Abstract
As cerebral mucormycosis is devastating in nature, here we discuss possible use of the intranasal route, in comparison to or in addition to intravenous administration, as a therapeutic approach to manage cases of mucormycosis with central nervous system involvement.
Graphical Abstract
Mucormycosis is a rare fungal infection that can produce serious human health issuesCitation1. Given their ubiquitous nature, we commonly encounter these fungi but a healthy immune system is able to eradicate them effectively. Given the opportunity and a weakened immune system (such as due to diabetes and use of steroids), mucormycosis can produce infection of the sinuses or the lungsCitation2. The etiological drivers of mucormycosis are a group of molds called mucormycetes that are able to cause this opportunistic infection, mostly in immunocompromised patientsCitation3,Citation4. These fungi are present in soil, and are often in association with decaying organic matter such as compost piles, and animal dungCitation5.
If not treated early and aggressively, it can spread to the eyes causing blindness, or possibly involve the central nervous system causing seizures and leading to deathCitation2. Pre COVID-19, the rate of prevalence of mucormycosis ranged between 0.005 and 1.7 per million population in different countries, except India, where the number of reported cases were far greater at 140 cases per million population. Now with ∼27 million COVID-19 cases in India (as of May 23, 2021), 77 million diabetics and the use of steroid for treatment, there has been an exponential increase in the reported cases of mucormycosisCitation2,Citation6. Of note, COVID-19 associated mucormycosis showed that 94% of patients had diabetes, suggesting the number of mucormycosis cases will continue to rise in India and globally. For the many patients affected with mucormycosis, the outcome is poor. About half of the affected patients will die and many will sustain permanent damage to their health.
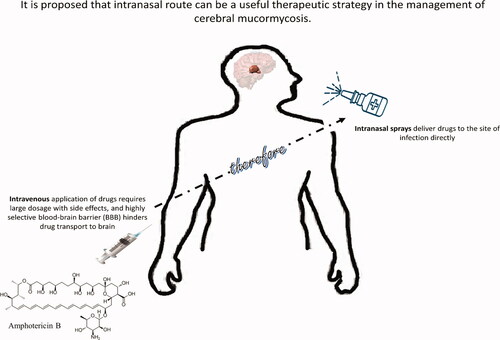
The recommended treatment against mucormycosis involves the intravenous application of amphotericin B (preferably liposomal formulation) in initial dose of 5 mg per kg body weight per day, and 10 mg per kg body weight per day at advanced stage of the disease. Depending on the severity of the disease, each patient needs 60–100 injections (each vial containing 50 mg)Citation2,Citation6,Citation7. With each injection costing from 5000 to 10,000 rupees, the projected cost of the treatment can be more than a million rupees, and even then, successful outcome is not certain. Such high dosage of drug is administered due to the intravenous application of amphotericin B, which leads to dilution of the drug in the plasma. In the case of cerebral mucormycosis, further complications are attributed due to the highly selective blood-brain barrier and poor penetration of amphotericin B to reach the central nervous system and target the fungi. This results in the application of higher doses of the drug to accomplish minimum inhibitory concentration to target the epicenter of infection. Moreover, usage of higher concentrations can result in various side effects such as nephrotoxicity and hepatotoxicity. Recently, the European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) discussed the clinical management of COVID-19 associated mucormycosis and several factors were considered comprising Diabetes mellitus and steroid usage as predisposing factors, the presence of spores in the environment, and alternative treatments strategies were discussedCitation8. As amphotericin B does not cross the blood-brain barrier effectively to counter neuropathologies, here we discuss the use of intranasal route as an alternative approach in the treatment of cerebral mucormycosis. The intranasal administration of amphotericin B has the potential to augment its’ efficacy as it will avoid systemic circulation, hence, reducing dosage to achieve minimum inhibitory concentration at the target site, reduce side effects such as nephrotoxicity and hepatotoxicity, and thus limit the brain damage and associated mortality. Other anti-fungal compounds such as voriconazole, fluconazole, and itraconazole do not depict reliable activity against mucormycosisCitation9,Citation10 even though voriconazole has the ability to cross the blood-brain barrierCitation11. In a previous study, it was shown that voriconazole prophylaxis is a risk factor for mucormycosisCitation12,Citation13.
The perivascular system is associated with the olfactory and trigeminal routes, and is utilized in the intranasal route, which can be employed as a therapeutic strategy for drug delivery to the central nervous system. In validation, a recent study depicted the rapid delivery of molecules from the imine group to the brain via the lymphatic system as an eminent routeCitation14. Moreover, utilizing the perivascular system, intranasal imines were able to reach the cortex and other brain regions readily. Drug delivery via the intranasal route is non-invasive, results in minimal damage to other tissues that are a result of systemic administration via the intravenous route, and thus is advantageous in comparison. This method for delivery of molecules is often preferable, and frequent administration is normally tolerated, with enhanced drug delivery to the brainCitation6. In fact, drug delivery via the intranasal route is an efficient way to avoid the blood-brain barrier, whilst using the paravascular system. Furthermore, the intranasal route is effective in achieving the MIC (minimum inhibitory concentration) at lower concentrations at the infection site by evading the haematogenous route. Importantly, intranasal delivery of amphotericin B was shown to be effective in the treatment of invasive mucormycosisCitation15. Remarkably, drugs that are vaporized have been observed to be more efficacious than in its liquid formCitation16. For instance, it was shown that the antifungal attributes of lemongrass (Cymbopogon citrates) in the vapor phase were elevated (32.7 mg/L) in comparison to the liquid phase (288 mg/L) leading to complete fungicidal efficacy against Candida albicans. It is speculated that when antimicrobials are in the vapor form, this allows their absorption within the membrane leading to increased cellular damage as revealed by deformities/ruptured cells on the surface, nonetheless, the exact underlying etiologies and mechanisms are not yet known. In addition, treatment using intravenous or intrathecal is invasive with possible associated complications. Intranasal route is currently utilized to deliver a variety of pharmaceutical drugs to treat a variety of health issues, such as for the management of endometriosis, migraine, sinus, and bone loss and breaks. However, various factors may affect nasal drug delivery, for instance hydrophobicity, molecular size (particularly with 1000 g/mol), surface charge and physiochemical properties. Moreover, there are various strategies that can be employed to elevate drug adsorptionCitation17. Overall, the management of cerebral mucormycosis using the intranasal route presents several advantages over the intravenous route such as (a) avoiding selectivity of the brain microvessels, (b) achieving MIC at reduced concentrations at the target site by avoiding venous drainage, (c) can allow delivery of drugs to the CNS, (d) allow evasion of undesirable damage due to intravenous administration, (e) delivery in the gas phase form to permeate porous cribriform, and (f) without affecting the blood-brain barrier integrity. Usage of nasal inhalers with specific drugs against cerebral mucormycosis to target fungi could provide a practical solution and may even prevent the need to carry out disfiguring surgical procedures, which may be necessitated despite aggressive therapyCitation12. Previously, a pediatric oncology patient suffering from mucormycosis, was treated with intranasal amphotericin B. Surgical intervention along with chemotherapy was initiatedCitation15. After 5 weeks post-surgery, another lesion was observed, and due to the concern of toxicities due to antifungal therapy, localized therapy comprising nasal liposomal amphotericin B was given, 13 months on, the patient showed no further fungal diseaseCitation15. All in all, the ease of intranasal administration of drugs to the brain makes this route particularly useful for simplicity of administration, even in a primary care setting, although future research using in vivo models is warranted, as well as investigating co-administration of amphotericin B via both the intranasal route and via intravenous administration. Furthermore, patient self-application can be conducted, which is particularly useful in developing countries for patients in rural areas, whereby access to tertiary hospitals may be difficult. Of note, it is thought that most of the COVID-19-associated mucormycosis cases are observed in patients with uncontrolled diabetesCitation18 originating from the lung or gut. The gut microbiome may influence cytokines, as well as increase chronic phase proteins and interferon signaling in lung cellsCitation19. Nasal administration may be utilized as a prophylactic as well as in post-exposure circumstances, especially if the prevalence of mucormycosis is >10% in a given population, thus making this an option in the design of preventative measures and/or therapeutic approaches against devastating cerebral mucormycosis, although future research is necessitated.
Transparency
Declaration of funding
No funding was received for this work.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RS and NAK conceptualized the work amid critical dialogue with MK, TI and MYA. MYA and RS reviewed the literature, and prepared the first draft of the manuscript and the figures under the supervision of NAK. All authors contributed to the manuscript and will act as guarantors.
Acknowledgements
None reported.
References
- Skiada A, Lass-Floerl C, Klimko N, et al. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):93–101.
- [cited 2021 May 22]. Available from: https://www.wsj.com/articles/rare-black-fungus-mucormycosis-infects-thousands-of-covid-19-survivors-in-india-11621614697.
- Mahalaxmi I, Jayaramayya K, Venkatesan D, et al. Mucormycosis: an opportunistic pathogen during COVID-19. Environ Res. 2021;201:e111643.
- Waldorf AR, Diamond RD. Aspergillosis and mucormycosis. In: Immunology of the fungal diseases. Boca Raton: CRC Press; 2020. p. 29–55.
- Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15:2–9.
- [cited 2021 May 22]. Available from: https://www.bbc.com/future/article/20210519-mucormycosis-the-black-fungus-hitting-indias-covid-patients.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421.
- Rudramurthy SM, Hoenigl M, Meis JF, et al. ECMM/ISHAM recommendations for clinical management of COVID‐19 associated mucormycosis in low‐and Middle‐income countries. Mycoses. 2021;64(9):1028–1037.
- Spellberg B, Ibrahim AS. Recent advances in the treatment of mucormycosis. Curr Infect Dis Rep. 2010;12(6):423–429.
- Pfaller MA, Messer SA, Hollis RJ, et al. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob Agents Chemother. 2002;46(4):1032–1037.
- Henry ME, Bolo NR, Zuo CS, et al. Quantification of brain voriconazole levels in healthy adults using fluorine magnetic resonance spectroscopy. Antimicrob Agents Chemother. 2013;57(11):5271–5276.
- Sundaram ASM, Sathanantham ST, Chinchole V, et al. Fostering a deeper understanding of COVID-19-associated mucormycosis–a commentary on the mucormycosis coinfection in the context of global COVID-19 outbreak: a fatal addition to the pandemic spectrum. Int J Surg. 2021;2021:e106031.
- Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191(8):1350–1360.
- Krishnan JKS, Arun P, Appu AP, et al. Intranasal delivery of obidoxime to the brain prevents mortality and CNS damage from organophosphate poisoning. Neurotoxicology. 2016;53:64–73.
- Khafagy R, Gupta S, Campisi P, et al. Treatment of localized mucormycosis using nasal amphotericin B irrigation in pediatric oncology. Pediatr Blood Cancer. 2020;67(4):e28175.
- Tyagi AK, Malik A. Liquid and vapour-phase antifungal activities of selected essential oils against candida albicans: microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement Altern Med. 2010;10:65.
- Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14(9):3754–3779.
- Hoenigl M, Seidel D, Carvalho A, et al. The Emergence of COVID-19 Associated Mucormycosis: analysis of cases from 18 Countries. 2021; [cited 2021 Oct 30]. Available from: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ppcovidwho-8778.
- Kalantar-Zadeh K, Ward SA, Kalantar-Zadeh K, et al. Considering the effects of microbiome and diet on SARS-CoV-2 infection: nanotechnology roles. ACS Nano. 2020;14(5):5179–5182.
