Abstract
Objective
This study evaluated body mass index (BMI) and weight changes in people living with human immunodeficiency virus (HIV-1; PLWH) initiated on single-tablet darunavir/cobicistat/emtricitabine/tenofovir alafenamide (DRV/c/FTC/TAF) or bictegravir/FTC/TAF (BIC/FTC/TAF).
Methods
Electronic medical record (EMR) data for treatment-naïve or virologically suppressed adults with HIV-1 who initiated treatment with DRV/c/FTC/TAF or BIC/FTC/TAF (index date) were obtained from Decision Resources Group’s EMRs (17 July 2017–1 March 2020). Inverse probability of treatment weighting was used to account for differences in baseline characteristics between the two cohorts. BMI and weight changes from pre-index to 3, 6, 9 and 12 months following the index date were compared using weighted mean differences (MDs). The time until an increase in BMI or weight ≥5% or ≥10% was compared using weighted hazard ratios (HRs).
Results
The weighted DRV/c/FTC/TAF and BIC/FTC/TAF cohorts comprised 1116 and 1134 PLWH, respectively (mean age = ∼49 years, females: ∼28%). Larger increases in BMI and weight from pre-index to each post-index time point were observed in PLWH initiating BIC/FTC/TAF vs DRV/c/FTC/TAF (12 months: MD in BMI = 1.23 kg/m2, p < .001; MD in weight = 2.84 kg [6.26 lbs], p = .008). PLWH receiving BIC/FTC/TAF were significantly more likely to experience weight gain ≥5% (HR = 1.76, p = .004) and ≥10% (HR = 2.01, p = .020), and BMI increase ≥5% (HR = 1.77, p = .004) and ≥10% (HR = 1.76, p = .044) than those receiving DRV/c/FTC/TAF.
Conclusions
BIC/FTC/TAF was associated with greater BMI and weight increases compared to DRV/c/FTC/TAF. Weight gain and its sequelae may add to the clinical burden of PLWH and should be considered among other factors when selecting antiretroviral single-tablet regimens.
Introduction
The incidence of human immunodeficiency virus (HIV-1) has declined in the last two decades in large part due to improved infection management through early detection and treatment with antiretroviral therapies (ARTs)Citation1. The use of ARTs has improved clinical outcomes, quality of life and survival in people living with HIV-1 (PLWH), although it has also been linked to weight gain, renal impairment, bone loss, cardiovascular disease and metabolic disordersCitation2–10. The US Department of Health and Human Services (DHHS) Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV recommend the use of ART regimens containing an integrase strand transfer inhibitor (INSTI) in most clinical situationsCitation10, although for PLWH who may be at risk of poor adherence or for those who require rapid initiation of ART before genotypic drug resistance test results become available, the use of boosted darunavir (DRV; a protease inhibitor [PI]), bictegravir (BIC; an INSTI), or dolutegravir (DTG; an INSTI) is specifically recommendedCitation10.
An increasing number of publications have reported that PLWH initiating an INSTI-based regimen gain more weight than those treated with other ART regimensCitation10–16, which may add to the clinical burden of HIV-1 by increasing the risk of sequelae such as diabetes mellitus, myocardial infarction, stroke and other cardiometabolic conditionsCitation17–19. Specifically, increased risks of weight gain have been associated with female genderCitation20, older ageCitation21,Citation22, Black raceCitation8,Citation23, baseline body mass index (BMI)Citation23, baseline CD4+ cell countCitation24, baseline HIV-1 viral loadCitation8 and certain genotypesCitation25. Consequently, the DHHS guidelines have now noted that among treatment-naïve PLWH, receiving a regimen containing an INSTI agent is linked to greater weight gain than receiving a regimen containing a boosted PI or non-nucleoside reverse transcriptase inhibitor (NNRTI)Citation10. Aside from the third agent (e.g. INSTI), some nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) – especially tenofovir alafenamide (TAF) – have been shown to cause weight gain as wellCitation10,Citation26,Citation27.
A previous assessment of weight-related outcomes based on linked electronic medical records (EMRs) and claims data showed that increases in BMI and body weight were greater among PLWH initiated on BIC/emtricitabine (FTC)/TAF compared to DRV/cobicistat (c)/FTC/TAFCitation28, two single-tablet regimens (STRs) that were approved by the US Food and Drug Administration (FDA) in 2018Citation29,Citation30. However, the conclusions were limited to only 9 months given the short observation period and small sample size.
The objective of this study was to build upon previous work and compare weight-related outcomes between a larger sample of ART-naïve and virologically suppressed ART-experienced (i.e. stable suppressed) PLWH newly initiating treatment with DRV/c/FTC/TAF or BIC/FTC/TAF using more recent data with longer follow-up.
Methods
Data source
EMR data from 17 July 2017 to 1 March 2020 from Decision Resources Group (DRG)’s Real World Data Repository (part of Clarivate) were used in the current study. DRG’s EMR data is primarily ambulatory and includes specialist and primary care visits. Information on individuals, encounters, written prescriptions, diagnoses and vitals (including BMI and weight) is available. In addition, the database is representative of the whole US population and includes individuals from every state. The data were de-identified and complied with the Health Insurance Portability and Accountability Act’s patient requirements. Institutional Review Board approval was not required for this study because this was a retrospective analysis of existing EMR data and did not involve intervention or interaction with the patients and additionally, no patient-identifiable information was included in the dataset.
Study design
This retrospective longitudinal study included ART-naïve and stable suppressed ART-experienced PLWH. The index date was defined as the date of DRV/c/FTC/TAF or BIC/FTC/TAF initiation on or after 17 July 2018 (US FDA approval date for DRV/c/FTC/TAF). A baseline period of 12 months of continued clinical activity prior to the index date was required and the follow-up period spanned from the index date to the end of continuous clinical activity (i.e. period from the first to the last record in the EMR database) or data availability, whichever occurred first.
Study sample
Individuals were included in the study if they met the following inclusion criteria: treated with DRV/c/FTC/TAF or BIC/FTC/TAF (i.e. index treatment), had an HIV-1 diagnosis on or before the index date, were ≥18 years old at the index date, had pre-index continuous clinical activity for ≥12 months, had ≥1 BMI or weight measurement in both the baseline and observation periods, and were either treatment-naïve (i.e. no ART during the baseline period) or virologically suppressed ART-experienced (i.e. PLWH treated with an ART at baseline who had no HIV-1 viral load measurement ≥50 copies/mL during the 6 months pre-index [stable switchers]; ).
Figure 1. Selection of the study population. Abbreviations. ART, Antiretroviral therapy; BIC, Bictegravir; BMI, Body mass index; c, Cobicistat; DRV, Darunavir; EMR, Electronic medical record; FTC, Emtricitabine; HIV-1, Human immunodeficiency virus type 1; ICD-9 CM/ICD-10 CM, International Classification of Disease, 9th/10th Revision, Clinical Modification; PLWH, People living with HIV-1; TAF, Tenofovir alafenamide.

The exclusion criteria for the study included the following: PLWH receiving ART but who were not stable or PLWH who had ≥1 diagnosis during the baseline period for HIV-2, chronic renal insufficiency (or a measurement for creatinine clearance <30 mL/min), liver disease (e.g. liver cirrhosis and hepatitis), malignant neoplasm (except for cutaneous Kaposi’s sarcoma, resected noninvasive cutaneous squamous carcinoma or basal cell carcinoma) or pregnancy.
Study measures
Demographic and clinical characteristics of PLWH included in the study were described during the 12 month baseline period. The pre-index BMI or body weight was defined as the measurement during the baseline period that was closest to the index date. In the absence of measurements during this period, the closest measurement within 30 days after the index date was used. The BMI or weight value at 3, 6, 9 or 12 months following the index date was considered to be the one closest to that point in time (within a 45 day window before or after the time point). For each post-index time point, the absolute and relative differences (i.e. increase >0%, ≥5% and ≥10%) in BMI and weight between the post-and the pre-index time point were assessed. To further understand the temporal trends in these changes, the time to BMI or weight increase of ≥5% or ≥10% was also evaluated over the complete follow-up for both study cohorts.
Statistical analysis
Baseline characteristics were summarized using means, standard deviations (SDs) and medians for continuous variables, while frequencies and proportions were reported for binary variables. Inverse probability of treatment weighting (IPTW) accounted for imbalances in characteristics evaluated during the baseline period between treatment cohorts. In this approach, each PLWH’s propensity score (PS) is evaluated using a multivariate logistic regression model, where the dependent variable is the index treatment (i.e. DRV/c/FTC/TAF or BIC/FTC/TAF) and characteristics evaluated during the baseline period are independent variables used to predict treatment assignment. The following baseline characteristics were included in the PS model: age; sex; race; geographic region; type of insurance plan; year of initiation of the index treatment; time since HIV-1 disease onset; presence of symptomatic HIV-1 and AIDS; Quan–Charlson Comorbidity Index (CCI) score (not including symptomatic HIV-1); obesity; pre-diabetes; type II diabetes; hypertension; use of ART, TAF or other drugs known to cause weight change (i.e. anticonvulsants, antidepressants, antidiabetics, lipid-lowering agents, antihypertensives, antipsychotics, appetite stimulants and suppressants, beta blockers, corticosteroids, and hormonal therapies); use of a PI, INSTI, NNRTI or TAF agent as part of the most recent regimen in the last 45 days pre-index; baseline BMI; and presence of a high-density lipoprotein (HDL) measurement. The weight for each PLWH was estimated as follows: 1/PS for the DRV/c/FTC/TAF cohort and 1/(1 − PS) for the BIC/FTC/TAF cohort. Weights derived from the IPTW were truncated at the 95th percentile of the distribution of weights to avoid extreme values. In addition, weights were normalized by the mean weight. As a result, even though the number of PLWH contributing to the analysis did not change, the post-IPTW weighted sample sizes differed from original sample sizes because of the different weight assigned to each PLWH (whereas the weight of each PLWH was one before IPTW). Therefore, post-IPTW, the sum of PLWH weights for each cohort yielded a different sample size than for the original unweighted cohorts. Differences in outcomes obtained after weighting the cohorts with IPTW thus reflect the average treatment effect. Standardized differences were used to compare baseline characteristics between the two cohorts; characteristics with a standardized difference <10% were considered well balancedCitation31.
Mean differences (MDs) estimated from weighted ordinary least squares regression were used to compare the two cohorts in terms of changes in mean BMI and weight from pre-index to each post-index time point (i.e. 3, 6, 9 and 12 months). The proportion of PLWH in each cohort with any, ≥5% and ≥10% increase in BMI and weight from the pre-index period to each of the post-index time points (i.e. 3, 6, 9 and 12 months) was compared using odds ratios (ORs) estimated from weighted logistic regression. As BMI and weight measurements were missing for some time points, the sample size included in the evaluation of changes in BMI or weight varied across time points. Therefore, in addition, the time to BMI or weight increase ≥5% and ≥10% included all PLWH and was compared between the two cohorts using hazard ratios (HRs) estimated from weighted Cox proportional hazard models. The weighted median time to BMI or weight increase ≥5% or ≥10% was reported using Kaplan–Meier curves. To account for variables that remained imbalanced after IPTW, all models were further adjusted for the following variables (doubly robust approach): baseline use of PIs, INSTIs, NNRTIs, beta blockers and insulins; and history of sleep-wake disorders, psychoses and insomnia. In addition, 95% confidence intervals (CIs) and p values for all models were calculated using nonparametric bootstraps with 499 replicates. The DRV/c/FTC/TAF cohort was the reference group in all regression models.
Results
A total of 223 and 2027 eligible PLWH were included in the unweighted DRV/c/FTC/TAF and BIC/FTC/TAF cohorts, respectively (). After weighting of the cohorts using IPTW to adjust for baseline characteristics, the weighted sample sizes (i.e. the sum of weights in each cohort) were 1116 for the DRV/c/FTC/TAF cohort and 1134 for the BIC/FTC/TAF cohort ().
Table 1. Baseline characteristics during the 12 month period prior to the index date among weighted DRV/c/FTC/TAF and BIC/FTC/TAF cohorts.
Baseline characteristics
Baseline characteristics included in the IPTW model were well balanced between the cohorts, based on standardized differences <10% (). The mean (SD) age was 49.2 (12.1) years in the weighted DRV/c/FTC/TAF cohort and 48.9 (12.8) years in the weighted BIC/FTC/TAF cohort, and the proportion of females was 27.9% and 28.6%, respectively. Most PLWH in this cohort were Whites (DRV/c/FTC/TAF: 36.3% vs BIC/FTC/TAF: 34.4%) or Blacks/African Americans (35.5% vs 38.1%), resided in the South (72.9% vs 71.9%) and had commercial insurance (67.9% vs 69.2%). The mean (SD) baseline Quan-CCI score (not including HIV-1 symptoms) in the two cohorts was 0.3 (0.6).
In the DRV/c/FTC/TAF and BIC/FTC/TAF cohorts, 61.4% (N = 685) and 60.3% (N = 684), respectively, were previously treated with an ART during the baseline period (i.e. these were considered ART-experienced PLWH), of whom 50.1% and 49.3% used TAF (). Among ART-experienced PLWH, 22.0% (151 of 685) and 25.6% (175 of 684) in the DRV/c/FTC/TAF and BIC/FTC/TAF cohorts, respectively, were still treated with their previous ART regimen in the last 45 days before initiating the index regimen. Among these patients, 33.1% in the DRV/c/FTC/TAF cohort were using a PI-based regimen in the last 45 days before initiating the index regimen, 57.0% an INSTI-based regimen and 21.9% an NNRTI-based regimen; these proportions were 20.6%, 57.1% and 28.6% in the BIC/FTC/TAF cohort. Unweighted baseline characteristics (i.e. prior to weighting the cohorts using IPTW) are presented in Supplemental Table 1.
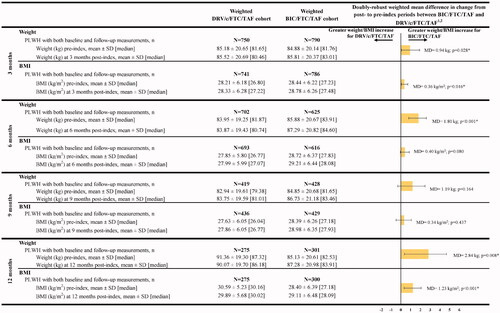
Comparison of BMI and weight changes at specific time points
Weight increases from the pre-index period to the 3, 6 and 12 month post-index time points were significantly greater among PLWH treated with BIC/FTC/TAF than among those treated with DRV/c/FTC/TAF (). The adjusted MD between pre- and post-index weights ranged from 0.94 kg (2.07 lbs) (BIC/FTC/TAF Δ3 months = 0.93 kg [2.05 lbs]; DRV/c/FTC/TAF Δ3 months = 0.34 kg [0.75 lbs]; p = .028) at 3 months to 2.84 kg (6.26 lbs) (BIC/FTC/TAF Δ12 months = 2.15 kg [4.74 lbs]; DRV/c/FTC/TAF Δ12 months = −1.29 kg [−2.84 lbs]; p = .008) at 12 months. A similar trend was observed for the increases in BMI from pre- to post-index periods, with the adjusted MD ranging from 0.36 kg/m2 (BIC/FTC/TAF Δ3 months = 0.34 kg/m2; DRV/c/FTC/TAF Δ3 months = 0.12 kg/m2; p = .016) at 3 months to 1.23 kg/m2 (BIC/FTC/TAF Δ12 months = 0.71 kg/m2; DRV/c/FTC/TAF Δ12 months = −0.70 kg/m2; p < .001) at 12 months.
Figure 2. Comparison of mean BMI or weight change between pre- and post-index periods. Abbreviations. BIC, Bictegravir; BMI, Body mass index; c, Cobicistat; CI, Confidence interval; DRV, Darunavir; FTC, Emtricitabine; MD, Mean difference; PLWH, People living with human immunodeficiency virus type 1; SD, Standard deviation; TAF, Tenofovir alafenamide. *p < .05. 1Doubly robust weighted MDs were obtained from weighted ordinary least squares regression adjusted for the following variables: baseline use of a protease inhibitor; use of an integrase strand transfer inhibitor; use of a non-nucleoside reverse transcriptase inhibitor; use of a beta blocker; use of insulin; and history of sleep–wake disorders, psychoses and insomnia. As the ordinary least square regression models included these variables, the doubly robust weighted MDs presented in the bar chart were not equal to the unadjusted difference in weighted means between the two cohorts that were calculated using the first two columns of results. MD >0 indicates that the BIC/FTC/TAF cohort had a larger BMI or weight gain than the DRV/c/FTC/TAF cohort. 2Nonparametric 95% CIs and p values were calculated based on 499 bootstrap resamples. At each bootstrap resample, the inverse probability of treatment weights were re-estimated.
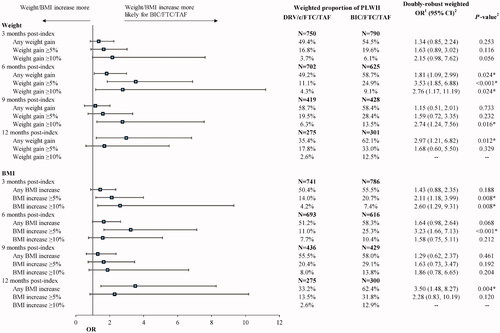
At the 12 month post-index time point, the proportion of PLWH initiated on BIC/FTC/TAF vs DRV/c/FTC/TAF who experienced any increase (i.e. >0%) in BMI or weight was significantly higher (weight: 62.1% vs 35.4%, OR = 2.97, p = .012; BMI: 62.4% vs 33.2%, OR = 3.50, p = .004; ). The proportion of PLWH initiated on BIC/FTC/TAF with increases ≥5% and ≥10% in BMI or weight was also significantly higher at 6 and 9 months for weight and at 3 and 6 months for BMI.
Figure 3. Proportions of PLWH with any increase or a ≥ 5% or ≥10% increase in BMI or weight from pre- to post-index periods. Abbreviations. BIC, Bictegravir; BMI, Body mass index; c, Cobicistat; CI, Confidence interval; DRV, Darunavir; FTC, Emtricitabine; OR, Odds ratio; PLWH, People living with human immunodeficiency virus type 1; SD, Standard deviation; TAF, Tenofovir alafenamide. *p < .05. 1ORs were estimated from weighted ordinary least squares regression adjusted for the following variables: baseline use of a protease inhibitor; use of an integrase strand transfer inhibitor; use of a non-nucleoside reverse transcriptase inhibitor; use of a beta blocker; use of insulin; and history of sleep–wake disorders, psychoses and insomnia. OR >1 indicates that the BIC/FTC/TAF cohort had a higher risk of a BMI or weight gain than the DRV/c/FTC/TAF cohort. ORs for BMI and weight increases ≥10% were not assessed at 12 months because of a lack of model convergence. 2Nonparametric 95% CIs and p values were calculated based on 499 bootstrap resamples. At each bootstrap resample, the inverse probability of treatment weights were re-estimated.
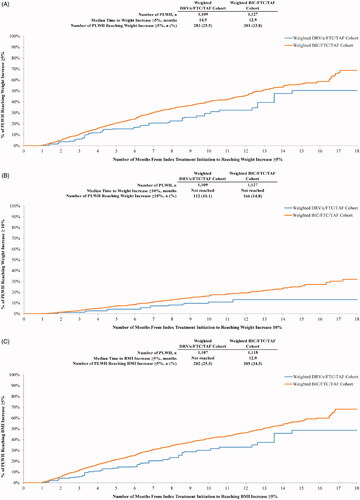
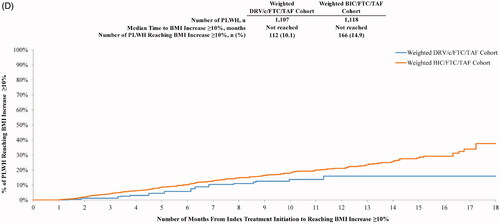
Time to BMI or weight increase
Over the entire follow-up period, PLWH initiated on BIC/FTC/TAF were 1.76 and 2.01 times more likely to experience a weight increase ≥5% (p = .004) and ≥10% (p = .020), respectively, relative to those receiving DRV/c/FTC/TAF (). Similar results were obtained for BMI increases ≥5% (HR = 1.77, p = .004) and ≥10% (HR = 1.76, p = .044).
Figure 4. Weighted HRs comparing weight gain or BMI increase ≥5% and ≥10% between the DRV/c/FTC/TAF and BIC/FTC/TAF cohorts. Abbreviations. BIC, Bictegravir; BMI, Body mass index; c, Cobicistat; CI, Confidence interval; DRV, Darunavir; FTC, Emtricitabine; HR, Hazard ratio; PLWH, People living with human immunodeficiency virus type 1; SD, Standard deviation; TAF, Tenofovir alafenamide. *p < .05. 1HRs were estimated from weighted Cox proportional hazards regression adjusted for the following variables: baseline use of a protease inhibitor; use of an integrase strand transfer inhibitor; use of a non-nucleoside reverse transcriptase inhibitor; use of a beta blocker; use of insulin; and history of sleep–wake disorders, psychoses and insomnia. HR >1 indicates that the BIC/FTC/TAF cohort had a higher risk of a BMI or weight gain than the DRV/c/FTC/TAF cohort. 2Nonparametric 95% CIs and p values were calculated based on 499 bootstrap resamples. At each bootstrap resample, the inverse probability of treatment weights were re-estimated.

The median time from index treatment initiation to weight gain ≥5% was shorter for the BIC/FTC/TAF cohort (12.9 months) than for the DRV/c/FTC/TAF cohort (14.5 months), and was not reached in either cohort for the ≥10% threshold (). A similar trend was observed for BMI whereby median time to BMI increase ≥5% was 12.9 months for PLWH initiated on BIC/FTC/TAF and was not reached for PLWH initiated on DRV/c/FTC/TAF (). Similar to weight gain ≥10%, median time to BMI increase ≥10% was not reached ().
Figure 5. (A) Time from initiation of the index treatment to weight increase ≥5%. (B) Time from initiation of the index treatment to weight increase ≥10%. (C) Time from initiation of the index treatment to BMI increase ≥5%. (D) Time from initiation of the index treatment to BMI increase ≥10%. Abbreviations. BIC, Bictegravir; BMI, Body mass index; c, Cobicistat; CI, Confidence interval; DRV, Darunavir; FTC, Emtricitabine; PLWH, People living with human immunodeficiency virus type 1; TAF, Tenofovir alafenamide.
Discussion
This study provides real-world evidence regarding BMI and weight changes among a diverse population of ART-naïve and virologically suppressed ART-experienced PLWH initiated on DRV/c/FTC/TAF or BIC/FTC/TAF. Results showed that after weighting PLWH to adjust for differences in baseline characteristics, the BIC/FTC/TAF cohort had significantly greater increases in BMI and weight than the DRV/c/FTC/TAF cohort. Moreover, the same trend was observed at all time points, with differences between cohorts being most pronounced at 12 months following initiation of the index treatment. Over the entire follow-up period, PLWH who received BIC/FTC/TAF were approximately twice as likely to experience BMI and weight increase ≥5% or ≥10% compared to those who received DRV/c/FTC/TAF.
Simplifying treatment regimens is an effective strategy proven to increase adherence to ART and improve prognosis among PLWHCitation32–34. As such, clinicians are encouraged to consider STRs either as a first-line ART or to simplify the current ART received by PLWHCitation10. This study provides a comprehensive assessment of changes in BMI and weight in PLWH receiving DRV/c/FTC/TAF or BIC/FTC/TAF, two currently FDA approved STRs, using recent and robust data derived from EMR. Using a larger sample with longer follow-up period for each cohort allowed for adequate adjustment of the imbalances between the cohorts (such as prior ART use) in this study, and it confirms previous results from a real-world study comparing DRV/c/FTC/TAF and BIC/FTC/TAFCitation28.
At each post-index time point examined, increases in BMI and weight were consistently higher for BIC/FTC/TAF vs DRV/c/FTC/TAF, and MDs tended to increase over time, from 0.94 kg (2.07 lbs) and 0.36 kg/m2 at 3 months to 2.84 kg (6.26 lbs) and 1.23 kg/m2 at 12 months, for weight and BMI increases, respectively. Previous studies have reported a similar positive relationship between time and BMI or weight increase among PLWH receiving ART. A large retrospective clinical study conducted using data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) reported an average weight increase of 4.4 kg (9.7 lbs) 1 year following initiation of an INSTI-based regimen among treatment-naïve PLWH, which further increased to 5.9 kg (13.0 lbs) at 5 years following initiationCitation35. The largest increases were observed among dolutegravir initiators, whose weight increased by 5.4 kg (11.0 lbs) at 1 year and 7.2 kg (15.9 lbs) at 2 years (data not available at 5 years)Citation35. A pooled analysis of over 5000 treatment-naïve PLWH enrolled in randomized clinical trials completed between 2003 and 2015 reported that initiators of INSTI-based regimens experienced a mean weight increase of 2.3 kg (5.1 lbs) at 48 weeks and 3.2 kg (7.1 lbs) at 96 weeks post-initiation, which was significantly higher than the 1.2 kg (2.6 lbs) and 1.4 kg (3.1 lbs) increase observed among initiators of PI-based regimensCitation8. The pooled analysis also suggested that, in addition to the type of regimen received, weight gain was also associated with certain demographic and disease characteristics of PLWH, such as gender, race, baseline CD4+ cell count and HIV-1 viral loadCitation8; these factors have also been described to be associated with weight gain in previous reportsCitation20,Citation22–24. Therefore, when interpreting results from the current study, one should consider the interactions among these characteristics as well as other confounders that may also have contributed to the observed weight gain differences between the cohorts.
The time-to-event analysis conducted in the current study, which included ART-naïve and virologically suppressed PLWH, demonstrated a clear trend for PLWH receiving BIC/FTC/TAF to experience clinically meaningful BMI and weight increases (i.e. ≥5% or ≥10%) more rapidly than those receiving DRV/c/FTC/TAF, further highlighting the growing challenge of managing weight gain in PLWH treated with ART in the real world. This time-to-event analysis had the advantage of including all eligible PLWH in the study and considering all follow-up BMI and weight measurements without having to separate the data into discrete time periods. Our findings showed that over the entire study period, BMI or weight increases ≥5% or ≥10% were 76%–101% more likely among PLWH receiving BIC/FTC/TAF vs DRV/c/FTC/TAF. The robustness of these findings was confirmed in several subgroup analyses, such as treatment-naïve PLWH, stable switchers, antidiabetic users at baseline, PLWH with baseline BMI <20 kg/m2 and PLWH with baseline BMI ≥35 kg/m2. For example, for the outcome of time to weight gain ≥5%, the results for all subgroups showed that PLWH initiated on BIC/FTC/TAF were more likely to have a weight gain ≥5% than those initiated on DRV/c/FTC/TAF (treatment-naïve: HR = 1.83, p < .001; stable switchers: HR = 1.73, p < .001; antidiabetic users: HR = 1.29; p = .388; BMI < 20 kg/m2: HR = 1.84, p = .029; BMI ≥ 35 kg/m2: HR = 1.84, p = .132). Of note, for some of the subgroups, results did not reach statistical significance, likely due to smaller sample sizes.
The mechanisms of ART-associated weight gain remain poorly understoodCitation36,Citation37. Apart from the “return-to-health” phenomenon, whereby weight gain is attributable to the clinical recovery from HIV-1–induced decreased adipogenesisCitation37, conventional ARTs have also been shown to exert off-target effects on adipose tissue and lipid and glucose metabolism, which leads to complications such as adipose dysfunction and insulin resistanceCitation38; and these conditions are closely linked to weight gain and obesityCitation36,Citation39. The impacts of PIs and INSTIs on metabolism appear to be different. PIs have been shown to be casually associated with lipodystrophy, which is characterized by peripheral fat loss and central fat accumulation, along with insulin resistanceCitation40,Citation41. Alterations in several regulatory proteins have been implicated in the metabolic complications associated with PIs, including reduction of sterol regulatory enhancer binding protein-1 required for adipocyte differentiationCitation42, inhibition of proteasome that prevents degradation of apolipoprotein BCitation43 and blockade of glucose transporter-4 that inhibits glucose uptake in adipocytesCitation40. The impacts of PIs on adipose functions and insulin sensitivity have been shown in vitro to be milder with newer PIs such as DRVCitation44. A recent post-hoc analysis of the phase 3 EMERALD study found that virologically suppressed PLWH who switched to DRV/c/FTC/TAF appeared to have minimal impact on the proportion of patients having metabolic syndrome (0.8 percentage point increase from baseline to week 48) compared with those who continued to receive conventional boosted PI-based regimen (4.2 percentage point increase from baseline to week 48)Citation45. Meanwhile, reports on the effects of INSTIs on metabolism vary. In vitro studies have shown differential and at times conflicting effects of INSTIs on adipose tissue and metabolismCitation46,Citation47. In an observational prospective cohort study, ART-controlled PLWH who switched from a PI-based regimen to raltegravir (an INSTI) or DTG showed improved insulin sensitivityCitation48, but in another randomized clinical trial, the proportion of ART-naïve PLWH treated with raltegravir experienced similar increases in insulin resistance as those treated with PI-based regimensCitation49. Mechanistically, INSTIs have been shown to interfere with the melanocortin receptor-4 (MCR4), a receptor that regulates metabolic energy balance and has been implicated in obesityCitation50; however, a later study concluded that clinical exposure to INSTIs was insufficient to exert an impact on MCR4 that could explain the INSTI-associated weight gainCitation51. In short, the mechanisms of INSTIs on metabolism and subsequent weight gain remain largely unknown. While the underlying mechanisms remain to be elucidated, the general observation is that INSTIs induce greater weight gain compared with PIsCitation8,Citation12,Citation13, which is in line with the findings of the current large cohort study.
In general, PLWH have an inherently higher risk of developing, or worsening, comorbid conditions (in particular, diabetes or cardiovascular diseases)Citation52–55, with studies suggesting that BMI and/or weight gains amplify those risksCitation18,Citation19,Citation56,Citation57, particularly when they are rapid or persist in the long-termCitation58,Citation59. With the effectiveness of ART prolonging life and resulting in HIV-1 becoming a chronic condition, an increasing proportion of PLWH survive well into old ageCitation60,Citation61, reinforcing the burden of comorbid age-associated health conditionsCitation54,Citation55. As a result, when considering long-term consequences on health, it is crucial to consider the potential for BMI or weight increase (which may be greatest in the first year post-initiation and typically continues to increase over timeCitation8,Citation35) associated with the use of certain ARTs when deciding the best course of treatment for PLWH. Further analyses are warranted to evaluate the long-term clinical consequences of ART-related BMI or weight increase in PLWH.
Limitations
Results from the current study should be interpreted in light of certain limitations. First, while prescriptions for ARTs are assumed to be filled by PLWH, they may not have adhered to the treatment regimen or refilled their prescriptions. Second, the use of IPTW only produces a valid causal estimate if all confounders are accounted for. Therefore, it is possible that unmeasured factors exist between the two cohorts that would explain some of the observed association between the two STRs and the observed changes in BMI and weight. Third, missing BMI or weight measurements could be informative as PLWH who do not experience significant BMI or weight changes may not undergo regular weight assessments. Therefore, it is possible that our analyses capture more extreme weight changes. However, this effect is likely to be non-differential as it is expected to impact PLWH in both treatment cohorts equally. Fourth, the treatment effect estimated in the current study is assumed to be related to the regimen as a whole (i.e. DRV/c/FTC/TAF or BIC/FTC/TAF) rather than the specific third agent (DRV or BIC) or the NRTI backbone used. Further research is needed to separate the impact of each regimen component on BMI increase and weight gain and in the real world. Fifth, for the study exclusion criteria, it was assumed that PLWH who did not have evidence of active viral replication (defined by HIV viral load ≥50 copies/mL) or renal insufficiency (<30 mL/min/1.73 m2) were eligible for inclusion, although the absence of any evidence for one of these two criteria does not guarantee that a PLWH did not have either of the conditions. Furthermore, drug resistance data was not available in this data source; therefore, it was not possible to determine whether stable suppressed PLWH had no known substitutions associated with resistance to components of the index regimen, as described in prescribing guidelines. Sixth, EMR data from DRG used in the current study did not contain information on waist-to-hip ratio and may have contained errors or omissions in variables of interest such as diagnoses, weight values, BMI values, HIV-1 viral load measurements and CD4+ cell count measurements.
Conclusions
PLWH initiating BIC/FTC/TAF were more likely to experience BMI or weight increases compared to DRV/c/FTC/TAF, and tended to reach a threshold of increases greater than 5% or 10% faster. Differences in BMI and weight change between the two treatment cohorts increased further at later time points. This study reinforces that the potential for ART-related BMI and weight gain and its sequelae may add to the clinical burden of PLWH and should be considered among other factors when selecting antiretroviral single-tablet regimens.
Transparency
Declaration of funding
This study was supported by Janssen Scientific Affairs, LLC. The sponsor was involved in all steps of the present work, including the design of the study; the collection, analysis and interpretation of data; the writing of the manuscript; and the decision to submit the article for publication.
Declaration of financial/other relationships
B.E., C.R., A.C.S., P.L. and M.H.L. are employees of Analysis Group, Inc, a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. B.B., D.A. and P.D. are employees of Janssen Scientific Affairs, LLC and stockholders of Johnson & Johnson. A reviewer on this manuscript disclosed that they have received grants for educational activities and personal fees for advisory boards and speakers bureau for Abbvie, Gilead Sciences and Janssen-Cilag. CMRO peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design of the study, analysis and interpretation of the data, drafting of the manuscript and revising it critically for intellectual content, and the decision to submit the manuscript for publication. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (23.5 KB)Acknowledgements
Medical writing assistance was provided by Loraine Georgy PhD and Janice Imai PhD, employees of Analysis Group, Inc, a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. The authors would like to thank Wing Chow PharmD MPH, a former employee of Janssen Scientific Affairs, LLC, for her contributions to the development of the study design and study protocol.
References
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2014–2018. HIV surveillance supplemental report 2020;25(No. 1). [cited 2021 Oct 28]. Available from: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317(4):185–191.
- Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–e447.
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
- Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013;64(2):183–189.
- Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai project study group. N Engl J Med. 2000;342(13):921–929.
- Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–181.
- Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379–1389.
- Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the international antiviral Society – USA panel. JAMA. 2020;324(16):1651–1669.
- U.S. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. [cited 2021 Oct 28]. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/what-start-initial-combination-regimens-antiretroviral-naive
- Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–1274.
- Chen YW, Hardy H, Pericone CD, et al. Real-world assessment of weight change in people with HIV-1 after initiating integrase strand transfer inhibitors or protease inhibitors. J Health Econ Outcomes Res. 2020;7(2):102–110.
- Chow W, Donga P, Cote-Sergent A, et al. An assessment of weight change associated with the initiation of a protease or integrase strand transfer inhibitor in patients with human immunodeficiency virus. Curr Med Res Opin. 2020;36(8):1313–1323.
- Mounzer K, Brunet L, Hsu R, et al. Changes in BMI associated with antiretroviral regimens in treatment-experienced, virologically suppressed individuals living with HIV. Paper presented at: IDWeek2019; Washington, DC.
- Norwood J, Turner M, Bofill C, et al. Brief report: weight gain in persons with HIV switched from Efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531.
- Wu KS, Anderson C, Little SJ. Integrase strand transfer inhibitors play the main role in greater weight gain among men with acute and early HIV infection. Open Forum Infect Dis. 2021;8(1):ofaa619.
- Koethe JR, Jenkins CA, Turner M, et al. Body mass index and the risk of incident noncommunicable diseases after starting antiretroviral therapy. HIV Med. 2015;16(1):67–72.
- Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes. Front Endocrinol. 2018;9(705):705.
- Achhra AC, Mocroft A, Reiss P, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med. 2016;17(4):255–268.
- Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666–e676.
- Nozza S, Malagoli A, Maia L, et al. Antiretroviral therapy in geriatric HIV patients: the GEPPO cohort study. J Antimicrob Chemother. 2017;72(10):2879–2886.
- Lake JE, Wu K, Bares SH, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis. 2020;71(9):e471–e477.
- Taylor BS, Liang Y, Garduno LS, et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr. 2014;65(2):e33–e40.
- Orkin C, Elion R, Thompson M, et al. Changes in weight and BMI with first-line doravirine-based therapy. AIDS. 2021;35(1):91–99.
- Leonard MA, Cindi Z, Bradford Y, et al. Efavirenz pharmacogenetics and weight gain following switch to integrase inhibitor-containing regimens. Clin Infect Dis. 2021;73(7):e2153–e2163.
- Gomez M, Seybold U, Roider J, et al. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015-2017. Infection. 2019;47(1):95–102.
- Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815.
- Emond B, Rossi C, Côté-Sergent A, et al. Weight change and predictors of weight change among patients initiated on darunavir/cobicistat/emtricitabine/tenofovir alafenamide or bictegravir/emtricitabine/tenofovir alafenamide: a real-world retrospective study. J Health Econ Outcomes Res. 2021;8(1):88–98.
- Janssen Pharmaceuticals. Symtuza – Prescribing Information. 2018 [cited 2018 Apr 27]. Available from: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SYMTUZA-pi.pdf
- Gilead Sciences. Biktarvy – Prescribing Information. 2018 [cited 2018 Apr 26]. Available from: https://www.gilead.com/-/media/files/pdfs/medicines/hiv/biktarvy/biktarvy_pi.pdf
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107.
- Altice F, Evuarherhe O, Shina S, et al. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. PPA. 2019;13:475–490.
- Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther. 2018;15(1):17.
- Sutton SS, Hardin JW, Bramley TJ, et al. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care. 2016;22(4):242–248.
- Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484.
- Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis. 2020;33(1):10–19.
- Shah S, Hindley L, Hill A. Are new antiretroviral treatments increasing the risk of weight gain? Drugs. 2021;81(3):299–315.
- Lagathu C, Bereziat V, Gorwood J, et al. Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin Drug Saf. 2019;18(9):829–840.
- Godfrey C, Bremer A, Alba D, et al. Obesity and fat metabolism in human immunodeficiency virus-infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis. 2019;220(3):420–431.
- Carr A. HIV lipodystrophy: risk factors, pathogenesis, diagnosis and management. AIDS. 2003;17(Suppl 1):S141–S148.
- Carr A, Samaras K, Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353(9170):2093–2099.
- Caron M, Auclair M, Vigouroux C, et al. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001;50(6):1378–1388.
- Liang JS, Distler O, Cooper DA, et al. HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med. 2001;7(12):1327–1331.
- Capel E, Auclair M, Caron-Debarle M, et al. Effects of ritonavir-boosted darunavir, atazanavir and lopinavir on adipose functions and insulin sensitivity in murine and human adipocytes. Antivir Ther. 2012;17(3):549–556.
- Dunn K, Gibson A, Van Landuyt E, et al. Low proportion of individuals develop metabolic syndrome (MetS) or hepatic fibrosis after switch to darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in virologically suppressed patients: a post hoc metabolic analysis. Paper presented at: International AIDS Society (IAS) Conference; 2021 Jul 18–21; virtual.
- Gorwood J, Bourgeois C, Pourcher V, et al. The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis. 2020;71(10):e549–e560.
- Moure R, Domingo P, Gallego-Escuredo JM, et al. Impact of elvitegravir on human adipocytes: alterations in differentiation, gene expression and release of adipokines and cytokines. Antiviral Res. 2016;132:59–65.
- Calza L, Colangeli V, Borderi M, et al. Improvement in insulin sensitivity and serum leptin concentration after the switch from a ritonavir-boosted PI to raltegravir or dolutegravir in non-diabetic HIV-infected patients. J Antimicrob Chemother. 2019;74(3):731–738.
- Dirajlal-Fargo S, Moser C, Brown TT, et al. Changes in insulin resistance after initiation of raltegravir or protease inhibitors with tenofovir-emtricitabine: AIDS clinical trials group A5260s. Open Forum Infect Dis. 2016;3(3):ofw174.
- Domingo P, Villarroya F, Giralt M, et al. Potential role of the melanocortin signaling system interference in the excess weight gain associated to some antiretroviral drugs in people living with HIV. Int J Obes. 2020;44(9):1970–1973.
- McMahon C, Trevaskis JL, Carter C, et al. Lack of an association between clinical INSTI-related body weight gain and direct interference with MC4 receptor (MC4R), a key central regulator of body weight. PLoS One. 2020;15(2):e0229617.
- Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–216.
- Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512.
- Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–1797.
- Bonnet F, Le Marec F, Leleux O, et al. Evolution of comorbidities in people living with HIV between 2004 and 2014: cross-sectional analyses from ANRS CO3 Aquitaine cohort. BMC Infect Dis. 2020;20(1):850.
- Isa SE, Oche AO, Kang’ombe AR, et al. Human immunodeficiency virus and risk of type 2 diabetes in a large adult cohort in Jos, Nigeria. Clin Infect Dis. 2016;63(6):830–835.
- Herrin M, Tate JP, Akgun KM, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr. 2016;73(2):228–236.
- Resnick HE, Valsania P, Halter JB, et al. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health. 2000;54(8):596–602.
- Luo J, Hodge A, Hendryx M, et al. BMI trajectory and subsequent risk of type 2 diabetes among middle-aged women. Nutr Metab Cardiovasc Dis. 2021;31(4):1063–1070.
- Manfredi R. HIV infection and advanced age emerging epidemiological, clinical, and management issues. Ageing Res Rev. 2004;3(1):31–54.
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med. 2007;23(3):567–583, vii.