Abstract
Objective
Since 2014, valproate has not been recommended for use in girls and women of childbearing potential unless other treatments are ineffective or not tolerated. Risk minimization measures (RMMs) of valproate were implemented to reduce the potential risks of developmental disorders among pregnant women. A drug utilization study was carried out to assess the effectiveness of RMMs.
Methods
This was a multinational, non-interventional cohort study. For the UK, existing data from the Clinical Practice Research Datalink database were used. The primary study endpoint was a change in the proportion of valproate initiations preceded by other medications relevant for valproate indications before and after implementation of RMMs.
Results
The proportion of valproate initiations preceded by medications related to valproate indications increased after RMM implementation in incident female users in the UK from 66.4% to 72.4%. The proportion of incident prescriptions for epilepsy and bipolar disorder with prior medication related to valproate indications increased, from 36.2% to 44.1% and 72.9% to 77.8%, respectively. The incidence rate of valproate-exposed pregnancies decreased from 16.9 to 10.9 per 1000 person-years in the pre- and post-implementation periods, respectively.
Conclusions
Results from this study indicated some improvement in physician prescribing and a potential reduction in valproate-exposed pregnancies in the UK. Given only modest improvement has been achieved, additional RMMs were implemented in 2018.
Introduction
Valproate is indicated for use in the treatment of epilepsy, as well as bipolar disorder, in the UKCitation1. Several formulations of valproate exist, and in the UK these include valproic acid, sodium valproate and semi-sodium valproate. Sodium and semi-sodium valproate are metabolized to valproic acid, which is responsible for the pharmacological activityCitation2. In this study, the term valproate describes magnesium valproate, sodium valproate, valproic acid, sodium valproate/valproic acid, valproate semi-sodium and valpromide.
Due to its efficacy, valproate is one of the treatment options for many patients with generalized and unclassified epilepsiesCitation3. Currently, the National Institute for Health and Care Excellence (NICE) recommends valproate as a treatment for appropriate patients for newly diagnosed generalized tonic–clonic seizures, myoclonic seizures, tonic or atonic seizures, Dravet syndrome, Lennox–Gastaut syndrome and idiopathic generalized epilepsies in boys, men and women who are not of childbearing potentialCitation4. Regarding bipolar disorder, NICE recommends valproate for use in appropriate types of patients where lithium has been ineffective or not toleratedCitation5. Valproate must not be offered to girls and women of childbearing potential (WOCBP) unless other options are ineffective or not tolerated and a pregnancy prevention programme is in place. WOCBP who are already taking valproate should be advised to gradually stop taking the drug with medical supervisionCitation5,Citation6. This recommendation in the summary of product characteristics is based on evidence that valproate has a high teratogenic potential and children exposed in utero to valproate have a high risk for congenital malformations and neurodevelopmental disordersCitation7. Folate supplementation (5 mg daily)Citation8 before the pregnancy may decrease the risk of neural tube defects, which may occur in all pregnancies. However, the available evidence does not suggest that it prevents the birth defects or malformations due to valproate exposureCitation7.
In October 2013, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) requested a review of valproate medicines and their use in pregnant women due to the emergence of new evidence from scientific literature on the risks of developmental disorders in children exposed to valproate and related substances in uteroCitation9.
In October 2014, the European Medicines Agency’s (EMA) Pharmacovigilance and Risk Assessment Committee (PRAC) conducted a review and made recommendations to minimize the risk of development disordersCitation6. Additionally, PRAC concluded that further risk minimization measures (RMMs), such as educational materials, are required to better inform patients and healthcare professionals on the potential risks of developmental disorders, along with a drug utilization study (DUS) to assess the effectiveness of the proposed RMMsCitation9. The objective of this DUS was to examine prescribing practices of valproate in female patients, particularly WOCBP, before and after dissemination of RMMs and assessment of effectiveness of RMMs. This multinational study took place across five European countries (France, Germany, Spain, Sweden and the UK) and the results are described elsewhereCitation10.
This paper focuses on data from the UK and provides insights into prescribing practices of valproate in female patients with a special focus on WOCBP regarding epilepsy and bipolar disorder before and after the implementation of RMMs.
Methods
Study design
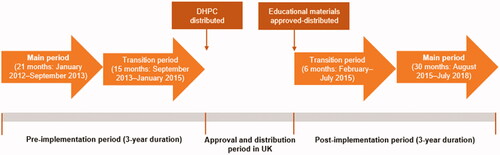
This was a multinational, non-interventional cohort study across France, Germany, Spain, Sweden and the UK. The study was registered in the EU Register of Post-Authorisation Studies (EUPAS11379). In the UK, existing data from the Clinical Practice Research Datalink (CPRD) database were used. The RMMs were implemented in the UK in January 2015 and included educational materials and direct healthcare professional communication (DHPC), approved by competent national authorities. A pre- and post-implementation period design was employed to examine the changes in the prescribing pattern of valproate. In the UK, a 36 month pre-implementation period (January 2012 to December 2014) and a 36 month post-implementation period (February 2015 to January 2018) were used. The pre- and post-implementation periods were divided into a main and transition sub-period. The pre-implementation transition period started at the time of the regulatory referral announcement and ended at the time of DHPC distribution (January 2015). The main pre-implementation period comprised the 21 months prior to the transition pre-implementation period. The post-implementation transition period comprised the first 6 months after the distribution of educational materials, while the main post-implementation period was defined as the subsequent 30 months after the end of the transition period ().
Figure 1. Study periods including entire, main and transition study periods for UK (figure adapted from Toussi et al.Citation10). Abbreviation. DHPC, Direct healthcare professional communication.
Study population and setting
During the pre- and post-implementation periods, all female patients receiving at least one valproate prescription in oral form, in the outpatient setting, as identified via the CPRD database, were included in the study. Incident prescriptions were defined as valproate prescriptions issued during the pre- or post-implementation period without prior prescription for valproate within 12 months before the prescription date. First-ever prescriptions were defined as valproate prescriptions issued during the pre- or post-implementation period without prior prescription for valproate within the patient’s entire available medical history before the prescription date. WOCBP were defined as female patients aged between 13 and 49 years, and all analyses were performed both in the overall population and in this subpopulation.
Objectives
The primary objective was to assess the effectiveness of RMMs in the outpatient setting by comparing the prevalence of prior medication use related to the indication of valproate initiation in women before and after implementation of RMMs. The secondary objectives were to describe prescribing patterns in the outpatient setting in women during the pre- and post-implementation periods, overall and in the WOCBP subgroup.
Endpoints
In accordance with the study objectives, the primary endpoint of this study was the change in proportion of valproate initiations preceded by other medications relevant for valproate indications, before and after implementation of RMMs, for incident and first-ever valproate prescriptions (overall, epilepsy and bipolar subgroups). Since valproate should be initiated if other treatments are not effective or not well tolerated, an increase in the proportion of initiations with pre-treatment (valproate not as first line treatment) indicates success.
Additionally, this study aimed to describe prescribing patterns in the outpatient setting in female patients during the pre- and post-implementation periods, overall and in WOCBP. This included the description of demographic characteristics, indication of valproate, valproate treatment characteristics (dosage and duration), concomitant use of hormonal contraceptives or intrauterine devices (IUD), and valproate exposure during pregnancy.
For analysis in incident prescriptions, data on relevant prior medication were obtained from the 12 month period prior to valproate initiation; for the first-ever prescriptions, data on relevant prior medication were obtained from patients’ entire medical history.
Data source
CPRD data consists of approximately 5 million active patients managed by GPs throughout the UKCitation11. Approximately 5.8 million pregnancies were registered in the CPRD database among 2.4 million women from January 1987 to February 2018.
Statistical methods
The main analyses of outcome parameters were performed on prescription level using descriptive statistical methods, with the main analytical unit being prescriptions. The primary analytical focus was on the main pre- and post-implementation periods. In addition, analysis of the primary endpoint in incident prescriptions was performed for the entire pre- and post-implementation periods to allow for a larger sample size.
Interrupted time series (ITS) analysis of the primary endpoint was performed as the secondary analysis in this study. The methodological approach described by Lopez Bernal et al. was usedCitation12. A segmented regression analysis based on the quasi-Poisson regression model was used. Overall, 24 quarterly data points were considered (12 in each pre- and post-implementation period). A level- and slope-change impact model was used, where level change (period effect, parameter [PERIOD]) corresponded to the overall impact of the intervention (RMMs), and reflected an overall change of the primary endpoint in the post-implementation period, compared with the pre-implementation period and change of slope (parameter [TIME]), indicating a general trend to a change of the primary endpoint over time after intervention. The effectiveness of RMMs in the ITS analysis was measured on the .05 significance level.
ITS analysis was performed for the incident and first-ever valproate prescriptions overall and in the subgroups of epilepsy and bipolar disorder. ITS and incidence rate of pregnancy were evaluated during the entire study periods.
Results
Number of patients and prescriptions of valproate
In total, this study included 12,356 female patients (178,938 prescriptions) with at least one prescription record of valproate during the 21 month main pre-implementation period, and 7952 patients (142,995 prescriptions) during the 30 month main post-implementation period. The proportion of incident prescriptions was 1.3% (2367 prescriptions) and 0.9% (1269 prescriptions) in the main pre- and post-implementation periods, respectively ().
Table 1. Number of valproate prescriptions in the main pre-implementation and post-implementation periods.
Demographic characteristics
In the overall study population, the mean age was 52 years in both main pre- and post-implementation periods; 40.8% and 36.6% of valproate users were WOCBP in the pre- and post-implementation periods, respectively ().
Table 2. Demographic characteristics of the analysis based on index prescription (patient level) in the UK.
In incident users, the mean age was 52 years in the main pre-implementation period and 50 years in the main post-implementation period. In the main pre- and post-implementation periods 39.5% and 41.0% of users, respectively, were WOCBP ().
Valproate initiations preceded by at least one medication for epilepsy and bipolar disorder (primary endpoint)
The proportion of valproate initiations preceded by medications related to valproate indications in the overall population increased after implementation of RMMs in incident female users in the UK (from 66.4% to 72.4%) (). This is in line with indication-specific data (epilepsy and bipolar disorder), which show increases in the post-implementation period for both epilepsy and bipolar disorder ().
Table 3. Number of valproate prescriptions and results on primary endpoint in the main study periods in UK.
Table 4. Results on primary endpoint in subgroups epilepsy and bipolar disorder for the main study periods in UK.
The overall proportions of incident prescriptions for epilepsy with prior medication related to epilepsy during the 12 months prior to the valproate prescription date were 36.2% (95% confidence interval [CI] 32.5–39.9%) and 44.1% (95% CI 38.8–49.4%) in the main study periods before and after implementation of RMM, respectively. In the WOCBP group, the corresponding proportions of incident prescriptions for epilepsy were 43.9% (95% CI 37.5–50.2%) and 47.7% (95% CI 39.0–56.4%).
The proportions of incident prescriptions for bipolar disorder with prior medication related to bipolar disorder were 72.9% (95% CI 67.7–78.2%) and 77.8% (95% CI 71.6–83.9%) in the main study periods before and after the implementation of RMM, respectively. In the WOCBP group, the corresponding proportions for bipolar disorder were 75.8% (95% CI 69.1–82.5%) and 78.4% (95% CI 69.6–87.2%) ().
In the first-ever valproate users (overall and subgroup WOCBP), the proportion of valproate initiations preceded by medications relevant for valproate indications during the patients’ entire medical history was higher compared with incident valproate users in both periods, whatever the indication.
Treatment characteristics of valproate
The mean (standard deviation [SD]) prescribed daily dose was 882.7 mg (530.7 mg), median 800 mg in the main pre-implementation period and 917.9 mg (580.6 mg), median 900 mg in the main post-implementation period in all valproate prescriptions. The estimated mean prescription duration in the main pre- and post-implementation periods was 28.4 days (20.1 days), median 28 days and 27.6 days (19.7 days), median 28 days in all prescriptions, respectively.
Indication for valproate prescription
Indication of interest, including epilepsy and bipolar disorder, was observed in 78.8% and 80.7% of all valproate prescriptions in the respective main pre- and post-implementation periods. Epilepsy was the indication for 56.0% and 55.7% of all valproate prescriptions in the main pre- and post-implementation periods, respectively. The corresponding proportions for epilepsy in the WOCBP population were 60.6% and 59.2%. Bipolar disorder was the indication for 18.0% and 20.2% of all valproate prescriptions in the main pre- and post-implementation periods, respectively, while the corresponding proportions in the WOCBP population were 18.0% and 19.0%.
Use of hormonal contraceptives or intrauterine devices
The proportion of overall valproate prescriptions in WOCBP with concomitant use of hormonal contraceptives or IUDs was 2.7% in the main pre-implementation period and 2.8% in the main post-implementation period. The corresponding proportion of incident prescriptions (in WOCBP) was 4.0% in the main pre-implementation period and 5.3% in the main post-implementation period.
Pregnancy
Overall, 293 pregnancies in 243 patients (1.8% of the overall number of valproate users) were identified in the main pre-implementation period; 146 (49.8%) of 293 pregnancies were exposed to valproate. The corresponding figures in the main post-implementation period were 145 pregnancies in 109 patients (1.4% of the overall number of valproate users); 61 (42.1%) of 145 pregnancies were exposed to valproate.
The incidence rate of all pregnancies from the entire pre- and post-implementation periods was 34.3 and 22.7 per 1000 person-years in the pre- and post-implementation periods (36 months), respectively. The incidence rate of exposed pregnancies was 16.9 and 10.9 per 1000 person-years in the pre- and post-implementation periods, respectively ().
Table 5. Incidence rate of pregnancy in the women of childbearing potential group during study.
In all (exposed and non-exposed) pregnancies, epilepsy was the indication for valproate in 49.5% and 50.3% of cases in the main pre- and main post-implementation periods, respectively. The corresponding proportions for bipolar disorder were 17.4% and 15.9%.
The mean age in pregnancy was 30 years in the main pre-implementation period and 32 years in the main post-implementation period. In the first trimester, 94.5% and 95.1% of pregnancies were exposed to valproate in the main pre- and main post-implementation periods, respectively; in the second trimester, 27.4% and 18.0%, respectively; in the third trimester, 30.1% and 21.3%, respectively ().
Table 6. Pregnancies exposed to valproate: demographic characteristics, characteristics of pregnancy; analysis on pregnancy level*.
The mean (SD) duration of exposure during pregnancy was 103.0 (88.1) and 77.8 (72.0) days in the main pre- and main post-implementation periods, respectively. The mean (SD) prescribed daily dose was 797.4 mg (431.7 mg) and 1015.7 mg (594.8 mg) in the main pre- and main post-implementation periods, respectively.
Interrupted time series analysis: all incident and first-ever valproate prescriptions
In all performed ITS analyses, no statistically significant changes in either investigated parameter, “PERIOD” (indicating overall change in the post-implementation period compared with the pre-implementation period) or “TIME” (indicating trend to change of primary endpoint over time after implementation of RMMs), were detected ().
Figure 2. ITS analysis of the primary endpoint. (A) All incident valproate prescriptions in the UK in overall population. (B) All first-ever valproate prescriptions in the UK in overall population. (C) All incident valproate prescriptions in the UK in epilepsy subgroup. (D) All first-ever valproate prescriptions in the UK in epilepsy subgroup. Abbreviation. Rx, prescription.
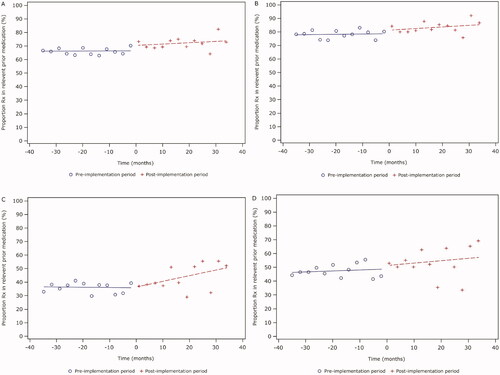
The results for the subgroup of bipolar disorder were not presented because the quarterly number of valproate prescriptions for this indication was very low (<30) and the analyses have not provided statistically meaningful information.
Discussion
RMMs are a commonly used intervention with the aim to minimize the occurrence of adverse drug reactions and optimize the safe and effective use of drugs throughout their life cycle. It is important to continue monitoring the effectiveness of RMMs to control and manage the benefit–risk balanceCitation11,Citation13.
The primary objective of this study was to evaluate the effectiveness of RMMs implemented in the UK in 2015 following the EMA PRAC assessment on valproate use. It is important to consider that the management of epilepsy during pregnancy is challenging and, up to now, valproate can be the only option to control life threatening seizures for some women. Valproate is now contraindicated in pregnancy for the treatment of epilepsy unless there is no suitable alternative availableCitation5,Citation6. Valproate is contraindicated in pregnancy for patients being treated for bipolar disorderCitation14,Citation15.
This study shows an increase in the proportion of incident valproate prescriptions with previous use of medication related to valproate indications (epilepsy and bipolar disorder), suggesting an improvement in the proportion of appropriate prescribing in the UK, i.e. prescriptions given in line with the requirements set out by the EMA and MHRA. Similarly, there was a higher proportion of appropriate prescribing with a previous use of medication related to valproate in the post-implementation period than in the pre-implementation period for first-ever users, which was also seen in the WOCBP age group for both incident and first-ever users. When considering indication-specific data, the epilepsy subgroup showed that the proportion of incident prescriptions with prior medications for epilepsy was higher in the post-implementation period than the pre-implementation period. The results were similar for the bipolar disorder subgroup, as a slight increase in incident prescriptions in the main post-implementation period was observed.
The reduction in the number of incident and first-ever prescriptions suggests a reduction of valproate initiation after RMM implementation. These results are in line with a previous study in the UK; despite an increase in anti-epileptic drug (AED) use, when focused on sodium valproate, the incidence of prescription decreasedCitation11.
This DUS also showed that the incidence rate of all pregnancies decreased from the pre-implementation period to the post-implementation period. Moreover, exposed pregnancy incidence also decreased between the pre- and post-implementation periods. These results are encouraging as they may indicate the positive impact of RMM in the UK.
However, data from one clinical audit in 2018 (following contraindication in pregnant women with epilepsy and bipolar disorder)Citation5,Citation6 suggest that valproate may still be being prescribed in girls and WOCBP; this audit of prescribing practice across England in WOCBP with diagnosed bipolar disorder found that 24% of women aged younger than 50 years were prescribed valproate-containing medicines, and in half of these women there was documented evidence that information was provided on the risks of teratogenicity and necessity for contraceptionCitation13. Furthermore, a UK study of 100 WOCBP showed that 55% of participants did not feel they were involved in decision-making regarding the AED they were prescribed. However, the same study did show that patients taking valproate were more informed about, and expressed an understanding of, the risks involved with their treatment, when compared with patients taking other AEDsCitation16.
Factors that may influence the results of this study include the fact that the data are derived from GPs, so prescribing patterns from other specialities might be limited. However, as GPs assume a “gatekeeper” role in the UK (meaning that patients have to see a primary care provider, who then decides whether specialist care is necessary), this had a limited impact on the study results. Patient exposure was determined based on written prescriptions as a proxy for actual usage, possibly leading to overestimation of exposure. However, such overestimation may not have a significant impact, as a previous study showed that over 90% of all written prescriptions recorded in CPRD were subsequently dispensedCitation17.
Conclusions
This study assessed the effectiveness of RMMs put in place in the UK following the 2014 EMA PRAC assessment. The decrease in the overall number of valproate initiations and in the incidence of pregnancies in the UK from the pre- to post implementation periods suggested a reduction in overall valproate exposure, and specifically in pregnancy. Similarly, the increased proportion of incident valproate prescriptions for epilepsy and bipolar disorder with prior relevant medication pointed in the same direction and suggest a positive effect of the RMMs. Following an additional regulatory referral in the EU and implementation of additional RMMs in 2018, monitoring of effectiveness of these RMMs will be continued.
Transparency
Declaration of funding
The study was funded by a consortium of companies including Alfsigma SpA, Apotex Europe BV, Aristo Pharma GmbH, Arrow Generiques, Betapharm Arzneimittel GmbH, Biogaran, Demo SA, Desitin Arzneimittel GmbH, Generis Farmacêutica SA, GL Pharma GmbH, Hexal AG, Mylan EMEA SAS, Neuraxpharm Arzneimittel GmbH, Noridem Enterprises Ltd, Orion Corporation, Sanofi Aventis Groupe, Stada Arzneimittel AG, TAD Pharma GmbH, Tecnifar Indústria Técnica Farmacêutica SA, Temmler Pharma GmbH, Teva Pharmaceuticals Europe BV and Wockhardt UK Ltd, and the medical writing support was funded by Sanofi.
Author contributions: B.E. and M.T. were involved in the conduct of the study and in data acquisition. All authors were involved in the discussion and interpretation of study results. All authors were involved in critically revising the manuscript, have provided final approval and take full accountability for the work, for all content and editorial decisions. Authors received no payment from any company of the consortium directly or indirectly (through a third party) related to the development/presentation of this publication.
Declaration of financial/other relationships
I.D. and D.G. have disclosed that they are employees of Sanofi and may hold shares and/or stock options in the company. C.N. has disclosed that she was an employee of Sanofi at the time the study was conducted and is currently an employee of IQVIA, France. S.K. has disclosed that she is an employee of Teva Pharmaceutical Industries Ltd. B.E. and M.T. have disclosed that they are employees of IQVIA, Germany and France, respectively, which was paid by a consortium of companies to perform the analyses.
Ethical approval: All procedures performed in this study involving human participants were in accordance with the ethical standards of the applicable institutional review board and ethics committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The protocol of the study was approved by the EMA and registered in the EU PAS register prior to the start of data collection.
The authors individually and collectively are responsible for all content and editorial decisions and received no payment from Sanofi directly or indirectly (through a third party) related to the development/presentation of this publication.
Acknowledgements
The authors would like to thank Margarita Shlaen for her valuable contributions to this study and Sinead Monaghan for her guidance in writing this publication. The authors would also like to thank Annie Hill from HealthCare21 Communications Ltd, Macclesfield, Cheshire, SK10 2XA, UK, a Lucid Group agency, for providing medical writing support.
Data availability statement
The analysis was done on secondary data sources. The authors are not the data owners and do not have the legal rights to share any data. Moreover, the data owners are not allowed to share any individual data according to European data privacy rules.
References
- NICE. Bipolar disorder: assessment and management. NICE Clinical Guidelines, No. 185 [Internet]. 2020 [cited 2020 Jun]. Available from: https://www.nice.org.uk/guidance/cg185
- Kapur S, Taylor D, Paton C. The Maudsley prescribing guidelines. Chichester (UK): CRC Press; 2009.
- Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1016–1026.
- NICE. Epilepsies: diagnosis and management [Internet] [cited 2020 Jun]. Available from: https://www.nice.org.uk/guidance/cg137/chapter/1-Guidance#sodium-valproate
- NICE. Bipolar disorder: assessment and management [Internet] [cited 2020 Jun]. Available from: https://www.nice.org.uk/guidance/cg185/chapter/1-Recommendations#managing-mania-or-hypomania-in-adults-in-secondary-care-2
- GOV.UK. Press release: valproate banned without the pregnancy prevention programme [Internet] [cited 2020 Feb]. Available from: https://www.gov.uk/government/news/valproate-banned-without-the-pregnancy-prevention-programme
- Sanofi. Depakote (valproate) summary of product characteristics [Internet] [cited 2021 Sep]. Available from: https://www.medicines.org.uk/emc/product/6102/smpc#gref
- Wockhardt UK Ltd. Folic acid summary of product characteristics [Internet] [cited 2021 Sep]. Available from: https://www.medicines.org.uk/emc/product/6998#gref
- European Medicines Agency. Procedure under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data: substances related to valproate. Assessment report [Internet] [cited 2020 Jun]. Available from: https://www.ema.europa.eu/en/documents/referral/valproate-related-substances-article-31-referral-prac-assessment-report_en.pdf
- Toussi M, Shlaen M, Coste F, et al. Effectiveness of risk minimisation measures for valproate: a drug utilisation study in Europe. Pharmacoepidemiol Drug Saf. 2021;30(3):292–303.
- Charlton R, Damase-Michel C, Hurault-Delarue C, et al; EUROmediSAFE consortium. Did advice on the prescription of sodium valproate reduce prescriptions to women? An observational study in three European countries between 2007 and 2016. Pharmacoepidemiol Drug Saf. 2019;28(11):1519–1528.
- Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–355.
- Baldwin DS, Amaro HJF. Prescription of valproate-containing medicines in women of childbearing potential who have psychiatric disorders: is it worth the risk? CNS Drugs. 2020;34(2):163–169.
- Macfarlane A, Greenhalgh T. Sodium valproate in pregnancy: what are the risks and should we use a shared decision-making approach? BMC Pregnancy Childbirth. 2018;18(1):200.
- EURAP Study Group. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66(3):354–360.
- Harris L, Lowes O, Angus-Leppan H. Treatment decisions in women of childbearing age on valproate. Acta Neurol Scand. 2020;141(4):287–293.
- Jick H, Jick SS, Derby LE, et al. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768.
