Abstract
Objective
Recombinant factor VIII and factor IX Fc fusion proteins (rFVIIIFc and rFIXFc) were developed with an extended half-life (EHL) to improve the management of people with hemophilia A (PwHA) and B (PwHB), respectively.
Methods
This survey gathered physician-reported treatment decisions and physician views on outcomes in PwHA or PwHB who switched to rFVIIIFc or rFIXFc in the 12 months prior to study completion.
Results
Physicians (N = 37) considered bleeds, pharmacokinetic parameters, joint health and adherence the most important factors to assess both in routine care and when deciding to switch to an EHL therapy. In the 12 months prior to study completion, 37 physicians switched 113 PwHA to rFVIIIFc and 25 physicians switched 36 PwHB to rFIXFc. Most PwH (>90%) had moderate or severe hemophilia and many (>60%) switched within 6 months of the survey. The main reason for switching PwHA to rFVIIIFc was to allow fewer injections (49%), while the main reason for switching PwHB to rFIXFc was the product becoming available for use (36%). Overall, 96% of PwHA and 89% of PwHB who were switched remained on these EHL products at the time of survey. Mean total weekly dose, injection frequency and annualized bleeding rate were reported to have reduced following switching.
Conclusion
This survey provides valuable insight into reasons for, and challenges to, the use of EHL products in clinical practice. Physicians perceived that switching to treatment with rFVIIIFc or rFIXFc can improve quality of life, treatment burden, disease control and adherence.
Background
Hemophilia A and B are rare bleeding disorders caused by an X-linked defect in the gene responsible for coding coagulation factor VIII (FVIII) and clotting factor IX (FIX) genes, respectivelyCitation1,Citation2. The severity of hemophilia is defined by plasma procoagulation levels of the respective factor and can be mild (>5–<40% of normal), moderate (1–5%) or severe (<1%)Citation3. In severe cases patients can experience frequent spontaneous bleeding without any identifiable hemostatic challenge; in moderate cases patients usually experience bleeding after mild to moderate injuries; whereas, in mild cases patients may not be diagnosed for years and may only bleed after surgery or major traumaCitation1,Citation4,Citation5. Prevalence (per 100,000 males) is estimated at 17.1 for hemophilia A (6.0 for severe cases) and 3.8 for hemophilia B (1.1 for severe cases) in Australia, Canada, France, Italy, New Zealand and the UK; in 2019 the estimated number of patients with hemophilia globally was 1,125,000 (418,000 estimated to have severe disease)Citation6.
Clotting factor concentrates are the recognised standard of care and, to prevent the musculoskeletal complications that can occur with severe haemophilia, prophylaxis should be initiated early in life (before the age of 3)Citation5. Extended half-life (EHL) factor concentrates extend the mean terminal half-life of treatments relative to that of standard half-life (SHL) productsCitation7. EHL products have the potential to address unmet patient needs as the lower clearance reduces infusion frequency, allowing treatment that is tailored for the individual patient needs. This can result in improved adherence and treatment outcomes, along with increased protection from bleeds, particularly in individuals with active lifestylesCitation8–15.
Recombinant FVIII Fc fusion protein (rFVIIIFc [Elocta]) and recombinant FIX Fc fusion protein (rFIXFc [Alprolix]) were developed using Fc fusion technology and were among the first EHL products approved in the Europe (rFVIIIFc in 2015 and rFIXFc in 2016) for the treatment and prophylaxis of bleeding in patients with hemophilia A (PwHA) and in patients with hemophilia B (PwHB), respectivelyCitation7,Citation16,Citation17. At the time of this survey (end of 2018), the availability of other EHL factor products was limited to rurioctocog alfa pegol (Adynovi) in Germany for the treatment of hemophilia A and to albutrepenonacog alfa (Idelvion) in Germany, the UK, Italy and Spain and nonacog beta pegol (Refixia) in Germany, the UK and Italy for the treatment of hemophilia B (Supplementary Table S1). With local pricing and reimbursement processes affecting availability in each country and patient visits with HCPs typically taking place once or twice a yearCitation18, many patients were still being switched to rFVIIIFc and rFIXFc during 2018.
The efficacy, safety and pharmacokinetics (PK) of rFVIIIFc has been assessed in two phase 3 trials: A-LONG (in patients ≥12 years old) and Kids A-LONG (in patients <12 years old), and the subsequent extension study, ASPIRECitation8,Citation14,Citation19. Five-year data from the ASPIRE study showed no development of inhibitors, a favorable safety profile and low annualized bleeding rate (ABR) in patients treated with rFVIIIFcCitation19. A post-hoc analysis of A-LONG showed that rFVIIIFc treatment resulted in lower ABR compared with patients’ previous FVIII regimensCitation9. Correspondingly, the phase 3 B-LONG and Kids B-LONG studies (in patients ≥12 and <12 years old, respectively), together with the B-YOND extension study, have provided information on the safety, efficacy and PK of rFIXFcCitation20–22. Five-year data from the B-YOND extension study showed a lack of inhibitor development, low ABR and a safety profile comparable with prior studies in patients treated with rFIXFcCitation22. A post-hoc analysis of B-LONG illustrated the value of rFIXFc in reducing ABRs compared with patients’ previous FIX regimensCitation23.
Limited data are available on the key reasons informing physicians’ decisions to switch patients to EHL products in real-world clinical practice. The aim of this survey was to explore physician-reported treatment decisions and their views on patient outcomes in PwHA and PwHB who were switched to rFVIIIFc and rFIXFc, respectively. The survey was intended to expand information on the use of EHL products in routine clinical practice to improve our understanding of the reasons for, and challenges and benefits to, real-world use of EHL products in managing hemophilia.
Methods
A survey was conducted to capture physicians’ views on long-term prophylaxis for adult PwHA and PwHB, and changes to treatment strategies during the 12 months prior to survey completion. The survey was also intended to collect information on physicians’ treatment decisions and their views on outcomes in PwHA and PwHB who were switched to rFVIIIFc and rFIXFc, respectively. The survey comprised an online questionnaire including >50 dichotomous, multiple-choice, Likert-scale and open-ended questions, which took 30–40 min to complete. To capture different angles and points of emphasis in the treaters’ beliefs, predefined answer alternatives as well as coding of open-ended questions allowed for partially overlapping constructs to be chosen by respondents. As part of the survey, physicians were asked to complete patient record forms (PRFs) for male PwHA and PwHB without active inhibitors who had switched from regular (continuous) prophylactic treatment with other therapies to regular (continuous) prophylactic treatment with either rFVIIIFc or rFIXFc, respectively, within the last 12 months. PRFs described actions and treatment decisions and one form was completed per patient. A minimum of two and maximum of seven forms could be completed by each respondent with the aim of collecting a total of 200 hemophilia A and 50 hemophilia B PRFs from a sample of 50 physicians across five countries.
Physicians in existing market survey panels in France, Germany, Italy, Spain and the UK were sent requests to participate. The questionnaire was translated into local languages and interested respondents who were eligible for participation subsequently provided data. To be eligible for participation, physicians were required to: work in a city with a hemophilia treatment centre as per the World Federation of Haemophilia list of centers; work in a hemophilia treatment centre the majority of the time; specialize in hematology, hematology–oncology or pediatric hematology; treat at least six PwH without active inhibitors annually; be personally involved in the choice of treatment strategy and factor replacement products, and have switched at least two patients who previously received prophylaxis with standard half-life FVIII to prophylaxis with rFVIIIFc (no minimum criteria were specified for rFIXFc); physicians were asked to complete a set of preliminary questions to ensure that they were eligible to participate in the survey. Physicians were asked for their opinions only; no patient records were accessed.
The survey was conducted between November and December 2018, and respondents received remuneration for their participation according to fair market value. The results of the survey were analyzed, and the data were summarized descriptively. For questions relating to collection of health outcomes and reasons for treatment switching, physicians were able to select multiple options; therefore, percentages do not add up to 100%.
Formal ethical approval was not required for this market research study as this was an opt-in survey, which was considered to be low risk. This market research followed the laws and guidelines related to the protection of personal data in each country included in the study (including British Healthcare Business Intelligence Association [BHBIA], Market Research Society [MRS], European Pharmaceutical Marketing Research Association [EphMRA]) and European Society for Opinion and Marketing Research [ESOMAR]). None of the findings from the research could be related back to the individual physician or their institution. All physicians provided informed consent and had the right to withdraw from the interview at any time.
Results
Survey population
Invitations to participate in the survey were sent to over 2500 hematologists, hemato-oncologists and paediatric hematologists (France [n = 600], Germany [n = 500], Italy [n = 600], Spain [n = 600], UK [n = 350]) and 518 surveys were returned (France [n = 80], Germany [n = 78], Italy [n = 141], Spain [n = 177], UK [n = 42]). After screening out 481 ineligible respondents (France [n = 73], Germany [n = 72], Italy [n = 130], Spain [n = 172], UK [n = 34]), data were obtained from a total of 37 physicians (France [n = 7], Germany [n = 6], Italy [n = 11], Spain [n = 5], UK [n = 8]; Supplementary Figure S1).
Treatment patterns
PwHA
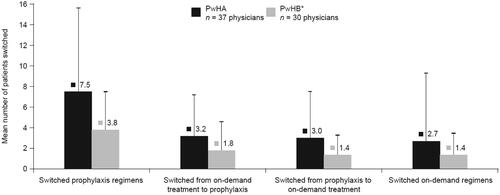
Half of the 37 physicians (51%), consulted with >25 PwHA at least once per year, while 38% and 11% of physicians managed 16–25 and 6–15 patients, respectively. During the previous 12 months, 37 physicians had switched 113 PwHA to rFVIIIFc. Overall, each physician (n = 37) had switched a mean (standard deviation [SD]) of 7.5 (8.1) patients from one prophylaxis regimen to another in the previous 12 months (). Furthermore, each physician had switched a mean (SD) of 3.2 (4.0) patients from on-demand treatment to prophylaxis and a mean of 3.0 (4.5) patients from prophylaxis to on-demand treatment. A mean (SD) of 2.7 (6.6) patients switched from one on-demand regimen to another on-demand regimen.
Figure 1. Profile of treatment switches in PwHA or PwHB in the 12 months prior to the survey. Note. This includes all treatment switches (switches to SHL or EHL treatments); error bars represent standard deviation. Relevant survey question(s): How many of these PwHA or PwHB had their factor replacement product switched in each of the following scenarios? *A total of 30 physicians switched treatment regimens for PwHB, 25 of whom switched patients to regular prophylaxis with rFIXFc. Abbreviations. EHL, extended half-life; PwHA, people with hemophilia A; PwHB, people with hemophilia B; rFIXFc, recombinant FIX Fc fusion protein; SHL, standard half-life.
PwHB
Half of the 37 physicians (51%), consulted with >15 PwHB at least once per year, while 19% and 30% of physicians managed 10–15 and four to nine patients, respectively. During the previous 12 months, 30 physicians switched treatments for PwHB, including 25 physicians who had switched 36 PwHB to rFIXFc. Overall, each physician (n = 30) had switched a mean (SD) of 3.8 (3.7) patients from one prophylaxis regimen to another in the previous 12 months (). Furthermore, each physician had switched a mean of 1.8 (2.8) patients from on-demand treatment to prophylaxis and a mean of 1.4 (1.9) patients from prophylaxis to on-demand treatment. A mean of 1.4 (2.1) patients switched from one on-demand regimen to another on-demand regimen.
Health outcomes evaluated
The proportions reported in this section refer to the number of physicians who used these measures and not to the number of patients for whom these measures were used. Across the entire cohort (N = 37 physicians), the majority (>80%) of physicians considered outcomes related to bleeds, PK parameters, joint health and adherence as the most important factors to assess both in routine care and when deciding to switch to an EHL therapy (). The outcomes that were collected for each of these parameters are shown in Supplementary Figure S2.
Figure 2. Collection of health outcomes data: information that physicians (n = 37) always/regularly collected during routine treatment and considered at the time of switching patients to an EHL therapy. Relevant survey question(s): How often do you collect the following health outcome data for your hemophilia patients as part of their routine treatment?/Which specific health outcome measurements do you take into consideration when making a decision to switch a patient’s hemophilia treatment to an EHL factor replacement treatment? [Select all that apply]. Abbreviations. EHL, extended half-life; PK, pharmacokinetic.
![Figure 2. Collection of health outcomes data: information that physicians (n = 37) always/regularly collected during routine treatment and considered at the time of switching patients to an EHL therapy. Relevant survey question(s): How often do you collect the following health outcome data for your hemophilia patients as part of their routine treatment?/Which specific health outcome measurements do you take into consideration when making a decision to switch a patient’s hemophilia treatment to an EHL factor replacement treatment? [Select all that apply]. Abbreviations. EHL, extended half-life; PK, pharmacokinetic.](/cms/asset/d89b19e7-3332-4efa-8fbd-80f9303583f7/icmo_a_1991901_f0002_b.jpg)
In routine care, 95% of physicians reported always or regularly collecting bleed data; information about the total number of bleeds per joint, whether bleeds were spontaneous or traumatic, and a patient’s total number of bleeds were collected by 84%, 78% and 76% of physicians, respectively. When deciding to switch a patient to an EHL therapy, bleed data (considered by 95% of physicians) primarily involved consideration of a patient’s total number of bleeds (81% of physicians); other outcomes included the total number of bleeds per joint and whether bleeds were spontaneous or traumatic (49% and 30%, respectively).
Almost all physicians (92%) assessed PK in routine patient care, either by conducting a full PK analysis (49%), by measuring factor trough levels (53%) or through measurement of both peak and trough levels (49%). PK evaluations were routinely (27%) or occasionally (54%) combined with a population PK model, such as that available via the Web-Accessible Population Pharmacokinetic Service. When deciding to switch a patient to an EHL therapy, the majority of physicians (89%) assessed PK, with 51% of physicians carrying out a full PK analysis.
Joint health data collected in routine care (by 84% of physicians), included physical examinations to determine joint functionality or range of motion (by 76% and 65%, respectively). Other data that physicians reported to collect always or regularly during routine care focused on the total number and location of target joints (62% of physicians), while joint scores were also determined by using both clinical (by 49% of physicians, all using the Hemophilia Joint Health Score)Citation24 and imaging scores (by 38% of physicians, including use of X-ray, ultrasound scans and magnetic resonance imaging [used for 63%, 60% and 51% of the scoring, respectively]). When deciding to switch, joint health data was considered by 76% of physicians, although less than half reported performing associated physical examinations of joint functionality (46%) or range of motion (32%); less than one-third of physicians collected clinical joint score data (32%), imaging joint score data (22%) or information about the total number and location of target joints (19%).
Adherence data (collected by 81% of physicians in routine care) was measured via a range of complementary techniques: physician’s “own” determinations, PK data and questionnaires (by 65%, 56% and 54%, respectively). When deciding to switch a patient to an EHL therapy, consideration of adherence (by 81% of physicians) mostly included physician’s “own” determinations and questionnaires (50% and 38% of physicians, respectively).
Switching to rFVIIIFc or rFIXFc: patient characteristics and reasons for switching
The decision to switch a patient onto a new treatment can be multi-faceted and, as such, the physicians were able to select multiple reasons for switching. Therefore, the following results highlight the most commonly selected reasons for switching treatment.
PwHA
The characteristics of patients switched to rFVIIIFc within the last 12 months before the survey are described in . Of the 113 PwHA switching from SHL factor replacement to rFVIIIFc, most had moderate or severe hemophilia (95%) and had switched within 6 months of the survey (64%). The discussion to switch was initiated by the physician for 88% of 113 patients, and the main reasons for switching were to allow fewer injections (49% of 113 patients), patient convenience (34% of 113 patients) and to support a patient’s lifestyle (32% of 113 patients; ).
Figure 3. Key reasons for switching PwHA or PwHB to rFVIIIFc and rFIXFc, respectively. Relevant survey question(s): What do you consider to be the main reasons behind switching to rFVIIIFc or rFIXFc for this specific patient? [Select all that apply]. *Other reasons included supply issues for PwHA and less vials to take abroad for PwHB. Abbreviations. PwHA, people with hemophilia A; PwHB, people with hemophilia B; rFVIIIFc, recombinant factor VIII Fc fusion protein; rFIXFc, recombinant factor IX Fc fusion protein.
![Figure 3. Key reasons for switching PwHA or PwHB to rFVIIIFc and rFIXFc, respectively. Relevant survey question(s): What do you consider to be the main reasons behind switching to rFVIIIFc or rFIXFc for this specific patient? [Select all that apply]. *Other reasons included supply issues for PwHA and less vials to take abroad for PwHB. Abbreviations. PwHA, people with hemophilia A; PwHB, people with hemophilia B; rFVIIIFc, recombinant factor VIII Fc fusion protein; rFIXFc, recombinant factor IX Fc fusion protein.](/cms/asset/effed1ea-885a-45ab-8bb1-678455a6ccd6/icmo_a_1991901_f0003_b.jpg)
Table 1. Characteristics of PwHA or PwHB switched to rFVIIIFc and rFIXFc, respectively, in the 12 months prior to the survey.
While the results above are given for the overall cohort of PwHA, on an individual level, reasons for treatment switches differed based upon patient characteristics and treatment practices. For instance, the switch to rFVIIIFc being more suited to the patient’s lifestyle was cited as a reason for switching in 41% of 74 patients who had a PK assessment versus 15% of 39 patients who did not. Furthermore, for 64% of 59 patients with severe disease and 35% of 49 patients with moderate disease, “fewer injections” was cited by physicians as a reason for switching.
For PwHA with a history of inhibitors (n = 20), the main reasons for switching were: too many spontaneous bleeding episodes with previous treatment (45% vs 25% of patients with no history of inhibitors [n = 93]); more suited to the patient’s lifestyle than the previous treatment (45% vs 29% of patients with no history of inhibitors) and aim to achieve higher trough levels (40% vs 19% of patients with no history of inhibitors). Physician satisfaction with the switch to rFVIIIFc was similar to the results for patients without a history of inhibitors (100% rating satisfied to very highly satisfied vs 90% respectively).
At the time of the survey, 96% of the 113 patients who had switched to rFVIIIFc in the previous 12 months remained on the product. Following the switch, the mean (SD) total weekly FVIII dose was reported to have decreased from 103.3 (48.2) IU/kg to 86.0 (35.6) IU/kg (based on the physicians’ views of the prescribed doses), and the median (range) injection frequency from 3.0 (0.7–7.0) to 2.0 (1.0–3.5) injections per week. Notably, fewer patients required the higher dosage (121+ IU/kg/week) after the switch (falling from 32% to 13%).
PwHB
Of the 36 PwHB switching from SHL factor replacement to rFIXFc (), most had moderate or severe hemophilia (91%) and had switched within 6 months of the survey (62%). The discussion to switch was initiated by the physician for 94% of 36 patients. The main reasons for switching PwHB to rFIXFc were due to the product becoming available for use in their country (36% of 36 patients), a high frequency of spontaneous bleeding episodes (33% of 36 patients), more convenient for the patient (28% of 36 patients) and to enable fewer injections (28% of 36 patients; ).
As with PwHA, reasons for treatment switches differed based on patient characteristics and treatment practices. For example, too many spontaneous bleeding episodes was cited as one of the most common reasons for switching to rFIXFc in certain subgroups, including 50% of the 20 patients in whom PK assessments were performed versus 13% in the 16 patients who did not receive PK assessments, and 50% of the 16 patients with severe disease compared with 12% of the 17 patients with moderate disease. Additionally, in 45% of the 20 patients for whom PK assessments were performed, “fewer injections” was cited as a reason for switching, compared with 6% of the 16 patients who did not receive PK assessments, and for 44% of the 16 patients with severe disease, “fewer injections” was cited as a reason for switching, compared with 12% of the 17 patients with moderate disease.
At the time of the survey, 89% of the 36 patients who had switched to rFIXFc in the previous 12 months remained on the product. Following the switch, physicians’ responses for PwHB suggested a mean (SD) total weekly FIX dose reduction from 117.8 (90) IU/kg to 71.2 (55) IU/kg (based on the physicians’ views on the prescribed doses) and median (range) reduction in weekly injection frequency from 2.2 (1.0–4.0) to 1.0 (0.7–5.0). Notably, fewer patients required the higher dosage (121+ IU/kg/week) after the switch (falling from 42% to 12%).
Switching to rFVIIIFc or rFIXFc: physician-estimated ABR
Physician-estimated ABR was collected for a subset of patients who had switched to and received rFVIIIFc (n = 33) or rFIXFc (n = 9) for at least 6 months, and for whom physicians were able to provide an estimate of ABR before and after the switch. In these patients, physician-estimated median (interquartile range) ABRs decreased from 3.0 (1.0–5.5) to 1.0 (0–3.0) for PwHA and from 3 (1.0–6.5) to 1 (0.5–4.0) for PwHB after switching. Furthermore, after switching, numerically greater proportions of patients were estimated to have an ABR of 0 and numerically lower proportions estimated to have an ABR ≥3 compared with before ().
Table 2. Physician-estimated ABR in PwHA or PwHB switched to rFVIIIFc and rFIXFc, respectively, and subsequently receiving at least 6 months of therapy.
Switching to rFVIIIFc or rFIXFc: physicians’ perceptions on impact of switching
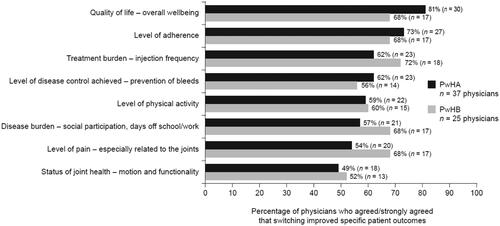
Of the responding physicians for PwHA (n = 37), most considered (i.e. agreed or strongly agreed) that switching improved specific outcomes, including quality of life (81%), adherence (73%), disease control (prevention of bleeds, 62%) and reduced treatment burden (62%). Of the responding physicians for PwHB (n = 25), the majority considered that switching resulted in reduced treatment burden (72%), pain (68%) and disease burden (68%), as well as improved quality of life (68%) and adherence (68%) ().
Figure 4. Physicians’ perception of the impact on PwHA or PwHB switched to rFVIIIFc and rFIXFc, respectively. Relevant survey question(s): Overall, following the switches to rFVIIIFc or rFIXFc described previously, please indicate to what extent you agree or disagree with the following statements on how these patients fared (relative to their previous treatments) – based on my experience with the switch to rFVIIIFc or rFIXFc, I believe that “(…) is improved”. Abbreviations. PwHA, people with hemophilia A; PwHB, people with hemophilia B; rFVIIIFc, recombinant factor VIII Fc fusion protein; rFIXFc, recombinant factor IX Fc fusion protein.
Switching to rFVIIIFc or rFIXFc: physicians’ views on EHL products
Overall, physicians (n = 37 for PwHA; n = 25 for PwHB) agreed or strongly agreed that switching patients to EHL products allowed for a more active lifestyle, without worrying about bleeds (57% and 48% for PwHA and PwHB, respectively), had the potential to provide individualized prophylaxis (54% and 44% for PwHA and PwHB, respectively) and improved adherence rates with prophylaxis (49% and 56% for PwHA and PwHB, respectively; Supplementary Figure S3). Twenty-seven percent of physicians treating PwHA believed the time taken to switch prevented them from changing patients’ therapies; the corresponding figure for physicians treating PwHB was 40%.
Discussion
The current survey explored physician-reported (n = 37) treatment decisions and patient outcomes in PwHA (n = 113) and PwHB (n = 36) who were switched to EHL rFVIIIFc and rFIXFc, respectively, in real-world setting. “More convenient for the patient” and “fewer injections” were among the most common reasons for switching to rFVIIIFc, and “availability of the product” and “too many spontaneous bleeding episodes” for switching to rFIXFc. According to physicians’ responses, switches were generally initiated by them and much less often by patients themselves. Although patients are generally well-informed, European patients have been found to exert less influence over their treatment decisions than their US counterpartsCitation25. The finding in our survey is in line with those of the HOPE study with UK patients switching to EHL factor productsCitation26. The results of the survey showed that weekly dosage, injection frequency and estimated ABR were reported to have decreased following the switch, and factors including patient quality of life, treatment burden, disease control and adherence were perceived to have improved. The results of this survey provide valuable insight into the management of PwHA and PwHB in routine clinical practice and suggest that switching to rFVIIIFc or rFIXFc provides several benefits to the patient.
Recommendations produced by the European Haemophilia Therapy Strategy Board (EHSTB) advocate that “appropriate performance indicators (outcome measures) of hemophilia care should be a part of routine practice”Citation27. The recommendations, which were based on data most commonly collected by physicians in daily clinical practice, suggest monitoring the following domains: bleeding frequency; hemophilia joint health score; at least one imaging technique; at least one activity score; days lost from everyday life; and quality of life (questionnaire)Citation27. Similarly, in our survey, 95% of physicians reported consideration of bleed outcomes. In addition, joint health data were collected by physicians in our survey as part of routine care (84%) and when deciding to switch a patient to an EHL therapy (76%).
Weekly dosage and injection frequency both decreased after switching to rFVIIIFc or rFIXFc as reported by physicians who participated in our survey. Physicians estimated that ABR decreased in comparison to patients’ previous prophylaxis regimen with SHL factor replacement therapy and believed that EHL products could enable patients to pursue a more active life, without worrying about bleeds. In addition, physicians perceived other benefits of switching to rFVIIIFc or rFIXFc to include reduced treatment burden, as well as improvements in quality of life and treatment adherence. Other studies have highlighted the benefits of rFVIIIFc and rFIXFc, including the positive impact on health outcomes and patient quality of lifeCitation10,Citation11,Citation28. Incorporating real-world data into an evaluation of the cost-effectiveness of rFVIIIFc has shown cost-savings and enhanced health-related quality of life compared with SHL FVIII treatment, based on lower ABRs and improved joint healthCitation10. A retrospective review of existing medical records for 17 PwHA and 19 PwHB switched to rFVIIIFc or rFIXFc, respectively, showed ABRs decreased after switching (from 2.3 to 1.3 and from 2.5 to 0.82, respectively), indicating benefits compared with SHL productsCitation11.
In a single-centre retrospective study designed to review data from PwHA who were switched to rFVIIIFc, infusion frequency and weekly dose were reduced, and management of bleeding was improved; the reasons for switching included reduced infusions and higher protection from bleeding risk, similar to our studyCitation13. Furthermore, preliminary real-world data from the Canadian Bleeding Disorders Registry involving 139 adult and pediatric patients switching to EHL products showed similar benefits to the present study with respect to injection frequencyCitation29. For example, switching to rFVIIIFc and rFIXFc, resulted in reductions of factor consumption by 19% in 62 PwHA and 50% in 17 PwHB, respectively. In 45 children with hemophilia A, weekly infusions decreased from a mean of three to two, and in seven children with hemophilia B, weekly infusions decreased from a mean of 2.5 to one, showing that switching benefits pediatric patientsCitation29.
In our survey, the perceived benefits of rFVIIIFc and rFIXFc were reflected in the high proportions of patients who remained on this treatment (96% and 89%, respectively). Similarly, in a retrospective analysis of existing medical records of patients switched to EHL products, only five of 36 patients reverted back to SHL factor treatmentCitation11. When considering patient perspective on the use of EHL products an interview-based study designed to assess patient considerations on novel treatments in hemophilia found that patients considered ease of use of medication, including injection frequency, important when considering a potential treatment switchCitation30. Furthermore, a web survey conducted to investigate preferences for treatment attributes indicated that injection frequency, the ability to take part in physical activity, and bleed control and prevention had an impact on quality of lifeCitation31. The HOPE study also found that the majority of patients were positive about EHL therapies, with the reduced injection frequency resulting in improvements in quality of life, and other benefits including greater protection, i.e. fewer bleedsCitation26. The physicians’ views and treatment outcomes collected in our study are similar to the patient views gathered in these patient-centered studies.
One benefit of the present study is that it involves the use of real-world data reflecting routine clinical practice. In addition, the study team was able to collect information on a wider patient group than possible in randomized-controlled trials as patient selection criteria was less strict. Limitations of the present study include the collection of data based on physicians’ opinions and retrospective estimates. Importantly, no patient-reported assessments were included and therefore the physician perceptions could not be compared with the actual patient experience. Since no patient records were accessed, the ability to confirm any of the outcomes reported by the physicians was limited. The low survey response rate (∼20%) may be perceived as a limitation of the study, given that 2500 physicians were invited to participate. However, this response rate was in the range expected and it included a large cohort of physicians who were screened out as, whilst they were specialists in hematology, they did not treat patients with hemophilia on a regular basis.
A-SURE, an ongoing non interventional European study, has been designed to compare treatment outcomes associated with EHL products with SHL products in routine clinical practice, and aims to use the three primary endpoints of bleeding rate, injection frequency and factor consumption to compare real-word treatment outcomesCitation32. Preliminary results have demonstrated that, for PwHA with a previous history of inhibitors, switching to rFVIIIFc does not result in recurrence of inhibitors (patients were negative for inhibitors at the time of switching)Citation33. In contrast to the present study, this study will collect data based on predefined outcomes and also assess patient-reported outcomes over a 24-month prospective period and a 12-month retrospective periodCitation32. The results of the ABR and injection frequency assessments will complement the results of our study and other similar studies, contributing to our understanding of the use of rFVIIIFc in routine clinical practice.
Conclusions
The results of the present study provide valuable information relating to the management of PwHA and PwHB in routine clinical practice, and the reasons for, perceived clinical benefits of, and challenges associated with, switching patients to rFVIIIFc or rFIXFc through data collected from physicians across five countries in Europe. The results of this survey have shown that these EHL treatments for PwHA and PwHB result in improvements in several key areas, including patient quality of life, treatment burden, disease control and adherence.
Transparency
Declaration of funding
The project was funded by Swedish Orphan Biovitrum AB (Sobi) and implemented by two independent customer insight companies who ran the market research study (boobook [https://www.boobook.world/] supported by Big Fish International [http://bigfish-international.com/]) who helped with questionnaire design and data interpretation.
Declaration of financial/other relationships
M. van der Sluijs is a shareholder in, and employee of, Swedish Orphan Biovitrum AB. S. Tawil is an employee of Swedish Orphan Biovitrum AB. N. Huyghe and C. Wood are employees of boobook and Big Fish International, respectively. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. M. van der Sluijs and S. Tawil designed the study. N. Huyghe ran the survey and collected and analyzed the data. C. Wood contributed to questionnaire design and data analysis. All authors interpreted the data, contributed to drafting and revising the article, provided their final approval of all content and agree to be accountable for all aspects of the work.
Ethics approval
Formal ethical approval was not required for this market research study as this was an opt-in survey, which was considered to be low risk. This market research followed the laws and guidelines related to the protection of personal data in each country included in the study (including BHBIA, MRS, EphMRA and ESOMAR). None of the findings from the research could be related back to the individual physician or their institution. All physicians provided informed consent and had the right to withdraw from the interview at any time.
Supplementary_information_Final_version_clean.docx
Download MS Word (341.3 KB)Acknowledgements
Medical writing and editorial support, funded by Sobi, was provided by Andy Lockley and Jilly Hope of Bioscript Medical. Data in this manuscript have previously been presented as posters at the XXVII Congress of the International Society on Thrombosis and Haemostasis (ISTH), Melbourne, Australia, 6–10 July 2019.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to their proprietary nature but are available from the corresponding author on reasonable request.
References
- Acharya SS. Advances in hemophilia and the role of current and emerging prophylaxis. Am J Manag Care. 2016;22(5 Suppl):s116–s125.
- Centers for Disease Control and Prevention. What is hemophilia? [cited Aug 2020]. Available from: https://www.cdc.gov/ncbddd/hemophilia/facts.html
- White GC, 2nd, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on thrombosis and haemostasis. Thromb Haemost. 2001;85(3):560.
- Mansouritorghabeh H. Clinical and laboratory approaches to hemophilia A. Iran J Med Sci. 2015;40(3):194–205.
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26 Suppl 6:1–158.
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–546.
- Lambert T, Benson G, Dolan G, et al. Practical aspects of extended half-life products for the treatment of haemophilia. Ther Adv Hematol. 2018;9(9):295–308.
- Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325.
- Shapiro AD, Ragni MV, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein: extended-interval dosing maintains low bleeding rates and correlates with von Willebrand factor levels. J Thromb Haemost. 2014;12(11):1788–1800.
- Bullement A, McMordie ST, Hatswell AJ, et al. Cost-effectiveness analysis of recombinant factor VIII Fc-fusion protein (rFVIIIFc) for the treatment of severe hemophilia a in Italy incorporating real-world dosing and joint health data. Pharmacoecon Open. 2020;4(1):133–142.
- Wang C, Young G. Clinical use of recombinant factor VIII Fc and recombinant factor IX Fc in patients with haemophilia a and B. Haemophilia. 2018;24(3):414–419.
- Peyvandi F, Garagiola I, Boscarino M, et al. Real-life experience in switching to new extended half-life products at European haemophilia centres. Haemophilia. 2019;25(6):946–952.
- Tagliaferri A, Matichecchia A, Rivolta GF, et al. Optimising prophylaxis outcomes and costs in haemophilia patients switching to recombinant FVIII-Fc: a single-Centre real-world experience. Blood Transfus. 2019;18:374–385.
- Young G, Mahlangu J, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13(6):967–977.
- Gringeri A, Wolfsegger M, Steinitz KN, et al. Recombinant full-length factor VIII (FVIII) and extended half-life FVIII products in prophylaxis-new insight provided by pharmacokinetic modelling. Haemophilia. 2015;21(3):300–306.
- European Medicines Agency. Alprolix summary of product characteristics. [cited Mar 2020]. Available from: https://www.ema.europa.eu/en/documents/product-information/alprolix-epar-product-information_en.pdf.
- European Medicines Agency. Elocta Summary of Product Characteristics. [cited Mar 2020]. Available from: https://www.ema.europa.eu/en/documents/product-information/elocta-epar-product-information_en.pdf.
- European Haemophilia Network (EUHANET). European guidelines for the certification of Haemophilia Centres. [cited Sep 2020]. Available from: http://www.euhanet.org/docs/euhanet-european_guidelines_for_the_certification_of_haemophilia_centres_2013.pdf.
- Nolan B, Mahlangu J, Pabinger I, et al. Recombinant factor VIII Fc fusion protein for the treatment of severe haemophilia A: final results from the ASPIRE extension study. Haemophilia. 2020;26(3):494–502.
- Fischer K, Kulkarni R, Nolan B, et al. Recombinant factor IX Fc fusion protein in children with haemophilia B (Kids B-LONG): results from a multicentre, non-randomised phase 3 study. Lancet Haematol. 2017;4(2):e75–e82.
- Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323.
- Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and sustained efficacy for up to 5 years of treatment with recombinant factor IX Fc fusion protein in subjects with haemophilia B: results from the B-YOND extension study. Haemophilia. 2020;26(6):e262–e271.
- Powell J, Shapiro A, Ragni M, et al. Switching to recombinant factor IX Fc fusion protein prophylaxis results in fewer infusions, decreased factor IX consumption and lower bleeding rates. Br J Haematol. 2015;168(1):113–123.
- Stephensen D, Drechsler WI, Scott OM. Outcome measures monitoring physical function in children with haemophilia: a systematic review. Haemophilia. 2014;20(3):306–321.
- Lamb CC, Wolfberg A, Lyytinen K. UK vs US physician decision-making in the treatment of haemophilia. Haemophilia. 2019;25(4):616–625.
- Khair K, Pollard D, Harrison C, et al. HOw patients view extended half-life products: Impressions from real-world experience (the HOPE study). Haemophilia. 2019;25(5):814–820.
- Hermans C, Klamroth R, Richards M, et al. Outcome measures in European patients with haemophilia: survey of implementation in routine clinical practice, perception of relevance and recommendations by European treaters in the EHTSB. Haemophilia. 2017;23(2):222–229.
- Iorio A, Krishnan S, Myren KJ, et al. Continuous prophylaxis with recombinant factor IX Fc fusion protein and conventional recombinant factor IX products: comparisons of efficacy and weekly factor consumption. J Med Econ. 2017;20(4):337–344.
- Keepanasseril A, Stoffman J, Bouskill V, et al. Switching to extended half-life products in Canada - preliminary data. Haemophilia. 2017;23(4):e365–e367.
- van Balen EC, Wesselo ML, Baker BL, et al. Patient perspectives on novel treatments in haemophilia: a qualitative Study. Patient. 2020;13(2):201–210.
- Steen Carlsson K, Andersson E, Berntorp E. Preference-based valuation of treatment attributes in haemophilia a using web survey. Haemophilia. 2017;23(6):894–903.
- Oldenburg J, Hay CRM, Jiménez-Yuste V, et al. Design of a prospective observational study on the effectiveness and real-world usage of recombinant factor VIII Fc (rFVIIIFc) compared with conventional products in haemophilia A: the A-SURE study. BMJ Open. 2019;9(5):e028012.
- Oldenberg J, Heller C, Malmström H, et al. No relapse in patients with previous inhibitors switched to rFVIIIFc in ongoing observational phase 4 studies. Res Pract Thromb Haemost. 2020;4:463–464.
