30-day supply cohorts assuming a 30-day permissible gap; our findings do suggest 90-day supply fills with rivaroxaban may be associated with improved persistency if decision-makers accept a gap of 14-days (a threshold that has frequently been used in the published persistence literature)Citation14. The improved medication-taking metrics observed in this study with 90- vs 30-days’ supply fills suggest there is an opportunity for insurance plans to improve adherence and potentially downstream patient outcomes by allowing and/or encouraging longer days’ supply fills with rivaroxaban for AF patients.
The above findings are consistent with a prior retrospective, observational adherence study by Taitel and colleagues utilizing data for 52,898 California Medicaid enrolleesCitation3. These investigators demonstrated that providing chronic medications, such as statins, antihypertensives, antidepressants, or oral hypoglycemics, as 90-day fills resulted in 20% better adherence (p < .001) and 23% better persistence (p < .001) to these treatments compared to 30-day fills.
The identification of successful strategies for improving medication-taking behavior in oral anticoagulant patients (such as using extended days’ supply fills) is of great importance since non-adherence to anticoagulation have been shown to increase NVAF patients’ risk of stroke or systemic embolism by 50%Citation2 and the composite of stroke or mortality by 7% per 10% decline in PDC (HR = 1.07; 95%CI = 1.03–1.12)Citation1. In addition to stroke, systemic embolism, and mortality risk, adherence to DOAC therapy have been shown to have reduced overall, mean, per-patient healthcare costs ($29,742 vs. $33,609, p = .005) compared to non-adherent patients; driven by lower combined mean, per-patient, inpatient and outpatient visit costs ($23,544 vs. $30,485, p ≤ .001)Citation15.
The strengths of our study included our utilization of the large IBM MarketScan data sets which are generally considered to be representative of the real-world use and performance of rivaroxabanCitation4. Moreover, propensity scores were estimated based upon a substantial number of demographics, comorbidities, and concurrent outpatient co-medications identified during the baseline period and were subsequently used to perform propensity score matching (and overlap weighting in sensitivity analysis) to adjust for potential confoundingCitation7,Citation12. Next, we include all patients that filled a prescription claim for rivaroxaban during May 2018, regardless of whether they received rivaroxaban prior. This allowed us to include a population of patients who were a mix of new- and experienced users of rivaroxaban, which may be optimal for an adherence studyCitation10,Citation11. A similar approach to assess the effect of 90-day prescriptions was performed by investigators in the above-mentioned Taitel et al. studyCitation3.
Our study has limitations worth noting as well. First, this study is subject to all the limitations of real-world, claims-based studiesCitation16. This includes dependence on pharmacy claims to estimate rivaroxaban adherence and their inability to explain why a patient was non-adherent. While measuring adherence using pharmacy claims is a standard method, it is only a proxy for actual medication use. The presence of prescription claims is not a guarantee that patients ingested the medication. The lack of direct observation of adherence in our study may have resulted in higher medication adherence estimates. That being said, on a macro-level, PDC has been associated with quality of care and shown to correspond well with other direct measures of patient adherence to therapyCitation11. Moreover, our observed proportion of patients with a PDC ≥80% and mean PDC values were consistent with estimates from a recently published meta-analysis on the topic (PDC ≥ 80% for rivaroxaban = 70%, 95%CI = 64–75%; mean PDC for rivaroxaban = 79%, 95%CI = 75–84%)Citation14. Second, after their second consecutive rivaroxaban prescription, patients may have experienced a change in their days’ supply but were still analyzed according to their initial cohort assignment. The requirement of the index and immediate subsequent prescriptions to be the same days’ supply fill may have had the unintended consequence of generating immortal time bias. Third, because multiple prior studies have suggested medications taken less frequently during the day are associated with better adherenceCitation2,Citation5,Citation17,Citation18, we only assessed adherence and persistence to rivaroxaban, a once-daily DOAC. Our findings are likely less generalizable to other DOACs that have different dosing administrations (i.e. twice daily instead of once daily). Next, while we implemented propensity score matching (and overlap weighting in sensitivity analysis) to reduce the risk of confounding, residual confounding resulting from unobserved variables (including patients’ feelings toward taking anticoagulation) cannot be ruled outCitation7,Citation16,Citation17. Finally, our study was performed using IBM MarketScan CCAE and MDCR data sets [Citation4] making our findings most applicable to commercially insured patients and those with Medicare supplemental plans. Our findings may not be generalizable to the uninsured or patients with other public or government insurance.
Conclusions
Dispensing 90-day supply fills with rivaroxaban increased the proportion of patients achieving acceptable levels of adherence as well as their mean adherence compared to 30-day supply fills. Ninety-day rivaroxaban fills may also result in improved persistence vs. 30-day fills when smaller permissible gaps are chosen to identify medication discontinuation.
Transparency
Declaration of funding
This study was supported by Janssen Scientific Affairs, LLC, Titusville, NJ, USA. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of financial/other relationships
CIC has received research funding and honoraria from Janssen Scientific Affairs, LLC; Bayer AG; and Alexion Pharmaceuticals. VA is an employee of Janssen Pharmaceuticals. TJB has not conflicts to declare. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors had a role in study design; data collection, analysis, and interpretation of data; writing the report; and approved the manuscript for submission.
Acknowledgements
None declared.
Ethical approval
All IBM MarketScan data are de-identified, and thus are in compliance with the Health Insurance Portability and Accountability Act of 1996 to preserve patient anonymity and confidentiality. This study was deemed exempt for institutional review board oversight.
References
- Borne RT, O'Donnell C, Turakhia MP, et al. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the veterans health administration. BMC Cardiovasc Disord. 2017;17:236.
- Alberts MJ, Peacock WF, Fields LE, et al. Association between once- and twice-daily direct oral anticoagulant adherence in nonvalvular atrial fibrillation patients and rates of ischemic stroke. Int J Cardiol. 2016;215:11–13.[
- Taitel M, Fensterheim L, Kirkham H, et al. Medication days' supply, adherence, wastage, and cost among chronic patients in medicaid. Medicare Medicaid Res Rev. 2012;2(3):mmrr.002.03.a04.
- Watson Health. IBM MarketScan Research Databases for life sciences researchers.© IBM Corporation; 2020. [cited April 6, 2021]. Available from: https://www.ibm.com/downloads/cas/OWZWJ0QO.
- McHorney CA, Peterson ED, Ashton V, et al. Modeling the impact of real-world adherence to once-daily (QD) versus twice-daily (BID) non-vitamin K antagonist oral anticoagulants on stroke and major bleeding events among non-valvular atrial fibrillation patients. Curr Med Res Opin. 2019;35:653–660.
- Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban versus warfarin among patients with non-valvular atrial fibrillation. Curr Med Res Opin. 2014;30:2461–2469.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- Centers for Medicare and Medicaid Services. CMS Chronic Conditions Data Warehouse Condition Categories. [cited 2021 Aug 7]. Available from: https://www2.ccwdata.org/web/guest/condition-categories
- Canfield SL, Zuckerman A, Anguiano RH, et al. Navigating the wild west of medication adherence reporting in specialty pharmacy. J Manag Care Spec Pharm. 2019;25:1073–1077.
- Pharmacy Quality Alliance. Adherence to Direct-Acting Oral Anticoagulants (PDC-DOAC). [cited 2021 Jan 31]. Available from: www.pqaalliance.org/measures-overview#pdc-doac
- Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418.
- Langan SM, Schmidt SA, Wing K, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ. 2018;363:k3532.
- Ozaki AF, Choi AS, Le QT, et al. Real-World adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2020;13:e005969.
- Deshpande CG, Kogut S, Willey C. Real-World health care costs based on medication adherence and risk of stroke and bleeding in patients treated with novel anticoagulant therapy. J Manag Care Spec Pharm. 2018;24:430–439.
- Gandhi SK, Salmon W, Kong SX, et al. Administrative databases and outcomes assessment: an overview of issues and potential utility. J Manag Care Spec Pharm. 1999;5(3):215–222.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497.
- Weeda ER, Coleman CI, McHorney CA, et al. Impact of once- or twice-daily dosing frequency on adherence to chronic cardiovascular disease medications: a meta-regression analysis. Int J Cardiol. 2016;216:104–109.